Keywords
False-positive; HIV; Immunochromatography; Screening; Constitutional disposition; p24 antigen; HIV antibody
Introduction
With recent advancements in HIV screening and treatment, the rate of detection of HIV-infected patients has been increasing, and this condition can now be kept under control. A false-positive result from an HIV screening test may become apparent when a confirmation test is negative after the initial HIV screening test is positive. The incidence of false-positive results in HIV screening is 0.1-2.2% [1,2].
When an HIV screening test is performed preoperatively, it is rare that the test is repeated. We investigated the occurrence of continued false-positive results in patients who had a single false-positive HIV screening. There is a limit to survey the detailed drug usage of individual patients; therefore, we aimed to grasp the overall trend of false-positive results over a period of time.
Methods
HIV screening tests were performed for 31,041 individuals at the Kawasaki Medical School Hospital (Okayama, Japan) over three years, from April 2016 to March 2019. There were 13 cases out of 31,041 in which initial HIV screening produced a false-positive, and subsequent tests were carried out at least twice over the three years. When HIV screening was carried out using the same assay for three years, we investigated the length of time for which the results of testing of these 13 cases continued to produce false-positive results.
This study was conducted in accordance with the guidelines of the World Medical Association's International Code of Medical Ethics. Informed consent was obtained from all patients prior to participation. HIV screening tests were performed using fourth generation lateral flow immunochromatography Daina Screen HIV Combo assays (Daina Screen; Alere Medical Co. Ltd., Tokyo, Japan) to detect HIV-1/2 antibodies and HIV-1 p24 antigens simultaneously. Measurements were performed using the equivalence lot of the Daina Screen kit during the review period. For the plasma specimens that had been measured using an old lot, we remeasured some cryopreserved plasma specimens using the current lot, and analyzed the applicability of the resulting values [3].
The Daina Screen HIV Combo assay is based on a sandwich immunoassay technique. The assay uses a nitrocellulose strip with a sample dripping site comprised of a biotin-labeled p24 antibody and a conjugate site comprised of a colloid-labeled p24 antibody and colloid-labeled HIV-1 and HIV-2 antigens. When an HIV-1 p24 antigen or HIV antibody-positive plasma specimen is dropped on the sample dripping site, a complex of “biotin-labeled anti-p24 antibody-p24 antigen-colloid-labeled anti-p24 antibody” or “colloid-labeled HIV antigen-HIV antibody” is formed, depending upon the constituents of the sample. The complex of “biotinlabeled anti-p24 antibody-p24 antigen-colloid-labeled anti-p24 antibody” becomes trapped in solid phase avidin at the antigen detection site, and a red line resulting from the colloid is formed. Similarly, any colloid-labeled HIV antigen-HIV antibody complex gets trapped in the solid phase HIV antigen in the antibody detection part, and a red line derived from the colloid is generated (Figure 1a). The required quantity of plasma specimen is 50 μL, and the measurement duration is 20 minutes.
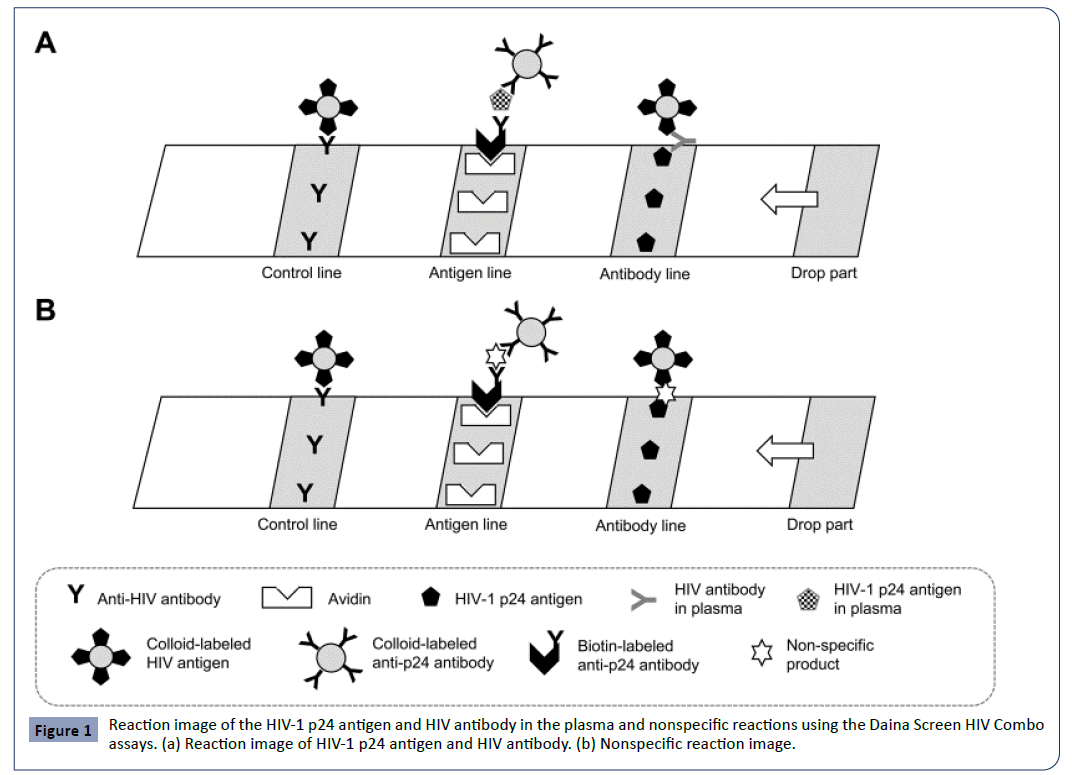
Figure 1: Reaction image of the HIV-1 p24 antigen and HIV antibody in the plasma and nonspecific reactions using the Daina Screen HIV Combo assays. (a) Reaction image of HIV-1 p24 antigen and HIV antibody. (b) Nonspecific reaction image.
When an HIV screening test was positive, the quantity of HIV-1 RNA was assayed using a COBAS TaqMan 48 Analyzer (COBAS TaqMan; Roche Diagnostics, Tokyo, Japan) [4], and the HIVspecific antibody from the Western blot kits LAV Blot I and II (WB; Bio-Rad Laboratories, Inc., Hercules, USA) [5] was used as a confirmation test. The characteristics of the 13 patients who were re-screened for HIV using the same assay for three years following false-positive results at the time of the first examination are shown in Table 1. The publication of this study was approved by the clinical research ethics committees of Kawasaki Medical School Hospital. IRB approval number: 3500, IRB approval date: 2019.5.21.
Table 1 Distribution of 13 cases for whom HIV test re-examination was performed following false positive HIV test results at the time of the first medical examination.
| Cases of false-positive HIV test results |
Age (years) |
Sex |
Clinical diagnosis operative procedure |
Pattern of the false-positive reaction |
At the first visit |
At the second visit |
At the third visit |
At the fourth visit |
| 1 |
80 |
M |
Sick sinus syndrome |
Transient |
F-pos. p24 antigen |
Negative result (295 days later) |
Not re-consultation |
|
| 2 |
12 |
F |
Superficial erosion |
Transient |
F-pos. p24 antigen |
Negative result (357 days later) |
Not re-consultation |
|
| 3 |
31 |
F |
Breast cancer |
Transient |
F-pos. p24 antigen |
Negative result (619 days later) |
Not re-consultation |
|
| 4 |
76 |
F |
Cerebral aneurysm |
Transient |
F-pos. antibody |
Negative result (304 days later) |
Not re-consultation |
|
| 5 |
20 |
F |
Pectus excavatum |
Transient |
F-pos. antibody |
Negative result (117 days later) |
Negative result (487 days later) |
|
| 6 |
48 |
F |
Breast cancer |
Transient |
F-pos. antibody |
Negative result (207 days later) |
Negative result (598 days later) |
Not re-consultation |
| 7 |
68 |
M |
Bronchiectasis |
Transient |
F-pos. antibody |
Negative result (268 days later) |
Negative result (618 days later) |
Not re-consultation |
| 8 |
65 |
F |
Revision arthroplasty |
Transient |
F-pos. antibody |
Negative result (220 days later) |
Negative result (515 days later) |
Not re-consultation |
| 9 |
77 |
M |
Excision of oral-maxillofacial malignancy |
Continuous ? |
F-pos. p24 antigen |
F-pos. p24 antigen (105 days later) |
Not re-consultation |
|
| 10 |
89 |
F |
Intraocular lens implantation |
Continuous ? |
F-pos. antibody |
F-pos. antibody ( 89 days later) |
Not re-consultation |
|
| 11 |
52 |
M |
Diabetic retinitis Skin abscess |
Continuous |
F-pos. antibody |
F-pos. antibody (145 days later) |
F-pos. antibody (523 days later) |
Not re-consultation |
| 12 |
76 |
F |
Intraocular lens implantation |
Continuous |
F-pos. antibody |
F-pos. antibody (269 days later) |
F-pos. antibody (800 days later) |
Not re-consultation |
| 13 |
75 |
F |
Tendon sheath incision |
Continuous |
F-pos. antibody |
F-pos. antibody (221 days later) |
F-pos. antibody (538 days later) |
F-pos. antibody (883 days later) |
Results
The time from the first medical examination to the second assessment ranged from 89 to 519 days, with an average of 235 days. The age of the patients at the time of the first medical examination in these 13 cases was 12–89 years, with a median of 68 years, and the male to female sex ratio was 4:9. In these 13 cases, tests for the HBs antigen, the HBc antibody, the HCV antibody, and the syphilis serum reactions were negative, and false-positive reactions for the serologic markers of hepatitis and syphilis were not observed. Of the 13 cases, four produced falsepositive results for the p24 antigen and nine were false-positive for the HIV antibody. Of the 13 cases, eight (Cases 1–8) produced negative reactions on serial reexamination, and five (Cases 9–13) continued to present false-positive reactions.
False-positive reactions to the p24 antigen or the HIV antibody were observed in Cases 1–8 at the time of the first medical examination, but were negative upon reexamination.
Case 1, an 80-year-old man, had sick sinus syndrome; Case 2, a 12-year-old girl, had skin sores; and Case 3, a 31-year-old woman, had undergone a mastectomy due to breast cancer. All of these cases presented false-positive reactions to the p24 antigen. Case 4, a 76-year-old woman, had a cerebral aneurysm; Case 5, a 20-year-old woman, had pectus excavatum; Case 6, a 48-year-old woman, had undergone mastectomy due to breast cancer; Case 7, a 68-year-old man, had bronchiectasis; and Case 8, a 65-yearold woman, had an artificial joint reimplantation. These cases produced false-positive reactions to the HIV antibodies.
In the two cases of mastectomy due to breast cancer, a falsepositive reaction to the HIV-1 p24 antigen was observed in Case 3, and a false-positive reaction to the HIV antibody was observed in Case 6. The false-positive reaction to the HIV-1 p24 antigen continued for 105 days in Case 9, a 77-year-old man with a resection of a malignant tumor of the chin. The falsepositive reaction to the HIV antibody continued from the first medical examination to days 89 and 800 in Cases 10, an 89-yearold woman, and 12, a 76-year-old woman with intraocular lens insertion.
In patients with ophthalmological problems, the false-positive reaction to the HIV antibody continued from the first medical examination to day 523 in Case 11, a 52-year-old man with diabetic retinopathy. The false-positive reaction to the HIV antibody continued from the first medical examination involving four hospital visits to consultation on day 883 in only one case, Case 13, a 75-year-old woman with a tendon sheath incision. Differences in false-positive results according to the classification of clinical evidence were not apparent. In 8 of the 13 cases the HIV false-positive result disappeared within three years. Five of the 13 cases continued to have false-positive results. In these five cases, we were able to confirm that the HIV false-positive results continued for 883 days from 89 days.
Discussion
The existence of false-positive results in immunoreaction has been reported in gynecological checkups [6], autoimmune diseases [7], preventive vaccination against infectious diseases [8], and lipid blood symptoms [9]. However, all these cases were discovered from isolated measurements. Therefore, even if HIV screening produces a false-positive result, these results are generally not followed up and investigated appropriately. It is difficult to analyze HIV false-positive results for every individual case, but it is important to understand the overall trend of screening results over time. The result of this study may lead to the production of recommendations for the follow-up of patients who generated a false-positive result.
In these 13 cases, therapeutic drugs such as painkillers were administered to the patients for the primary disease, and the HIV screening test was performed before the operation, following pretreatment. Therefore, the prescribed drugs and their metabolites may have affected the HIV screening test results. Fibrin microclots in the serum sample of the plasma specimens used for HIV screening tests, which might interfere with the assay, were removed [10].
We believe that “biotin-labeled anti-p24 antibody-nonspecific product (p24 antigen-like reaction material in a sample)-colloidlabeled anti-p24 antibody” or “colloid-labeled HIV antigennonspecific product (antibody-like reaction material in a sample)” may have been formed in the reaction process of the Daina Screen HIV Combo assay, and this might have resulted in falsepositive reactions (Figure 1b).
The HIV screening results of eight cases (Cases 1–8) were negative upon rescreening. We believe that a nonspecific product was formed because of the interaction between a therapeutic drug and the patients’ metabolisms, leading to transient false-positive reactions. These eight cases were reevaluated after an average of 298 days after the first false-positive result was obtained, and the nonspecific product formed might have disappeared within this time.
In Cases 9 and 10 monitoring will be continued to check whether false-positive results are persistent in the future. Of the 105 patients who underwent intraocular lens insertion over three years, only two cases (Cases 10 and 12) produced false-positive HIV testing results that were persistent over a long term. The constitution of individual patients may be a factor responsible for the false-positive reactions observed in Case 11 of diabetic retinitis, Case 12 of an intraocular lens insertion, and Case 13 of a tendon sheath incision, in whom false-positive HIV test results persisted for a long term.
It was challenging to continue HIV screening using the same assay for three years for these 13 cases, in comparison with obtaining the result of the one-time false-positive reaction. We were able to confirm that eight of the 13 cases tested as true-negative results by the end of this long term follow-up survey. In addition, for five of the 13 cases, we were able to confirm that the false-positive reaction continued long term.
By recording the time at which blood was drawn for repeat testing, we could correlate the conversion from false-positive to true-negative results with other factors in the patents’ lives. In approximately 61.5% (8/13) of the cases the false-positive results disappeared at around the time when doses of treatment drugs decreased. In approximately 38.5% (5/13) of cases, the falsepositive result continued over the long term. Individual differences in the metabolism of these patients may have contributed to the prolongation of the false-positive results.
Conclusions
It is important to understand the dynamics of HIV screening test results. A positive result causes stress to patients, and increases the cost and workload of the hospital, which needs to conduct follow-up tests to confirm or refute the results of the original test. Ideally, there should be a very low level of false-positive results returned by post-operative screening of patients. Investigations such as that reported here can help to provide an understanding of the circumstances, such as the administration of prescription drugs, which may lead to false-positive results, and hence enhance the efficiency of patient treatment.
Acknowledgments
We are grateful to Alere Medical Co., Ltd., Tokyo, for their generous technical support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of Interest
None.
26899
References
- Duong YT, Mavengere Y, Patel H, Moore C, Manjengwa J, et al. (2014) Poor performance of the determine HIV-1/2 Ag/Ab combo fourth-generation rapid test for detection of acute infections in a national household survey in Swaziland. J Clin Microbiol 52: 3743-3748.
- Rosenberg NE, Kamanga G, Phiri S, Nsona D, Pettifor A, et al. (2012) Detection of acute HIV infection: A field evaluation of the Determine® HIV-1/2 Ag/Ab combo test. J Infect Dis 205: 528-534.
- Alere HIV combo (2018) WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT.WHO PQ Public Report PQDx0243-013-00. 4.0 version.
- Pyne MT, Brown KL, Hillyard DR (2010) Evaluation of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test and identification of rare polymorphisms potentially affecting assay performance. J Clin Microbiol 48: 2852-2858.
- Davey RT, Deyton LR, Metcalf JA, Easter M, Kovacs JA, et al. (1992) Indeterminate western blot patterns in a cohoet of individuals at high risk for human immunodeficiency virus (HIV-1) exposure. J Clin Immunol 12: 185-192.
- Profitt MR, Yen-Lieberman B (1993) Laboratory diagnosis of human immunodeficiency virus infection. Infect Dis Clin North Am 7: 203-219.
- Bylund DJ, Ziegner UH, Hooper DG (1992) Review of testing for human immunodeficiency virus. Clin Lab Med 12: 305-333.
- Challakere K, Rapaport MH (1993) False-positive human immunodeficiency virus type 1 ELISA results in low-risk subjects. West J Med 159: 214-215.
- Schochetman G, George J (1992) AIDS testing. Methodology and management issues. Serologic tests for the detection of human immunodeficiency virus infection. Springer-Verlag, New York.
- Akl P, Blick KE (2014) A case of false-positive test results in a pregnant woman of unknown HIV status at delivery. Lab Med 45: 259-263.






