Keywords
Multiple sclerosis; 1H-MRS; Volumetric MRI; Clinical trials; Observational study (Cohort, Case-control)
Abbreviations
ARCS: Audio Recorded Cognitive Screen; Cr: Creatine; tCho: Total Choline; CRLB: Cramer-Rao Lower Bounds; tCr: Total Creatine; CSF: Cerebrospinal Fluid; DASS-21: Depression Anxiety Stress Scale; DMF: Dimethyl Fumarate; EDSS: Expanded Disability Status Scale; FLAIR: Fluid-Attenuated Inversion Recovery; Glu: Glutamate; Glx: Glutamate+Glutamine; GM: Gray Matter; GSH: Glutathione; 1H-MRS: Proton Magnetic Resonance Spectroscopy; LCModel: Linear Combination of Model Spectra; LSD: Least Significant Difference; mI: Myo-Inositol; MFIS: Modified Fatigue Impact Scale; MPRAGE: Magnetization- Prepared Rapid Gradient Echo; MSSS: Multiple Sclerosis Severity Score; MS: Multiple Sclerosis; NAA: N-Acetylaspartate; PBVC: Percentage Brain Volume Change; PCr: Phosphocreatine; PRESS: Point Resolved Spectroscopy; RRMS: Relapsing-Remitting Multiple Sclerosis; SPMS: Secondary Progressive Multiple Sclerosis; SDMT: Symbol Digit Modalities Test; WM: White Matter
Background
The mode of action of Dimethyl Fumarate (DMF) [1] in Multiple Sclerosis (MS) is thought to be partly due to its antioxidative properties, through modulation of systemic glutathione (GSH) activation, thereby attenuating oxidative stress and cellular loss in active MS [2]. To date, the impact of DMF on metabolic changes and oxidative status in the MS brain has not been extensively investigated.
The development of novel and non-invasive MR techniques such as 1H-MRS, enable monitoring of a number of chemical entities in the MS brain to be explored, including GSH [3,4]. Srinivasan et al. [5], demonstrated GSH levels to be significantly higher in gray matter (GM) compared to white matter (WM) in healthy controls (HCs) and reported a significant reduction in GM GSH in relapsing-remitting MS (RRMS). Also, regions of higher oxidative stress, within the WM T2 lesions in RRMS, have shown a depletion in GSH compared to normal appearing white matter [6]. In frontoparietal regions of secondary progressive MS (SPMS) patients, GSH levels are reduced compared to that measured in HCs [7,8]. Other studies confirmed lower GSH concentrations not only in SPMS but also in the total frontoparietal regions of primary progressive MS patients. Moreover, there was a positive association between the levels of GSH in this region with cognitive function, including memory and processing speed [9].
In the current study, we applied 1H-MRS to investigate longitudinal metabolic changes in the hippocampus of RRMS patients following the initiation of DMF treatment. We also explored if hippocampal neurometabolite changes in RRMS were associated with severity of clinical and neuropsychological symptoms.
Methods
Patients and healthy control subjects
Twenty patients with confirmed RRMS, in accordance with the McDonald criteria [10], aged between 20 to 55 years, who were considered eligible to commence DMF treatment by their treating neurologist, were included in this study. HCs (N=20) were age (± 2 years) and sex-matched to the RRMS cohort. Out of the remaining 20 RRMS patients, 7 could not be evaluated at 2 years due to various reasons such as pregnancy, having stopped DMF due to side effects or patient’s choice.
All patients were recruited from the John Hunter Hospital, Newcastle, Australia, MS outpatient clinic. Age and sex-matched HC were derived from the Hunter Medical Research Institute (HMRI) research register and needed to comply to the study inclusion criteria, which included passing an MRI safety clearance, as well as being able to comply with all study procedures. Institutional Review Board approval was obtained from the Hunter New England Local Health District Human Research Ethics Committee, with written informed consent obtained from all subjects prior to undertaking any study-related procedures. All scans were conducted between December 2015 and March 2018.
Study design
In the RRMS cohort, an open-label longitudinal observational study was conducted to evaluate the impact of DMF treatment on the hippocampal metabolite profile. We also conducted a cross-sectional evaluation prior to and at 24 months post DMF treatment inception, between RRMS patients and HCs. MRI/MRS data was acquired from the RRMS cohort at five different time points; Baseline (T0, pre-DMF onset), 1 month (T1), 6 months (T6), 12 months (T12) and 24 months (T24) post inception of DMF treatment onset. HCs were scanned at baseline and 24 months. DMF dosing was escalated over the first month of treatment, to achieve a therapeutic dose of 480 mg/day according to the following regime: 120 mg/day week 1, 240 mg/day week 2, 360 mg/day week 3 and 480 mg/day from week 4 onwards.
MRI acquisition and structural assessments
All MRI/MRS scans were undertaken on a 3T Prisma (Siemens Healthineers, Erlangen, Germany) MRI scanner equipped with a 64-channel head and neck coil located at the HMRI, Newcastle, NSW, Australia. Experimental parameters of the threedimensional isotropic T1-weighted Magnetization-Prepared Rapid Gradient Echo (MPRAGE) were as follows; sagittal orientation, TR/TE/TI=2000/3.5/1100 ms, 7o flip angle, Field of View (FOV)=256 × 256 mm, pixel size=1 × 1 × 1 mm3, NEX=4 and acquisition time=5 minutes. Three-dimensional T2 FLuid- Attenuated Inversion Recovery (T2-FLAIR) sequence, TR/TE/ TI=5000/386/1800 ms, 12o flip angle, FOV=256 × 256 mm, pixel size=1 × 1 × 1 mm3, echo train duration=858 ms, NEX=1 and acquisition time=4 minutes.
Quantification of hyperintense WM lesions were performed using T2-FLAIR data, where total lesion volumes were derived using the SPM platform. Annualized atrophy changes in Percentage Brain Volume Change (PBVC) for 13 RRMS patients in (Baseline, T12, and T24) were assessed using SIENA [11].
SPM [12] was used to segment the spectroscopic voxel into the cerebrospinal fluid (CSF), GM and WM. For accuracy in the MRS voxel re-positioning, during longitudinal re-assessment, MPRAGE data was reconstructed into 1 mm coronal and axial slices on the scanner. Lesions within the MRS voxel were segmented using the lesion growth algorithm described by Quadrelli et al. [13].
1H-MRS acquisition, post-processing, and analyses
One-dimensional (1D) 1H hippocampal MRS was applied using a Point resolved Spectroscopy (PRESS) sequence at short echo time, acquired from the Region Of Interest (ROI), as shown in Figure 1.
Figure 1: Hippocampal voxel size and position: T1-weighted MR images from a multiple sclerosis patient in coronal, sagittal and axial planes demonstrating the hippocampal voxel size and position (white box).
The following parameters were used: TR/TE=2000/30 ms, hippocampal voxel size=30 × 15 × 15 mm3, averages=96, vector size=1024 points, preparation scans=4, RF offset frequency=3.02 ppm and water suppression with scan time 3.2 minutes. Water reference was also acquired (4 averages, scan time=8 sec) from the same voxel position and size after disabling RF part of water suppression module.
Although non-edited MRS is less commonly used than Jedited MEGA-PRESS sequences [14] for detection of low concentration metabolites such as GSH, others have shown that non-edited MRS can be used to measure GSH reliably [15,16]. We validated non-edited MRS (TE=30 ms) by acquiring MRS data from two phosphate-buffered GSH phantoms (3 and 6 mM, pH=7.4) and fitting them using an automated linear combination of model spectra (LCModel, v6.2-2B) [17] software package. Both phantoms contained equal amounts of NAA (12.5 mM), creatine (Cr) (10 mM) and choline (Cho) (3 mM). LCModel fitting yielded the correct ratios of GSH/Cr in both phantoms. Samples of in-vivo MR spectra from hippocampus and in-vitro MRS data analyzed by LCModel are shown in Figure 2.
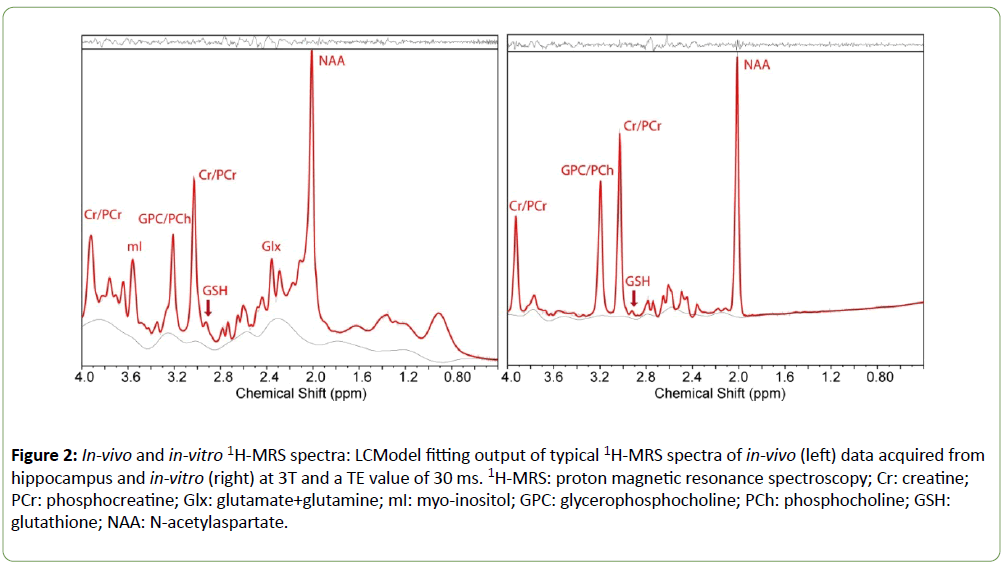
Figure 2: In-vivo and in-vitro 1H-MRS spectra: LCModel fitting output of typical 1H-MRS spectra of in-vivo (left) data acquired from hippocampus and in-vitro (right) at 3T and a TE value of 30 ms. 1H-MRS: proton magnetic resonance spectroscopy; Cr: creatine; PCr: phosphocreatine; Glx: glutamate+glutamine; mI: myo-inositol; GPC: glycerophosphocholine; PCh: phosphocholine; GSH: glutathione; NAA: N-acetylaspartate.
Single voxel 1D MRS was transferred offline and analyzed with LCModel using a basis set specifically designed for 3T and TE=30 ms with water normalization. This technique allowed the estimation of overlapping resonant metabolites such as glutamine+glutamate (Glx) at short TE. A water reference scan was used for eddy-current correction as well as partial volume correction in LCModel by adjusting ‘WCONC’ parameter based on percentages of WM, GM, and CSF as described in the LCModel manual. Concentrations of the brain metabolites were expressed as a ratio with respect to total creatine (Cr +phosphocreatine (PCr)=tCr) with Cramer-Rao lower bound (CRLB (SD%) less than or equal 20% accepted.
Quality control
Maintenance of quality control for MRI and MRS data was carried out by weekly scanning of the American College of Radiologists (ACR) phantom and spherical GE spectroscopic phantom [18] containing stable brain metabolites at physiological pH and concentrations.
Clinical assessments
Disability status was evaluated in the RRMS group, prior to and at 12 and 24 months following the inception of DMF treatment, by applying the Expanded Disability Severity Scale (EDSS). All EDSS evaluations were performed by a neurologist who had undertaken appropriate neuro status certification training. The Multiple Sclerosis Severity Score (MSSS) was calculated using the EDSS and duration of disease for each patient according to the algorithms provided by Roxburgh et al. [19].
Similarly, all study participants (RRMS and HCs) were assessed for cognitive performance at baseline, 12 and 24 months using the Audio Recorded Cognitive Screen (ARCS), which is a valid and reliable instrument for administering neuropsychological tests of cognitive function to unsupervised individuals [20]. The ARCS assesses performance in the domains of memory, verbal fluency, language (object naming), visuospatial function and attention with elements from each domain score then used to derive an overall ‘global’ cognitive performance score. The Symbol Digit Modalities Test (SDMT) was undertaken concurrently as a measure of attention and information processing speed presented in the visual modality.
The mental health status of participants was assessed using the short version of the Depression Anxiety Stress Scale (DASS-21) [21]. Higher scores were indicative of higher levels of depression, stress, and anxiety. All scores, derived from the 21- point scale, were multiplied by 2 to enable comparison to the full 42-point scale DASS and determine clinical cutoffs for symptom severity.
Fatigue status was determined using the Modified Fatigue Impact Scale (MFIS), a modified form of the Fatigue Impact Scale [22]. The questionnaire was based on items derived from interviews with MS patients concerning how fatigue impacts their lives. This instrument provided an assessment of the effects of fatigue in terms of physical and cognitive functioning.
Statistical analysis
To investigate the significant difference between MS and HCs groups, T-tests were applied using SPSS, for independent and paired sample analyses. Major brain metabolites (NAA, Cr, total choline (tCho), mI, GSH and Glx) were analyzed from MS patients and HCs at different time points. The level of significant change in metabolite levels associated with the onset of DMF treatment was assessed using repeated measures ANOVA, adjusted for appropriate covariates, followed by post hoc testing using the Least Significant Difference (LSD). Additionally, the correlation between clinical symptoms and metabolite levels was performed using the correlation coefficient for nonparametric correlations (Spearman’s rho).
Results
Participant demographics and characteristics
All of the 20 recruited RRMS patients met the enrollment criteria. Patients were predominantly female, early in their disease course with mild disability (EDSS 2 ± 0.18) (Table 1). The cross-sectional evaluation showed significant differences in the severity of mood symptoms, fatigue status and cognition impairment in the RRMS patients compared to age and sexmatched HCs at baseline and at two years (Table 1). There were no significant changes in levels of clinical symptoms for HCs during the 24-months period (baseline and 24 months) (Table 1). Reliable data were obtained from HCs and MS participants at each time point. Additionally, using repeated measures of ANOVA, longitudinal analysis (treatment effect) showed no statistical change was observed in the level of any clinical symptoms, EDSS, MSSS and disease duration for DMF treatment for 13 RRMS patients at three time points (T0, T12 and T24) over the two years (p>0.4, F<0.7). We observed no statistical changes in the level of severity of disability at DMF treatment onset and at the 24 months post-DMF treatment.
| Characteristics |
Baseline |
2 yrs follow up |
| HCs |
MS |
p-value |
HCs |
MS |
p-value |
| (N=20) |
(N=20) |
|
(N=13) |
(N=13) |
|
| Sex (% female) |
80% |
80% |
0.884 |
85% |
85% |
0.684 |
| Age |
35 ± 1.62 |
35 ± 1.64 |
0.921 |
37 ± 1.62 |
37 ± 1.64 |
0.721 |
| Disease duration (yrs) |
- |
5 ± 1.3 |
- |
- |
6 ± 1.50 |
- |
| EDSS |
- |
2 ± 0.18 |
- |
- |
2 ± 0.41 |
- |
| MSSS |
- |
3.75 ± 0.4 |
- |
- |
3 ± 0.71 |
- |
| Total ARCS |
93 ± 2.84 |
82 ± 3.44 |
0.015 |
95 ± 4.05 |
83 ± 5.33 |
0.096 |
| Memory |
92 ± 2.78 |
87 ± 4.74 |
0.346 |
96 ± 2.34 |
83 ± 7.94 |
0.173 |
| Fluency |
92 ± 4.02 |
81 ± 3.44 |
0.053 |
92 ± 5.72 |
84 ± 5.84 |
0.333 |
| Visuospatial |
100 ± 0.6 |
102 ± 0.92 |
0.04 |
100 ± 1.54 |
101 ± 1.01 |
0. 211 |
| Language |
92 ± 4.16 |
81 ± 4.43 |
0.066 |
94 ± 6.12 |
80 ± 3.83 |
0.066 |
| Attention |
101 ± 2.43 |
91 ± 3.42 |
0.015 |
101 ± 2.37 |
97 ± 4.62 |
0.437 |
| SDMT |
63 ± 3.27 |
52 ± 2.51 |
0.01 |
64 ± 2.49 |
53 ± 3.47 |
0.02 |
| DASS-21 |
10 ± 1.76 |
36 ± 5.95 |
0 |
8 ± 1.67 |
23 ± 4.57 |
0.006 |
| Stress |
6 ± 1.29 |
17 ± 2.54 |
0.001 |
5 ± 0.82 |
15 ± 2.71 |
0.003 |
| Anxiety |
2 ± 0.53 |
10 ± 2.07 |
0 |
1 ± 0.41 |
5 ± 1.27 |
0.004 |
| Depression |
2 ± 0.34 |
10 ± 1.94 |
0 |
2 ± 0.97 |
6 ± 1.81 |
0.044 |
| MFIS |
12.95 ± 2.48 |
37.4 ± 3.87 |
0 |
12 ± 4.44 |
29 ± 4.47 |
0.01 |
| Physical fatigue |
5 ± 1.06 |
18.1 ± 2.03 |
0 |
5 ± 2.27 |
13.1 ± 2.36 |
0.021 |
| Cognitive fatigue |
7.9 ± 1.64 |
19.32 ± 2.06 |
0 |
7 ± 2.27 |
16 ± 2.34 |
0.008 |
Note: Data are expressed as mean values ± SEM; MFIS: Modified Fatigue Impact Scale; SDMT: Symbol Digit Modalities Test; DASS-21: Depression Anxiety Stress Scales; EDSS: Expanded Disability Status Scale; MSSS: Multiple Sclerosis Severity Score; ARCS: Audio Recorded Cognitive Screen
Table 1: Cross-sectional analysis of mean demographic scores and disease-related variables for MS and HCs groups at baseline and 2 years.
Morphology (whole brain and voxel characteristics)
There was a significant variation in the MRS voxel composition (GM, WM, and CSF fractions) between HCs and MS patients using cross-sectional analysis, with a significant reduction in the WM fraction within the hippocampal voxel in the RRMS cohort at baseline (pre-DMF onset) compared to the HC group. We observed no significant difference at 24 months follow up post- DMF treatment start in voxel composition between HCs and MS patients. In addition, we saw no significant change in the total brain volume between the RRMS cohort and HCs at baseline and also at 2 yrs (Table 2). Interestingly, within the RRMS cohort, the longitudinal analysis showed the onset of DMF treatment had a significant impact on the WM voxel composition (F=2.65, p=0.04) up to 24 months post-treatment. We saw no significant change in the whole brain T2-FLAIR lesion load, during the 24- month DMF treatment period (F=1.24, p=0.12) (Table 2). There were no morphological changes during the 24-months DMF treatment period.
| |
Regions |
HCs |
MS baseline |
T1 |
T6 |
T12 |
T24 |
HCs
(2 yrs) |
F |
p |
| Sample size |
N=20 |
N=13 |
|
|
| MRS voxel |
CSF% |
3.2 ± 0.008 |
4.7 ± 0.006 |
3.8 ± 0.005 |
3.8 ± 0.005 |
3.5 ± 0.004 |
3.9 ± 0.006 |
4.4 ± 0.019 |
1.33 |
0.272 |
| GM% |
40 ± 0.009 |
46 ± 0.025 |
47.8 ± 0.006 |
47.6 ± 0.007 |
45 ± 0.02 |
46.5 ± 0.008b |
40.1 ± 0.02 |
2 |
0.101 |
| WM% |
55.7 ± 0.011 |
46 ± 0.024a |
48 ± 0.007 |
48 ± 0.008 |
51 ± 0.022 |
49.6 ± 0.01 |
55.4 ± 0.045 |
2.65 |
0.04 |
| lesion voxel (mm3) |
- |
0.011 ± 0.005 |
0.030 ± 0.012 |
0.024 ± 0.01 |
0.016 ± 0.009 |
0.023 ± 0.006 |
- |
1.82 |
0.152 |
| Whole brain (mm3) |
Whole brain volume |
1615.3 ± 27.4 |
1596.9 ± 14.3 |
- |
- |
- |
1601.2 ± 16.5 |
1621.6 ± 24.1 |
- |
- |
| T2 lesion volume |
- |
4.9 ± 0.9 |
4.83 ± 0.88 |
4.82 ± 0.98 |
4.7 ± 0.98 |
4.7 ± 0.98 |
- |
1.24 |
0.12 |
Note: a: p ≤ 0.001 MS Vs Hcs At Baseline; b: p ≤ 0.01 MS Vs HCs At 2 Year Follow Up; CSF: Cerebrospinal Fluid; GM: Gray Matter; MRS: Magnetic Resonance Spectroscopy; T1: 1 Month Of Treatment; T6: 6 Months Of Treatment; T12: 12 Months Of Treatment; T24: 24 Months Of Treatment; WM: White Matter
Table 2: Mean values of spectroscopic voxel segmentation and volume of brain fractions for RRMS patients compared to HCs showing treatment effect across time points.
Annualized atrophy changes of PBVC were analyzed for 13 RRMS patients at three time periods (T0 vs T12, T12 vs T24 and T0 vs T24), yielding -0.35 ± 0.14, -0.40 ± 0.12 and -0.55 ± 0.19, respectively. No substantial change was observed in the average annualized rate of brain volume loss between 1st and 2nd year of treatment with DMF. PBVC which was analyzed for the matched 13 HCs for only one time period (T0 vs T24), yielding (-0.19 ± 0.11), is significantly lower atrophy rate than that observed in the patient cohort.
MR spectroscopy
Using single voxel 1D MRS, the cross-sectional analysis identified a statistically significant reduction in hippocampal NAA (-13%, p=0.0001) and increase in mI (+9%, p=0.02) in the total RRMS cohort at baseline, compared to HCs (Figure 3). In contrast, we did not observe any significant difference in the level of hippocampal Glx, Cr, tCho or GSH between the RRMS group at baseline compared to HCs (data not shown). When assessing only the cohort that was followed up (N=13), the cross-sectional analysis showed no statistical significance (p>0.05) in hippocampal metabolites (NAA, Glx, Cr, tCho, mI, and GSH) between the RRMS cohort (N=13) and matched HCs (N=13) at the 24-month time point. No statistically significant difference was observed in MRS data between HCs at baseline compared to HCs at 2 yrs and between MS at baseline compared to MS at 2 yrs.
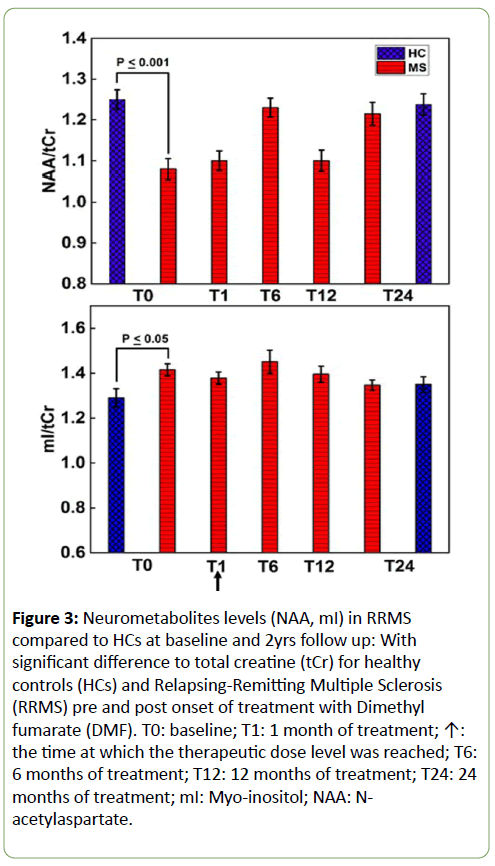
Figure 3: Neurometabolites levels (NAA, mI) in RRMS compared to HCs at baseline and 2yrs follow up: With significant difference to total creatine (tCr) for healthy controls (HCs) and Relapsing-Remitting Multiple Sclerosis (RRMS) pre and post onset of treatment with Dimethyl fumarate (DMF). T0: baseline; T1: 1 month of treatment; ↑: the time at which the therapeutic dose level was reached; T6: 6 months of treatment; T12: 12 months of treatment; T24: 24 months of treatment; mI: Myo-inositol; NAA: Nacetylaspartate.
Longitudinal analysis showed the onset of treatment with DMF did not significantly impact on the levels of hippocampal NAA, mI, Glx, tCho or Cr in the RRMS group. However, as the DMF treatment progressed, the mean hippocampal GSH levels were altered significantly over the 24-month treatment period (F=3.5, p<0.03) (Figure 4). Post hoc tests revealed that there was a statistically significant reduction of -28% in GSH levels from the baseline pre-treatment time point to T1 (0.6 ± 0.061 vs 0.43 ± 0.039, p=0.014). This reduction remained statistically significant at T6 (0.45 ± 0.03, p=0.035), but slightly increased at T12 (0.48 ± 0.045, p=0.15) and T24 (0.49 ± 0.029, p=0.18) to approach levels seen in the HC group.
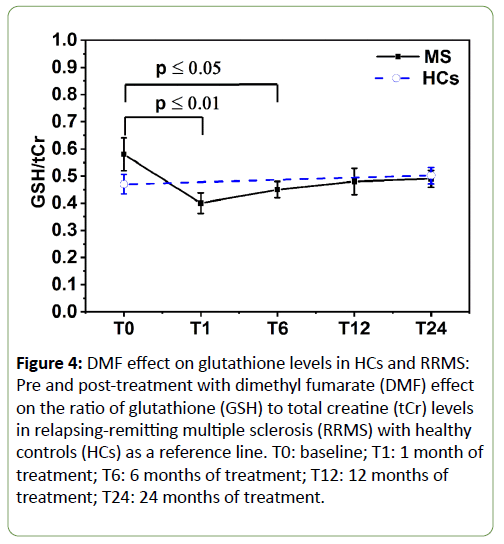
Figure 4: DMF effect on glutathione levels in HCs and RRMS: Pre and post-treatment with dimethyl fumarate (DMF) effect on the ratio of glutathione (GSH) to total creatine (tCr) levels in relapsing-remitting multiple sclerosis (RRMS) with healthy controls (HCs) as a reference line. T0: baseline; T1: 1 month of treatment; T6: 6 months of treatment; T12: 12 months of treatment; T24: 24 months of treatment.
Correlation of metabolites with clinical and volumetric measures
We investigated associations between clinical symptoms and hippocampal metabolite levels at the baseline, 12-month and 24-month post-DMF treatment onset. The clinical symptoms that showed the strongest associations with hippocampal metabolite levels were related to mood status at T12 and T24 (Table 3).
| Time points |
Metabolite/tCr |
Clinical parameters |
| T0 |
Glx |
EDSS |
MSSS |
D D |
WM |
- |
| -0.593* |
0.663* |
-0.754** |
-0.577* |
| NAA |
- |
MSSS |
WBV |
WM |
- |
| 0.637* |
0.657* |
-0.654* |
| T12 |
mI |
SDMT |
DASS-21 |
Depression |
Anxiety |
Stress |
| -0.551* |
0.695** |
0.634** |
0.687** |
0.549* |
| tCho |
- |
DASS-21 |
Depression |
Anxiety |
Stress |
| 0.554* |
0.543* |
0.647* |
0.540* |
| Glx |
- |
- |
- |
Anxiety |
- |
| 0.499* |
| T24 |
Cr |
- |
DASS-21 |
Depression |
- |
Stress |
| 0.691* |
0.639* |
0.631* |
| mI |
- |
- |
D D |
- |
- |
| 0.595* |
| Glx |
- |
- |
D D |
- |
- |
| -0.572* |
| NAA |
TARCS |
Attention |
- |
- |
- |
| 0.624* |
0.626* |
Note: *p ≤ 0.05; **p ≤ 0.01. Cr: creatine; DASS-21: depression anxiety stress scales; D D: disease duration; EDSS: expanded disability status scale; Glx: glutamate+glutamine; NAA: N-acetylaspartate; mI: myo-inositol; MSSS: multiple sclerosis severity score; SDMT: symbol digit modalities test; TARCS: total audio recorded cognitive screen; tCho: total choline; T0: baseline; T12: 12 months of treatment; T24: 24 months of treatment; WBV: whole brain volume; WM: white matter
Table 3: Spearman’s correlation between neurometabolites ratios and cognitive functions in RRMS across 24 month study period. Only statistically significant changes are listed.
There was a positive correlation between the levels of hippocampal tCho, mI and Cr with the overall severity of mood symptoms (total DASS-21 scores), as well as the levels for each domain, depression, anxiety and stress (Table 3), while Glx levels showed an association with anxiety levels. The cognitive domains evaluated by the ARCS displayed associations with hippocampal NAA only at T24, while processing speed and attention, determined by the SDMT, and were negatively correlated with hippocampal mI at T12. Other clinical symptoms that showed the associations with hippocampal metabolite levels were related to disability status, MSSS score, at baseline and T24 (Table 3). There was a positive correlation between the levels of hippocampal Glx and NAA with the MSSS score at baseline (Table 3), while Glx levels showed an association with duration of disease at baseline and T24. There was evidence for hippocampal axonal integrity (NAA/tCr) to be negatively correlated with voxel CSF (r=-0.47) as well as whole brain lesion volume score (r=-0.66) at T12 and positively correlated with total brain volume (r=0.65 and 0.68) at baseline and T12.
Discussion
Our cohort of 20 RRMS patients was clinically stable while undergoing treatment with DMF. The hippocampus is involved in learning and memory function and has the highest degree of neuroplasticity in the brain, with regional neurogenesis occurring throughout adult life. In MS, changes in hippocampal proliferation, lesion load, volume, and connectivity have been associated with alterations in cognitive and mood function in the EAE mouse model of MS [23] and in MS patients [24]. Despite their relatively short disease duration and low disability score, our RRMS cohort displayed cognitive impairment, evidenced by poorer visuospatial, fluency and attention scores, as well as lower overall performance scores on the ARCS than the HCs. Similarly, the RRMS cohort had worse attention and processing speeds in comparison to HCs, resulting in lower SDMT scores. Our study demonstrated no statistical change in the level of clinical symptoms, EDSS, MSSS for the study periods (T0-T12 and T12-T24) while being treated with DMF over the two years. These results cannot be interpreted as supportive of predicting a reduction of progression of disability later on. However, the protective value of treatment on disability may be evident when evaluated over a longer period of time.
In spite of the fact that the whole brain lesion load was low in our patient group, our findings indicated a reduction in total brain volume and partial GM volume within the hippocampal voxel, compared to healthy controls in cross-sectional and longitudinal studies. Reduction in brain volumes has been correlated to an increased risk of disease progression rates and a decrease of treatment effect in MS [25].
Previous literature [26] reported a faster rate of atrophy in MS patients (0.5-1.35% per year) compared to age-matched HCs (0.1-0.3% per year), which is consistent with our annual PBVC value of -0.4 and -0.55 for RRMS cohort and -0.19 for HCs group over 2 years. Therefore, the average annualized rate of brain volume loss over 2 years while receiving treatment with DMF, might suggest an effect of DMF on brain volume, which was described in the pivotal trials [27]. A lower rate of brain volume loss has been associated with a benefit on disease progression in MS [28].
Using single voxel MRS, we confirmed the importance of NAA and mI as indicators of axonal loss and gliosis [29]. We found a significant reduction in hippocampal NAA and an increase in mI in RRMS in comparison to age and sex-matched healthy individuals in the cross-sectional analyses. Furthermore, these changes were associated with morphological changes within the brain including increased CSF volume, total brain volume loss, and T2 lesion volume. The use of NAA as a marker of neuronal integrity was further supported by the reduction in NAA levels associated with an 18% lower white matter content in the hippocampal voxel compared to that seen in the same region in healthy controls. Indeed, others have shown an increase in white matter NAA content following treatment with MS diseasemodifying therapy [30]. Reduced level of NAA in the grey matter of MS patients has also been recently demonstrated [31] further supporting the importance of NAA as a disease marker. Increased levels of mI have been detected by others in the CSF from MS patients [32] and also in the T1-weighted hypo-intense chronic MS lesions [33], the latter thought to be associated with astrogliosis around the lesion [34].
Administration of DMF did not result in changes in the levels of NAA and mI during the 24-month treatment period. This may suggest that hippocampal neuronal integrity and the level of microglial gliosis was not significantly impacted on during this time frame consistent with no significant change in clinical parameters over this time period.
There were no metabolic changes in the HCs brain between baseline and 2 years follow up. This confirmed the reliability of the MRS technique, and that changes observed in the MS cohort are clinically meaningful.
We observed a trend for higher hippocampal GSH in the RRMS group at baseline compared to the HCs. However, the variability in these levels was high and hence it did not reach significance. This finding may suggest a variable level of oxidative status in the hippocampus within our RRMS cohort, at the pre-treatment onset time point, although none of the patients had received disease-modifying therapies within the preceding month, or steroid treatment in the preceding three months.
A significant reduction in GSH levels in the frontoparietal region has been reported by others in secondary progressive MS patients compared to RRMS [9]. The same study also reported a trend for reduced levels of GSH in this same region in RRMS compared to HCs. We know that our hippocampal voxel was comprised of approximately equal portions white and grey matter, with an increase of WM fraction during the duration of our study, however, the cellular source of the GSH cannot be determined with certainty. Others have shown at 7T, a differential reduction of GSH in the grey matter of MS patients, with no impact on white matter GSH [5]. In studies where reduced levels of GSH in progressive MS have been shown [9], spectral editing techniques were applied to optimize GSH localization, this differed in our study in which we have undertaken a series of in-vitro scans, using GSH phantoms, to validate our LCModel post-processing quantification techniques, to confirm an accurate measurement of GSH.
It has been hypothesized that a possible mode of action of DMF is by its anti-oxidative effects via modulation of GSH activity [2,35]. This has been illustrated in-vitro [35] following DMF administration, however, the current study is the first in vivo investigation of the impact of DMF treatment on hippocampal GSH metabolism in MS patients. We saw an initial decline in GSH levels during the first month of treatment. It should be noted that the dosing regimen for DMF involves a weekly dose escalation over a 4 week period until the recommended therapeutic level (480 mg daily) is achieved. Between the first and 24 months of treatment, we observed an increasing trend for GSH levels within the hippocampus, approaching that seen in age and sex-matched healthy controls. Although not conclusive, our findings do support in-vitro studies which have demonstrated an increase in GSH levels in astrocytes following the addition of DMF [35]. A corresponding decreasing trend in lesion volume within the voxel was observed during this period but did not reach significance. It may be warranted to investigate a longer DMF treatment period in a larger cohort to determine an association between hippocampal GSH levels and treatment efficacy but our study suggests that GSH is a more sensitive marker than morphological changes.
We saw a correlation between attention and processing speed, with an increase in hippocampal mI levels over 12 months. This may suggest an increase in hippocampal gliosis with altered cognitive function in our MS cohort. It also indicates that hippocampal mI levels may be a surrogate marker for cognitive function, although larger longitudinal studies are warranted to verify this association. The cognitive domains evaluated by the ARCS displayed associations with hippocampal NAA of RRMS at T24 only. This result is consistent with a previous study [29] that showed that decreasing NAA correlated with cognitive dysfunction as well as with disability in RRMS patients using 1H-MRS. We have recently shown that there is a complex interplay between mood disorders such as depression, anxiety, and stress with cognitive performance evidenced by lower ARCS scores in RRMS [36].
Using Positron Emission Tomography (PET) imaging, Colasanti et al., [37] have demonstrated hippocampal microglial activation to be associated with brain connectivity and depressive symptoms. Changes in connectivity with the hippocampus have also been demonstrated in depressed MS patients with alterations being more prominent in depressed compared to non-depressed patients [38]. As expected, the DASS self-report questionnaire confirmed higher levels of depression, anxiety, and stress in the RRMS cohort compared to HCs. We also observed an association between mI, tCho and Cr levels with mood symptoms at T12 and T24. This may represent an increase in gliosis in the hippocampus with increased depression, anxiety and stress symptoms in our cohort.
Despite not detecting any alterations in hippocampal Glx levels, we did observe a positive correlation between hippocampal Glx and anxiety at T12, and between Glx and MSSS levels at baseline. However, Glx was negatively correlated with disease duration at baseline and T24, and with EDSS at baseline. Glx correlation with EDSS level at baseline has been reported by Chard et al. in normal appearing white matter and normal appearing cortical grey matter [39]. However, no correlation between Glx and disease duration was found by Chard et al. Others have demonstrated altered levels of glutamate (Glu) in multiple sclerosis, although regional variations have been noted [40]. Elevation in Glu has been observed in white matter lesions but not in normal appearing white matter [41] while in the hippocampus, a depletion in Glu and Glx was measured [40], which was in turn shown to be associated with visual and verbal memory impairment. The underlying pathophysiological and metabolic changes associated with MS anxiety have not been evaluated as extensively as those for MS depression. Further studies are needed to fully appreciate the relevance of a link between hippocampal Glx and anxiety in MS.
This study is the first to illustrate a change in hippocampal metabolism associated with the onset of treatment with DMF in RRMS patients. However, there are a number of limitations to this study. The findings are preliminary and need to be confirmed in a larger patient cohort over an extended treatment period to enable longer-term impacts of DMF on disease outcomes and metabolic changes to be more fully explored. In the current study, we did not employ spectral editing schemes to optimize detection of GSH as utilized in other studies which have investigated the changes in GSH associated with MS [5,42]. We have instead, undertaken a series of in-vitro investigations to support the validity of spectral post-processing and quantification using LCmodel for our MRS spectral analysis. This is supported by ensuring that CRLB of GSH quantification produced by LCModel was always less than 20%, thus reducing the contribution of any possible contaminants such as Gammamino Butyric Acid (GABA).
Conclusion
This study demonstrated that MRS is a sensitive marker of disease activity with several metabolites correlated with clinical parameters, but also capable to detect a treatment effect prior to the volumetric change. We have shown that treatment with DMF may impact on hippocampal metabolism, specifically glutathione levels, which supports its assumed anti-oxidant mode of action, resulting in an anti-inflammatory effect in the MS brain following DMF treatment.
Acknowledgement
The authors thank the patients and controls who participated in this study and the Imaging Centre of the University of Newcastle and Hunter Medical Research Institute.
Funding
Independent investigator-initiated grant provided by Biogen Pharmaceuticals Pty Ltd.
Availability of Data and Materials
Availability of data and materials are subject to guidelines of local Hunter New England Local Health District Human Research Ethics Committee.
Authors’ Contributions
OA has been involved in writing, compiling and revising the manuscript critically to suit publication standards. JLS, KR, RL, and SR contributed significantly on study design, data collection, revising, literature and critical suggestions to reshape the manuscript. All authors read and approved the final version of the manuscript.
Ethics Approval and Consent to Participate
This study was approved by the Institutional Review Board from the Hunter New England Local Health District Human Research Ethics Committee (HNEHREC Reference No: 4/09/10/3.01), Newcastle, NSW, Australia.
Consent for Publication
Written informed consent was obtained from the patients/ healthy subjects for publication of their individual details and accompanying images in this manuscript. The consent forms are held by the corresponding author in the patients’ clinical notes and are available for review by the Editor-in-Chief upon request.
Competing Interests
OA, KR, RL, and SR have no competing interests. JLS has accepted travel compensation from Novartis, Biogen, and Merck. Her institution receives the honoraria for talks and advisory board commitment and as well as research grants from Biogen, Genzyme Sanofi, Merck, Novartis, Roche, and TEVA.
23800
References
- Bomprezzi R (2015) Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: An overview. Ther Adv Neurol Disord 8: 20-30.
- Ferreira B, Mendes F, Osorio N, Caseiro A, Gabriel A, et al. (2013) Glutathione in multiple sclerosis. Br J Biomed Sci 70: 75-79.
- De Stefano N, Bartolozzi ML, Guidi L, Stromillo ML, Federico A (2005) Magnetic resonance spectroscopy as a measure of brain damage in multiple sclerosis. J Neurol Sci 233: 203-208.
- Katsavos S, Anagnostouli M (2013) Biomarkers in multiple sclerosis: an up-to-date overview. Mult Scler Int 2013.
- Srinivasan R, Ratiney H, Hammond-Rosenbluth KE, Pelletier D, Nelson SJ (2010) MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magn Reson Imaging 28: 163-170.
- Henry R, Li Y, Zhu A, Leppert D, Seneca N, et al. (2015) 1-H MRSI in patients with relapsing multiple sclerosis at 7 Tesla (P6. 121). Neurol 84: P6-121.
- Choi C, Ganji SK, DeBerardinis RJ, Dimitrov IE, Pascual JM, et al. (2011) Measurement of glycine in the human brain in vivo by 1H?MRS at 3 T: application in brain tumors. Magn Reson Med 66: 609-618.
- Choi IY, Lee P, Hughes AJ, Denney DR, Lynch SG (2017) Longitudinal changes of cerebral glutathione (GSH) levels associated with the clinical course of disease progression in patients with secondary progressive multiple sclerosis. Mult Scler 23: 956-962.
- Choi IY, Lee P, Adany P, Hughes AJ, Belliston S, et al. (2017) In-vivo evidence of oxidative stress in brains of patients with progressive multiple sclerosis. Mult Scler 24: 1029-1038.
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, et al. (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69: 292-302.
- Smith SM, De Stefano N, Jenkinson M, Matthews PM (2001) Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr 25: 466-475.
- Ashburner J, Barnes G, Chen C, Daunizeau J, Flandin G, et al. (2014) SPM12 manual. Welcome Trust Centre for Neuroimaging, London, UK.
- Quadrelli S, Mountford C, Ramadan S (2016) Hitchhiker's guide to voxel segmentation for partial volume correction of in vivo magnetic resonance spectroscopy. Magn Reson Insights 9: 1- 8.
- Terpstra M, Henry PG, Gruetter R (2003) Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference?edited spectra. Magn Reson Med 50: 19-23.
- Duffy SL, Lagopoulos J, Hickie IB, Diamond K, Graeber MB, et al. (2014) Glutathione relates to neuropsychological functioning in mild cognitive impairment. Alzheimers and Dement 10: 67-75.
- Wijtenburg SA, Gaston FE, Spieker EA, Korenic SA, Kochunov P, et al. (2014) Reproducibility of phase rotation STEAM at 3T: focus on glutathione. Magn Reson Med 72: 603-609.
- Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30: 672-679.
- Schirmer T, Auer DP (2000) On the reliability of quantitative clinical magnetic resonance spectroscopy of the human brain. NMR Biomed 13: 28-36.
- Roxburgh RHSR, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, et al. (2005) Multiple Sclerosis Severity Score Using disability and disease duration to rate disease severity. Neurol 64: 1144-1151.
- Lechner-Scott J, Kerr T, Spencer B, Agland S, Lydon A, et al. (2010) The Audio Recorded Cognitive Screen (ARCS) in patients with multiple sclerosis: a practical tool for multiple sclerosis clinics. Mult Scler 16: 1126-1133.
- Henry JD, Crawford JR (2005) The short?form version of the Depression Anxiety Stress Scales (DASS?21): Construct validity and normative data in a large non?clinical sample. Br J Clin Psychol 44: 227-239.
- Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, et al. (1994) Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis 18: 79-83.
- Kim TW, Sung YH (2017) Regular exercise promotes memory function and enhances hippocampal neuroplasticity in experimental autoimmune encephalomyelitis mice. Neuroscience 346: 173-181.
- Roosendaal SD, Moraal B, Pouwels PJW, Vrenken H, Castelijns JA, et al. (2009) Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult Scler 15: 708-714.
- Sormani MP, Kappos L, Radue EW, Cohen J, Barkhof F, et al. (2017) Defining brain volume cutoffs to identify clinically relevant atrophy in RRMS. Mult Scler 23: 656-664.
- Giorgio A, Battaglini M, Smith SM, De Stefano N (2008) Brain atrophy assessment in multiple sclerosis: importance and limitations. Neuroimaging Clin N Am 18: 675-686.
- Arnold DL, Gold R, Kappos L, Bar-Or A, Giovannoni G, et al. (2014) Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study. J Neurol 261: 1794-1802.
- De Stefano N, Stromillo ML, Giorgio A, Bartolozzi ML, Battaglini M, et al. (2016) Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry 87: 93-99.
- Rovira À, Alonso J (2013) 1H magnetic resonance spectroscopy in multiple sclerosis and related disorders. Neuroimaging Clin N Am 23: 459-474.
- Khan O, Seraji?Bozorgzad N, Bao F, Razmjou S, Caon C, et al. (2017) The relationship between brain MR Spectroscopy and Disability in Multiple Sclerosis: 20?Year Data from the US Glatiramer Acetate Extension Study. J Neuroimaging 27: 97-106.
- Donadieu M, Le Fur Y, Lecocq A, Maudsley AA, Gherib S, et al. (2016) Metabolic voxel?based analysis of the complete human brain using fast 3D?MRSI: Proof of concept in multiple sclerosis. J Magn Reson Imaging 44: 411-419.
- Reinke SN, Broadhurst DI, Sykes BD, Baker GB, Catz I, et al. (2014) Metabolomic profiling in multiple sclerosis: insights into biomarkers and pathogenesis. Mult Scler 20: 1396-1400.
- Davitz MS, Wu WE, Soher BJ, Babb JS, Kirov II, et al. (2017) Quantifying global-brain metabolite level changes with whole-head proton MR spectroscopy at 3 T. Magn Reson Imaging 35: 15-19.
- Sajja BR, Wolinsky JS, Narayana PA (2009) Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimage Clin N Am 19: 45-58.
- Brennan MS, Matos MF, Li B, Hronowski X, Gao B, et al. (2015) Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS One 10: e0120254.
- Ribbons K, Lea R, Schofield PW, Lechner-Scott J (2016) Anxiety levels are independently associated with cognitive performance in an Australian multiple sclerosis patient cohort. J Neuropsychiatry Clin Neurosci 29: 128-134.
- Colasanti A, Guo Q, Giannetti P, Wall MB, Newbould RD, et al. (2016) Hippocampal neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol Psychiatry 80: 62-72.
- Nigro S, Passamonti L, Riccelli R, Toschi N, Rocca F, et al. (2015) Structural ‘connectomic’ alterations in the limbic system of multiple sclerosis patients with major depression. Mult Scler 21: 1003-1012.
- Chard DT, Griffin CM, McLean MA, Kapeller P, Kapoor R, et al. (2002) Brain metabolite changes in cortical grey and normal?appearing white matter in clinically early relapsing-remitting multiple sclerosis. Brain 125: 2342-2352.
- Muhlert N, Atzori M, De Vita E, Thomas DL, Samson RS, et al. (2014) Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J Neurol Neurosurg Psychiatry 85: 833-839.
- Srinivasan R, Cunningham C, Chen A, Vigneron D, Hurd R, et al. (2006) TE-averaged two-dimensional proton spectroscopic imaging of glutamate at 3 T. Neuroimage 30: 1171-1178.
- Choi IY, Lee SP, Denney DR, Lynch SG (2011) Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3 T. Mult Scler 17: 289-296.









