Key words
|
| |
| Diltiazem Hydrochloride, Pectin, Guar gum, In-vitro release |
| |
Introduction
|
| |
| Diltiazem hydrochloride is a calcium channel antagonist widely used in the treatment of angina pectoris, systemic hypertension and supra ventricular arrhythmias. It is a BCS class I (highly soluble, highly permeable) drug with extensive and highly variable hepatic first pass metabolism following oral administration, with systemic bio-availability of between 36-50% and half life of 3.5 ± 1.2 hours[1]. Its short biological half life calls for frequent daily dosing (3-4 times).The development of sustained release formulation of Diltiazem hydrochloride is therefore therapeutic relevance and can be used to provide a consistent dosage through sustaining an appropriate level of the drug over time[2, 3]. |
| |
| Hydrophilic polymer matrix systems are widely used in oral sustained drug delivery because of their flexibility to obtain a desirable drug release profile, cost effectiveness, and broad regulatory acceptance [4]. The ability of the hydrophilic polymer matrices to release and entrapped drug in aqueous medium and to regulate the release of such drug by control of swelling and cross linking makes them particularly suitable for sustained release application [5, 6]. Natural polysaccharide and their derivatives (like sodium alginate, agar beads, carrageenan, chitosan, pectin, guar gum, karaya gum etc.) represent a group of polymer widely used in pharmaceutical dosage forms [7]. |
| |
| Pectin (high methoxylated) is important ionic polysaccharides found in plant cell walls. They contain linear chains of (1-4)-linked α-D galacturonic acid residues. Its gelling ability and solubility strongly depends upon the pH of the surrounding media. The nontoxicity and the low production cost of pectin make them of great interest for the formulation of controlled release dosage forms [8]. |
| |
| Guar gum is an interesting polymer for the preparation of hydrophilic matrix tablets because of its high water swellability, nontoxicity and low cost. Various groups of workers have used guar gum as a controlled release carrier [9, 10]. In different formulations guar gum has been used as a binder and disintegrants [11, 12]. In spite of the wide pharmaceutical application of guar gum, its use in sustained release dosage form is limited due to uncontrolled rate of hydration and initial slow gelling, resulting into burst effect. Thus, the sustained release of drugs especially water soluble is difficult to formulate using guar gum alone. Thus, the main objective of this work was to use high methoxylated pectin in combination with guar gum that control the burst effect by promoting gelation (radial-axial expansion) and also to examine the release mechanism of guar-pectin combination matrix system. |
| |
Material and Methods
|
| |
| The drug Diltiazem hydrochloride was procured as gift sample from (Dr. Reddy’s Lab., Hyderabad), Pectin and Guar gum was procured from (TIC Gums, USA.), Lactose, Starch and Emcompress® was procured from (S. D. Fine Chemicals, Mumbai). All other chemicals purchased and were of analytical grade. |
| |
|
Preparation of Matrix Tablets:
|
| |
| Matrix tablets were prepared by wet granulation method using pectin and guar gum as per the formula given in the table 1. Diltiazem hydrochloride, pectin and, guar gum were mixed in a poly bag and the mixture was passed through the mesh #60. Granulation was done using sufficient quantity of granulating agent (distilled water) to get wet mass. The wet mass was passed through mesh #14. The wet granules were dried at 60 oC for 2 hours in an oven. The dried granules were then sized by mesh #18 and mixed with magnesium stearate. The granules of Diltiazem hydrochloride were compressed by a single punch tabletting machine (Kilburn-manestry) with 11 mm flat shaped punches. Seven different formulas, having different diluents (F1, F2 and F3 without diluents) at same concentration were developed to study the effect of diluents on drug release. |
| |
|
Evaluation of Tablets:
|
| |
|
Evaluation of physical properties:
|
| |
| The prepared matrix tablets were evaluated for uniformity of weight, hardness, friability and thickness. Uniformity of weight was performed according to official method [13]. Hardness of the tablets was tested using a Monsanto hardness tester [14]. Friability of the tablets was determined in a Roche friabilator [14]. Thickness was measured by Vernier caliper as per USP XXIV monograph. The readings were carried out in triplicate and average value was noted [15]. The physical properties are shown in table 2. |
| |
|
Determination of drug content:
|
| |
| Twenty tablets from each formulation were accurately weighed and average weight per tablet was determined. The tablets were powdered and powder equivalent to 400 mg of Diltizem hydrochloride was accurately weighed and extracted four times with 20 ml portions of distilled water. The extract was filtered through Whatman filter paper no. 41 into 100 ml volumetric flask and volume was made up to the mark with distilled water. The solution was suitably diluted with distilled water and analyzed against blank (distilled water) for the drug content double beam spectrophotometrically at 237 nm. Results are shown in table 2. |
| |
|
In vitro drug release studies:
|
| |
| The in vitro drug release studies were carried out using USP XXIV dissolution apparatus type II (paddle) at 100 rpm. The dissolution medium consisted of 0.1N HCl for the first 2 hours and the phosphate buffer pH 6.8 from 3 to 10 hours (900 ml), maintained at 37°C±0.5°C. Five ml of samples were withdrawn and analyzed spectrophotometrically at 237 nm using a UV-visible double beam spectrophotometer (Shimadzu, Japan, Model-1701) after suitable dilution. Fresh dissolution medium was replaced after each withdrawal. The study was performed in triplicate. |
| |
|
FTIR studies:
|
| |
| It was used to study the interactions between the drug and polymers. The drug and polymers must be compatible to produce a stable product. Drug and polymer interaction were studied by using FTIR (Shimadzu, Japan, Model-8001). IR spectral analysis of pure Diltiazem hydrochloride, pectin, guar gum, and Diltiazem hydrochloride with pectin and guar gum were carried out by KBr pellet method. The peaks and pattern produced by the pure drug were compared with combination of polymer [16]. |
| |
|
DSC studies:
|
| |
| Thermal study of Diltiazem hydrochloride, pectin, guar gum and Diltiazem hydrochloride with pectin and guar gum were assessed by DSC using DSC Q10 V9 instrument. Heating of sample was done from 50 to 250°C at rate of 10°C/min |
| |
|
Drug release kinetics:
|
| |
| The mechanism of drug release from binary polymeric gum matrix tablets during dissolution test in 0.1N HCl and phosphate buffer pH 6.8 was determined using zero-order, first-order and Higuhi equation. These models fail to explain drug release mechanism due to swelling along with gradual erosion of the matrix. Therefore, the dissolution data were also fitted to the well known exponential equation (Korsmeyer –Peppas equation), which is often used to describe the drug release behaviour from polymeric systems, when the mechanism is not well known or when more than one types of release phenomena is involved. Korsmeyer et al’s equation can be represented by |
| |
| Mt/M∞ = k tn |
| |
| Where, k is a constant incorporating the structural and geometric characteristics of the matrix tablets, n is the release exponent; indicative of the release mechanism [17] and Mt/M∞ represents the drug dissolved fraction at time t. For cylindrical matrix tablets, if the exponent n= 0.45, then the drug release mechanism is fickian diffusion, and if 0.45 |
| |
|
Stability studies: [19]
|
| |
| The optimized Diltiazem hydrochloride formulation (batch F3) were packed and subjected to accelerated stability studies as per ICH guidelines (40°C±2°C/75%RH±5%RH). The sample were withdrawn periodically (0, 15, 30, 60, 90 days) and evaluated for the different physico-chemical parameters viz. appearance, weight variation ,thickness, hardness , drug content and in vitro drug release studies. |
| |
Results and Discussion:
|
| |
| The physical properties of different batches of developed matrix tablet are given in table 2. The average percentage deviation of 20 tablets of each formulation was less than (5%), and hence all formulations passed the test for uniformity of weight as per official requirements (Pharmacopoeia of India 1996). The hardness of the tablets of all the formulations ranged from (4.0 0.0 to 6.33 0.57) kg/cm2, friability (0.47 ± 0.04 to 0.85 ± 0.03 %), thickness (3.29 ± 0.03 to 3.70 ± 0.047 mm), and drug content (97.22 ± 1.78 to 100.06 ±2.92 %)All were found within the acceptable official limits. |
| |
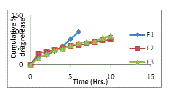
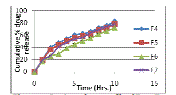
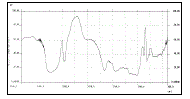
| The data obtained from in-vitro release studies for formulation batches F1 to F3 & F4 to F7 are shown in the figure 1 & 2 respectively. The cumulative percentage drug release of formulation batch F1 (drug to pectin ratio, 1:2) was 99.58 % at the end of 6 hours. Initially pectin controlled the drug released rate due to its high gelling property but after 3-4 hours, due to its eroding property drug release reached the level of 99.58% at the end of 6 hours. The cumulative percentage drug release of formulation batch F2 and F3 was 76.73% and 87.54 % at the end of 10 hours respectively. In batch F2 (drug to guar gum ratio,1:2), an initial burst release may be due to uncontrolled rate of hydration and an initial slow gelling of guar gum but after 4-5 hours, released rate become slow due to high swelling of guar gum. In batch F3, pectin was used in combination with guar gum that might control the burst effect by promoting gelation both in radial and axial expansion and after 4-5 hours, pectin maintained the release rate by acting as an eroding material and controlled the drug release up to 10 hours. |
| |
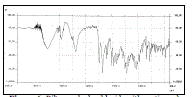
| Effects of diluents on in-vitro drug release from diltiazem hydrochloride matrix tablet are shown in table 3 and fig. 2. The lowest release (71.86 %) was seen with tablets containing Emcompress® while tablets containing lactose showed high drug release rate (82.58 %). Lactose containing tablets exhibited a single crack on the sides of tablets during dissolution process and drug diffusion was promoted due to the pores and channels that were created following the solubilization of the lactose (batch F4). Emcompress® is insoluble, non-swelling and tablets are intact throughout the dissolution process and drug released through diffusion via small inter and intra granular spaces (batch F6). Tablets containing avicel pH 101 and starch absorbed water through the goodness of fit for various models investigated for ditiazem hydrochloride tablets ranked in the order of Korsmeyer-Peppas>Higuchi>first order>Hixson-Crowell>zero order. As shown in table 4, the Korsmeyer-Peppas model gave the highest value of the squared correlation coefficient (r2) for the dissolution profile of diltiazem hydrochloride tablets (batch F3). The value of ‘n’ was found to be 0.6322 means it follows anomalous non-fickian diffusion, which coincides with swelling erosion study i.e. drug release was controlled by an intermediate of both diffusion and erosion mechanism. |
| |
| According to ICH guidelines , three months accelerated study (40°C±2°C/75%±5%RH) for the optimized formulation ( batch F3) showed negligible change over time for the parameters like appearance, weight variation ,thickness, hardness , drug content and in vitro drug release. Results are shown in table 5 |
| |
|
IR spectral analysis:
|
| |
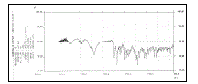
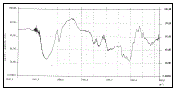
| The FTIR studies of pure diltiazem hydrochloride, pectin, guar gum and formulations containing pectin and guar gum were carried out to study the interaction between the drug and polymers used (shown in figure 3, 4, 5 & 6 respectively). |
| |
| -NH stretching, -CH aliphatic stretching, aromatic - CH stretching, esteric -CO stretching, -C=O stretching of pure Diltiazem hydrochloride and the Diltiazem hydrochloride sustained release tablets formulations containing pectin and guar gum were almost in the same region of wave number ranging from 3350 cm-1 to 1700 cm-1. The results proved that there were no significant interactions between the drug and polymers. |
| |
|
DSC studies:
|
| |
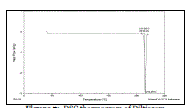
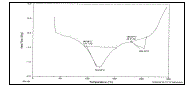
| DSC thermograms of pure drug diltiazem hydrochloride, pectin, guar gum and diltiazem hydrochloride sustained release tablets containing pectin and guar gum are shown in figure 7, 8, 9 & 10 respectively. |
| |
| DSC thermograms of diltiazem hydrochloride showed sharp endothermic peak at 215.25C, indicating the melting point of stable crystalline drug. However, the DSC thermograms of diltiazem hydrochloride sustained release tablets containing pectin and guar gum showed only a negligible shift in their position with some peak broadening in the latter (at 204.23C). Therefore, this study revealed that there were no significant interaction between the drug and polymers. |
| |
Conclusion
|
| |
| Previous studies have shown that guar gum alone cannot efficiently control the drug release. This study demonstrates that its combination with pectin is synergistic in controlling the diltiazem hydrochloride release. Use of hydrophilic polymers like guar gum and pectin was successful in the formulation of matrix and at the same time it is effective in retarding the drug release. Among all the formulations, F3 shows 87.54% release at the end of 10 hours. Furthermore, mathematical modeling and statistical analysis proved that the Korsmeyer-Peppas kinetic model can be described the dissolution profile. Stability studies shown that there was no significant change in appearance, weight variation, thickness, hardness, drug content, and in vitro drug release of selected formulation (F3). Thus, a suitable combination of two natural gums (pectin and guar gum) may be successfully employed for formulating sustained release matrix tablets of Diltiazem hydrochloride. |
| |
Acknowledgment
|
| |
| The authors acknowledge the facilities provided at University Deptt. Of Pharmaceutical Sciences, Nagpur (M.S) in carrying out the work. The authors would like to thank Dr.Reddy’s Lab, Hyderabad and TIC Gums, USA for providing gift sample of the drug and gums respectively. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |
 |
 |
 |
 |
 |
| Figure 6 |
Figure 7 |
Figure 8 |
Figure 9 |
Figure 10 |
|
| |
















