Keywords
Procalcitonin (PCT); Newborns; Preterm newbornsRespiratory distress syndrome (RDS)
Introduction
Prematurity is the leading cause of death globally, and the second largest contributor to all deaths under the age of 5 [1]. The group of premature newborns includes all newborns born under 37 weeks of gestation or less than 259 days from the first day of the last menstrual cycle. Risk factors for premature birth: having a previous premature birth, vaginal bleeding caused by placental abruption or placenta preavia, multiple gestations, an interval of less than six months between pregnancies, polyhydramnios, oligohydramnios, maternal medical disorders: thyroid disease, asthma diabetes, and hypertension, conceiving through in vitro fertilization; some infections, particularly of the amniotic fluid and lower genital tract, underweight or overweight before pregnancy, stressful life events, such as the death of a loved one or domestic violence [2,3]. Other risk factors for premature birth include: uterine abnormalities, such as septum; data on previous secondary or malignant changes of the uterine cervix and interventions of the uterine cervix, as well as interventions of the uterine cervix, such as cervical cone biopsy sample or loop electrocautery excision procedures; physical injury or trauma; multiple miscarriages or abortions [4,5].
Respiratory distress syndrome is also referred to as hyaline membrane disease or disorder due to a lack of surfactant in the lungs in preterm newborns. It is most common in preterm newborns under 37 weeks of gestation, as surfactant is produced in the largest amount at 34 to 37 weeks of gestation [6,7]. Risk factors that contribute to respiratory distress syndrome include male sex, white race, caesarean section, prematurity, multiple short pregnancies and preterm newborns from mothers with diabetes [8,9]. Radiological studies show diffuse atelectasis which is described as ground-glass appearance with visible air bronchograms and low lung expansion [10,11]. Differential Diagnosis for an infant with respiratory distress and/or cyanosis are: transient tachypnoea of newborn, pneumothorax, aspiration of blood/mucus, meconium aspiration, congenital pneumonia, pulmonary hypoplasia, persistent pulmonary hypertension, hydrops fetalis, congenital malformation, cystadenomatoid, malformation, congenital heart disease etc. [12]. Complications of this disease are: bronchopulmonary dysplasia, pneumothorax, hypoxia, persistent ductus arteriosus, retinopathy, intraventricular haemorrhage, cardiovascular arrest [13,14]. The therapy consists of supportive measures (electrolyte balance, intravenous fluid administration, glycemic control) and initial placement of the newborn on Nasal CPAP with a PEEP of 3–8 cm H2O. If respiratory failure persists, endotracheal intubation and mechanical ventilation are performed and a surfactant is administered endotracheally within two hours of delivery [15,16].
Definition of neonatal sepsis varies among studies. Sepsis is defined as systemic inflammatory response syndrome, SIRS, in the presence of, or as a result of proven or suspected infection, and it is leading cause of death in preterm newborns with RDS [16,17]. Neonatal sepsis is classified as early onset sepsis (EOS) and late onset sepsis (LOS), depending on the time of onset of the sepsis episode [17,18]. EOS is defined as a condition when the onset of sepsis occurs within 72 hours of postnatal life and transmission of bacteria is vertical from mother to preterm newborn. LOS is definded as a condition when the onset of sepsis occurs after 72 hours of postnatal life and transmission of bacteria is horizontal from health care personel topreterm newborn [19,20]. Diagnosing newborns with RDS suspected of sepsis is both challenging and complex. Biomarkers can play an important role in providing a timely diagnosis of sepsis, helping the differential diagnosis with non-infectious SIRS and the decision-making in the initial management [21]. Biomarkers have been shown to be useful in the diagnosis of infection and a good predictor of mortality, severe sepsis and septic shock in newborn with sepsis before organ dysfunction has advanced too far.Rapid elevation in the concentration of procalcitoninPCT is a promising indicator of sepsis in newly admitted critically ill newborns capable of complementing clinical signs and routine laboratory parameters, makes it an ideal biochemistry markerfor bacterial infection [22].
Material and Methods
The study were included 85 newborns with respiratory distress syndrome (RDS) and proven sepsis admitted in the Intensive Care Unit at the University Children Hospital-Skopje in period of December 2018 till May 2020 y. Diagnosis of sepsis in newborns with respiratory distress syndrome (RDS) diagnosed according to standard protocols for diagnosis of disease. Sepsis was defined as the identification of two or more Systemic Inflammatory Response Syndrome criteria in addition to present source of infection or suspected infection. The clinical criteria taken as indicative of sepsis were: respiratory distress syndrome, lethargy, apnea, tachypnea, bradycardia, seizures, poor perfusion, feeding intolerance, temperature instability, low birth weight, gestational age, gender. Tachypnea is due to an attempt to increase minute ventilation to compensate for a decreased tidal volume and increased dead space. Retractions occur as the newborns is forced to generate a high intrathoracic pressure to expand the poorly compliant lungs.
Medical data records of admitted newborns with RDS for sepsis were analyzed. All the laboratory examination were analyzed in the Clinical Laboratory at the University Children Hospital-Skopje. Sample for blood culture and PCT were taken the first at the admission, the second on 3-5 day.
Blood culture media were incubated at 37°C for 5 days in Bact Alert 3D 360. Positive blood culture were proven with the new multiplex polymerase chain reaction–based rapid diagnostic test (BioFire FilmAray Blood Culture Identification).
Procalcitonin was determined by immunoassay: patented ELFA (Enzyme-linked fluorescent assay) technology, automated Vidas Biomerieux immunoassay (ng/ml).
Statistical analysis
Data analysis is performed in a Statistic program 7.1 for Windows and SPSS Statistics 23.0, to compare the means of the variables, one-way ANOVA test. For all analyses p value <0.05 was taken for statistical significance.
Results
In the study we included 75 (M:F=45:30) newborns with respiratory distress syndrome (RDS) and proven sepsis admitted in the Intensive Care Unit at the University Children Hospital- Skopjein period of December 2018 till May 2020 y (Figure 1).
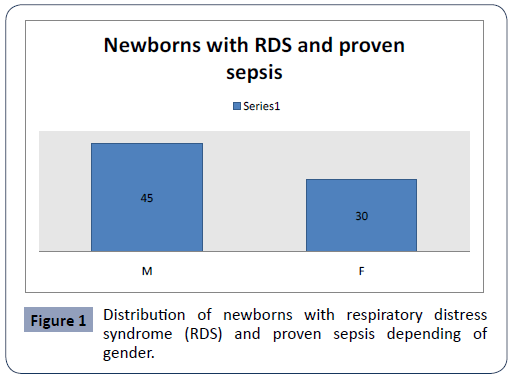
Figure 1: Distribution of newborns with respiratory distress syndrome (RDS) and proven sepsis depending of gender.
The newborns with respiratory distress syndrome (RDS) and proven sepsis admitted in the Intensive Care Unit at the University Children Hospital-Skopje have been divided into two groups. The first group included 32 preterm newborns with RDS and proven sepsis and the second group included 43 term newborns with RDS with and proven sepsis .
The identified bacteria included Staphylococcusaureus (n=48) mecA, Streptococcus (n=3), Acinetobacter baumannii (n=5), Serratia marcescens (n=4) and Entrobacteriaceae (n=35).
The average gestational age of the preterm newborns with RDS and proven sepsis was 36.04 ± 3.2 weeks and the term newborn with RDS 36.23 ± 3.5 weeks. There is no statistically significant difference in average gestational age between the two groups (p<0.01).
The average birth weight of the preterm newborns with RDS and proven sepsis was 2290.5 ± 731.2 grams, and the term newborn with RDS and proven sepsis was 2785.2 ± 588.1 grams. There is no statistically significant difference in average birth weight between the two groups (p<0.01).
Average serum albumin values in preterm newborns with RDS and proven sepsis showed lower values, compared to the term newborns with RDS and proven sepsis (26.23 ± 3.6 g/L vs. 32.87 ± 5.2 g/L). Average total serum protein values in preterm newborns with RDS and proven sepsis, compared to the term newborns with RDS and proven sepsis ( 47.03 ± 6.6 g/L vs. 50.47 ± 7.2 g/L).
Statistical analysis confirmed significantly different values of PCT in the analyzed time period in first group preterm newborns with RDS and proven sepsis p<0.001 (Figure 2). The highest average values (45.31 ± 41.29) were measured during admission with a subsequent sharp jump. After the second measurement at 3-5 days, the average values of PCT slowly decreased (33.02 ± 42.21)
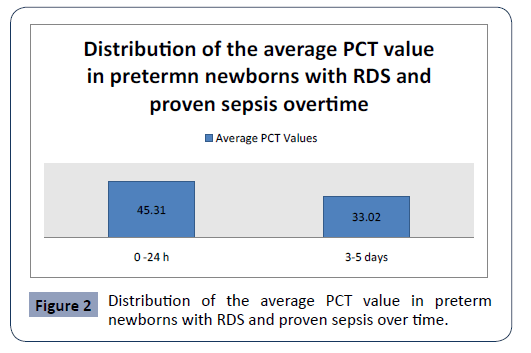
Figure 2: Distribution of the average PCT value in preterm newborns with RDS and proven sepsis over time.
Statistical analysis confirmed significantly different values of PCT in the analyzed time period in second group with term newborns with RDS and proven sepsis, p<0.001 (Figure 3). The highest average values (34.03 ± 34.19) were measured during admission with a subsequent sharp jump. After the second measurement at 3-5 day, the average values of PCT slowly decreased (29.36 ± 29.14).
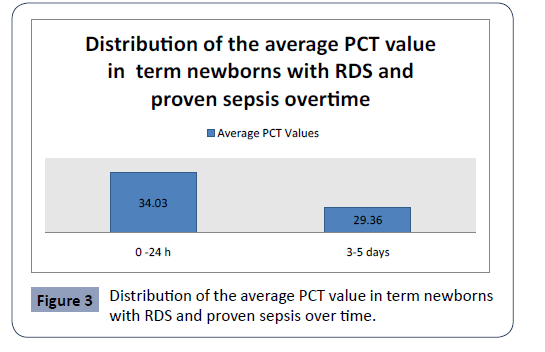
Figure 3: Distribution of the average PCT value in term newborns with RDS and proven sepsis over time.
There is statistically significant difference in average PCT between the two groups overtime (p<0.05).
Statistical analysis confirmed significantly different values of PCT in the analyzed time period in first group in preterm newborns with RDS and proven sepsis with mechanical ventilation (MV) and bubble continuous positive airway pressure (BCPAP) p<0.001 (Figure 4). The highest average values (45.31 ± 52.50) were measured in newborns with preterm newborns with RDS and proven sepsis with MV during admission, with a subsequent sharp jump compared to preterm newborns with RDS and proven sepsis with BCPAP. After the second measurement at 3-5 days, the average values of PCT slowly decreased (32.61 ± 42.85). Statistical analysis confirmed significantly different values of PCT in the analyzed time period in second group newborns with RDS and proven sepsis with MV and BCPAP p<0.001 (Figure 5). The highest average values (35.35 ± 42.51) were measured in newborns with RDS and proven sepsis with MV during admission, with a subsequent sharp jump compared to preterm newborns with RDS and proven sepsis with BCPAP. After the second measurement at day 3, the average values of PCT slowly decreased (30.71 ± 42.75)
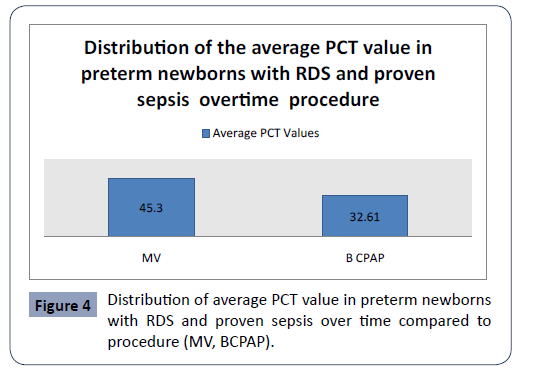
Figure 4: Distribution of average PCT value in preterm newborns with RDS and proven sepsis over time compared to procedure (MV, BCPAP).
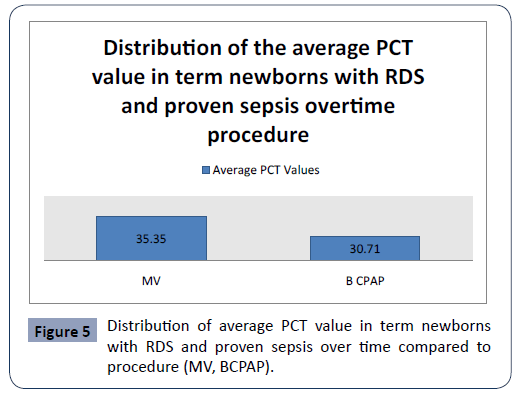
Figure 5: Distribution of average PCT value in term newborns with RDS and proven sepsis over time compared to procedure (MV, BCPAP).
There is statistically significant difference in average PCT between the two groups overtime procedure (MV, BCPAP) (p<0.05).
Discussion
Sepsis in newborns with RDS is essential for life-threatening condition and still represents an important cause of mortality and morbidity [23]. It is the systemic response to infection by microbial organisms and it is a leading cause of major cause of mortality and morbidity in newborns with the highest incidence occurring among newborns of very low birthweight and gestation. For pediatrician early identification of infections is still a challenge. Preterm newborns are at higher risk for sepsis than term newborns, as they tend to require more invasive procedures than term newborns [24]. Respiratory distress syndrome is a common cause of hospitalization in the intensive care unit. It is the leading cause of death in preterm newborns. Respiratory distress syndrome most commonly occurs in preterm newborns as a result of surfactant deficiency and lung underdevelopment [25,26]. Risk factors for sepsis in the postnatal period include: male gender, hypogammaglobulinemia, birth weight <1000 grams, central venous catheters, prolonged duration of mechanical ventilation and intravenous alimentation. Mechanical ventilation can effectively improve the arterial oxygen partial pressure and reduce the mortality of newborns with RDS. Early biomarkers to diagnose sepsis in ICU are widely used in clinical practice and they are useful for monitoring the infectious process, and can reduce the risk of death in newborns with RDS. An ideal biomarker for sepsis should have high sensitivity and specificity with early phase elevation, low cost and quick result [27,28]. The diagnostic performance of PCT in numerous studies from literature has suggested PCT to be a useful marker in the diagnosis of sepsis [29,30] in newborns with RDS. The diagnostic performance of PCT in numerous studies from literature has suggested PCT to be useful marker in diagnosis of sepsis. In our study, we examined PCT values, in newborns with RDS and two or three clinical signs of sepsis. Statistical analysis confirmed significantly different values of PCT in the analyzed time period in first group preterm newborns with RDS and two or three clinical signs of sepsis p<0.001. The highest average values (45.31 ± 41.29) were measured during admission with a subsequent sharp jump.
Statistical analysis confirmed significantly different values of PCT in the analyzed time period in second group with term newborns with RDS and two or three clinical signs of sepsis p<0.001. The highest average values (34.03 ± 34.19) were measured during admission with a subsequent sharp jump. There is statistically significant difference in average PCT between the two groups overtime (p<0.05). Statistical analysis confirmed significantly different values of PCT in the analyzed time period in first group in preterm newborns with RDS and two or three clinical signs of sepsis with MV and BCPAP p<0.001. The highest average values (35.35 ± 42.51) were measured in term newborns with RDS and two or three clinical signs of sepsis with MV during admission, with a subsequent sharp jump compared to preterm newborns with RDS and two or three clinical signs of sepsis with BCPAP. Statistical analysis confirmed significantly different values of PCT in the analyzed time period in second group term newborns with RDS and two or three clinical signs of sepsis with MV and BCPAP p<0.001. The highest average values (35.35 ± 42.51) were measured in term newborns with RDS and two or three clinical signs of sepsis with MV during admission, with a subsequent sharp jump compared to preterm newborns with RDS and two or three clinical signs of sepsis with BCPAP. There is statistically significant difference in average PCT between the two groups overtime procedure (MV, BCPAP) (p<0.05).
Conclusion
Neonatal sepsis is a life-threatening condition and it is a serious medical condition defined as a systemic inflammatory response, occurring in the first four weeks of life, caused by the body's response to an infection. Rapid identification and treatment of sepsis has a major impact on the clinical course, management, and outcome of critically ill newborns admitted in the intensive care unit (ICU). PCT is a useful biomarker for early diagnosis, treatment and for monitoring response to therapy of sepsis in newborns with RDS and proven sepsis. From our study we can conclude that PCT is promising sepsis markers in preterm newborns with RDS. PCT levels measured in the first 24 hours in preterm newborns with RDS and proven sepsis are significantly higher, especially those on invasive treatment (MV), compared to term newborns with RDS and proven sepsis. PCT measurement appears to be a better predictor to distinguish preterm newborns with RDS and proven sepsis and term newborns with RDS and proven sepsis when compared to clinical sign and procedure (MV, BCPAP). Also in this study we can conclude that the value of procalcitonin decreases with the implementation of adequate treatment. This is important for monitoring the effectiveness of the therapy. Thus, our data raise the possibility that the addition of serum PCT to the standard work-up of critically ill patients with suspected sepsis could increase diagnostic certainty and improve patient management.
32734
References
- Howson CP, Kinney MV, McDougall L, Lawn JE (2013) Born too soon: preterm birth matters. Reprod Health 10: S1.
- Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. Lancet 371: 75-84.
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, et al. (2006) The preterm parturition syndrome. BJOG: An International Journal of Obstetrics &Gynaecology 113: 17-42.
- Goldenberg RL, Andrews WW, Faye-Petersen O, Cliver S, Goepfert AR, et al. (2006) The Alabama Preterm Birth Project: placental histology in recurrent spontaneous and indicated preterm birth. Am J Obstet Gynecol 195: 792-796.
- Mercer BM, Goldenberg RL, Moawad AH, Meis PJ, Iams JD, et al. (1999) The preterm prediction study: effect of gestational age and cause of preterm birth on subsequent obstetric outcome. Am J Obstet Gynecol 181: 1216-1221.
- Gower WA, Nogee LM (2011) Surfactant dysfunction. Paediatr Respir Rev 12: 223-229.
- Hermansen CL, Lorah KN (2007) Respiratory distress in the newborn. American family physician 76: 987-94.
- Mehrabadi A, Lisonkova S, Joseph KS (2016) Heterogeneity of respiratory distress syndrome: risk factors and morbidity associated with early and late gestation disease. BMC Pregnancy Childbirth 16: 281.
- Reuter S, Moser C, Baack M (2014) Respiratory distress in the newborn. Pediatrics in review 35: 417-429.
- Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, et al. (2010) Respiratory morbidity in late preterm births. JAMA 304: 419-425.
- Bak SY, Shin YH, Jeon JH, Park KH, Kang JH, et al. (2012) Prognostic factors for treatment outcomes in transient tachypnea of the newborn. Pediatrics International 54: 875-880.
- Warren JB, Anderson JM (2010) Newborn respiratory disorders. Pediatrics in review 31: 487.
- Course C, Chakraborty M (2020) Management of Respiratory Distress Syndrome in preterm infants in Wales: A full Audit cycle of a Quality improvement project. Scientific reports 10: 1-7.
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, et al. (2019) European consensus guidelines on the management of respiratory distress syndrome–2019 update. Neonatology 115: 432-450.
- Bohlin K, Gudmundsdottir T, Katz-Salamon M, Jonsson B, Blennow M (2007) Implementation of surfactant treatment during continuous positive airway pressure. J Perinatol 27: 422-427.
- Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M (2017) Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 102: F17-23.
- Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG (2005) Seventy-five years of neonatal sepsis at Yale: 1928–2003. Pediatrics 116: 595-602.
- Shane AL, Stoll BJ (2014) Neonatal sepsis: progress towards improved outcomes. J Infect 68: S24-32.
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, et al. (2002) Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med 347: 240-247.
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, et al. (2002) Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110: 285-291.
- Wynn JL, Wong HR, Shanley TP, Bizzarro MJ, Saiman L, et al. (2014) Time for a neonatal–specific consensus definition for sepsis. Pediatr Crit Care Med 15: 523.
- Ugarte H, Silva E, Mercan D, DeMendonca A, Vincent JL (1999) Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med 27: 498-504.
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, et al. (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315: 801-810.
- Weinschenk NP, Farina A, Bianchi DW (2000) Premature infants respond to early-onset and late onset sepsis with leukocyte activation. J Pediatr 137: 345-350.
- Hermansen CL, Lorah KN (2007) Respiratory Distress in the Newborn. Am Fam Physician 76: 987-994.
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, et al. (2002) Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med 347: 240-247.
- Whicher J, Bienvenu J, Monneret G (2001) Procalcitonin as an acute phase marker. Ann Clin Biochem 38: 483–493.
- Riedel S, Melendez JH, Amanda T, Rosenbaum JE, Zenilman JM, et al. (2011) Procalcitonin as a marker for the detection of bacteremia and sepsis in the emergency department. Am J Clin Pathol 135: 182-189.
- Schuetz P, Christ-Crain M, Müller B (2008) Procalcitonin and other biomarkers for the assessment of disease severity and guidance of treatment in bacterial infections. Adv Sepsis 6: 82-89.
- Rhodes A, Evans LE, Alhazzani W, Levy MW, Antonelli M, et al. (2016) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 3: 304-377.










