Keywords
Pterygium recurrence; MMC; Stromalysis
Introduction
The pterygium was first described by an ophthalmic surgeon called Susruta in 1000 by AC [1]. It is one of the common ocular surface diseases, which is a triangular-shaped growth that invades the cornea more commonly at the nasal bulbar conjunctiva side but can occur at the temporal side also [2-4]. Back to Hippocrates' time, this illness had been identified, but till now, no actual pathogenesis had been described [4]. Contribution of environmental factors like ultraviolet radiation, hot climate and dusts specially cement dusts; Ali and Omar, conducted in their study the relation of cement dust with the pterygium [5]. Hill and Maske, regarded this illness as a proliferative rather than a degenerative process [6]. Recently hereditary and vascular endothelial growth factor A (VEGFA) have been suggested to play roles [7].
Till now, there is no medical therapy for pterygium; therefore, the only treatment of choice is surgical removal, which might end with recurrence, and for this reason, many factors had been implicated like skin color and geography [8]. Since the operation for pterygium started till now, many trials had done to remove the pterygium like using human's secretion and excretion; bile and urine, respectively, experiments with acids performed, removal of pterygium with horsehair had been tried [7]. Excising the pterygium and making a bare sclera “bare-sclera technique” first committed and performed by A D'Ombrain [9], but this procedure was associated with a high recurrence rate [10]. Then in 1963, Kunitomo and Mori used MMC to prevent the recurrence [11].
Recently, in adjunction to the surgical excision, the anti-vascular endothelial growth factor [8], amniotic membrane, conjunctival autograft in adjuvant with 5-fluorouracil or MMC performed [7]; although, it’s thought that the duration of exposure more important than the concentration [12] but till now the ideal duration of MMC exposure remained questionable.
The mitomycin C is an alkylating agent, and it’s an antibiotic which is taken from Streptomyces caespitosus, a strain of actinomyces, for the first time by Wakaki et al [13]. It Inhibits pterygium cell proliferation and migration, and eventually, cell death; the cell death is higher in pterygium than in conjunctival cells [14]. The mechanism of cell inhibition is through inhibiting the synthesis of nuclear DNA, RNA transcription, and the synthesis of protein and, it is a potent fibroblast inhibitor, but also studies show that it can damage the mitochondrial DNA [15,16]. When used in high concentration acts as a chemotherapeutic agent but in low doses acts as an antifibrotic agent that prevents scar formation this feature made the MMC famous in many ophthalmological surgeries like pterygium surgery, it can have both systemic and local adverse effects; the systemic absorption can occur when the dose is higher than 0.2 mg but 0.2 mg even if remain for 60 seconds not found to be associated with systemic absorption, it’s half-life in plasma is around 20 minutes, Yulshi et al [17]. Also, one of the devastating local side effects is scleral stromalysis in various doses, even with a low dose of 0.02 mg which is used by Lindquist and Lee, in their study [15], and might happen even after decades as demonstrated by Wan Norliza et al [18].
Subjects and Methods
It is an observational retrospective study that comprised of a total of 144 subjects, had been divided into three groups, each group consisting of 48 patients who underwent pterygium surgery with MMC and graft. The same surgeon performed all operations. The permission and approval confirmed by the local ethics committee under the number 623. All subjects had verbal informed consent for their participation in the study after the procedure been explained. All groups had received the same postoperative treatment.
Regarding the surgical procedure; it is performed under local anesthesia using 2% lidocaine, which was injected into the subtenon to achieve anesthesia. The pterygium tissue was removed from the cornea up to its base with the help of a crescent knife and scissor followed by cauterization of the bleeders. Then the conjunctiva undermined, and the tenon dissected totally from the conjunctiva around 2-3 mm starting from the free edges of conjunctiva inward also the bare sclera freed from all tissues. In all groups, MMC used followed by copious irrigation of bare sclera and the space between undermined conjunctiva and episclera, for this purpose balanced salt solution used [19]. Then bare sclera was covered with conjunctival autograft transplantation taken from the superior bulbar conjunctiva, the size of the graft is adjusted to the size of the bare sclera and sutured with 8-0 vicryl. All groups had the same amount of MMC exposure, but the dose and the duration were adjusted according to the groups:
In group A, many pieces of surgically sterile sponges immersed in 1 cc of 0.2 mg MMC solution was placed under the conjunctiva in the upper, central and lower parts then the free edge of the conjunctiva hold with serrated forceps brought up to the limbus to cover the bare scleral surface for 30 seconds followed by removal of the sponges and copious irrigation.
Group B, just irrigation of the bare sclera and under conjunctival space with 1 ml, 0.1 mg MMC followed by immediate copious irrigation.
Group C, just irrigation of the bare sclera and under conjunctival space with 1 ml, 0.2 mg MMC followed by immediate copious irrigation.
Results
Statistical analysis was performed using SPSS version 25, which summarized the data’s in percentage for qualitative variables. The ANOVA, Chi-square test, T-test, and Post Hoc Test used to compare variables between the groups, and the variables with P-value ≤ 0.05 considered significant statistically.
The study included a total of 144 eyes of 72 patients. The range of the age among all groups was from 18 to 69, with a mean of 38.66, the standard deviation of 15.33. Among them, 88.2% were males, and 11.8% were females. The mean age for Group A was 34 years, for Group B was 40 years, and Group C was 42 years.
Each patient was observed for two years postoperatively, and slit-lamp examination was performed to check for recurrence at every visit; any fibrovascular proliferation from the previous pterygium site was regarded recurrence. Scleral stromalysis defined by the presence of seeing black-brown appearance through the sclera in the operated area.
The incidence of recurrence was 13.5%; 13.5% in total groups, among which, 12.5% for group B and, 0.7% for group A and no recurrence in Group C. The p-value=0.000.
The recurrence was more among males with non-significant p-value=0.5 and more among > 50 years of age with highly significant p-value=0.000, Figure 1.
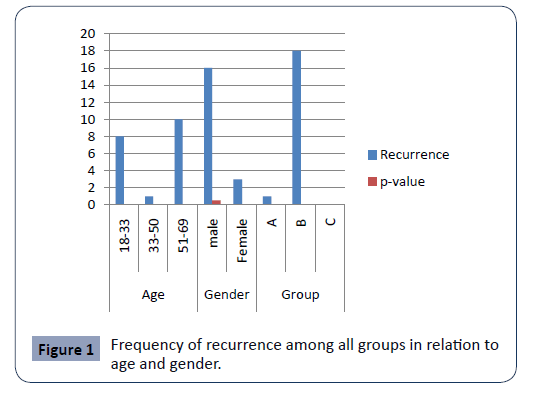
Figure 1: Frequency of recurrence among all groups in relation to age and gender.
Regarding stromalysis; it is incidence was stromalysis 0.71%, a mild scleral stromalysis confirmed in Group A only, who was 65 years old male and was high myopic, the p-value for stromalysis in relation to both gender and the age was p-value=0.07 nonsignificant, but the correlation with the groups were significant p-value=0.03, i.e. the longer the duration more chances for the stromalysis to happen but the shorter the duration is more protective, the Post Hoc Test showed a significant relation, Figure 2.
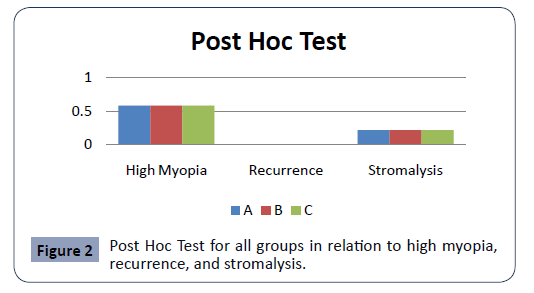
Figure 2: Post Hoc Test for all groups in relation to high myopia, recurrence, and stromalysis.
The incidence of high myopia was 3.5% in all groups. The Post Hoc Test showed a none significant relation, Figure 2.
Discussion
Since 2015 our protocol for pterygium surgery is excision, intraoperative MMC 0.2 mg/mL (0.02%) application for 30 seconds with conjunctival autograft. But, the lack of universal guidelines, unavailability of an ideal procedure to prevent recurrence and stromalysis, the presence of hot, dry climate, thin sclera in myopic patients, and old age groups all together might cause both recurrence, Lei G showed in his study, [20] and stromalysis.
The commonest postoperative challenging encountered worldwide, for pterygium, is recurrence which necessitates multiple interventions, and each intervention leaves some scar which finally may end with fibrosis of the conjunctival fornices; the symblepharon, Mohammed I conducted in his study [21], while the most devastating postsurgical challenging is fibrinolysis due to repeated using of MMC, which might end with total scleral melting; the scleromalacia, Lee JS et al [22]. While Solomon A et al [23] revealed that the application of MMC for one time to the bare sclera is not associated with a decrease in the scleral thickness, up to 6 years of follow up. It is well understood that combining conjunctival autograft with MMC reduces recurrence, as proven by Ha SW et al [24]. At the same time, various doses can cause scleral stromalysis, Lindquist TP and Lee WB, [15] and Ha SW et al postulated that the long exposure (1-2 minutes) can cause systemic absorption and toxicity [24] but Cheng HC et al evaluated the lower dose (0.2 mg/ml) for 30 seconds, which is not the ideal dose and duration to prevent recurrence [11], and the current study showed the same result.
Sebban A and Hirst LW study showed, the incidence of recurrence in subjects operated with 0.2 mg MMC and conjunctival autograft procedure is around 12-13% [25], also in our study showed nearly the same incidence 13.5% in whole groups, among which, 12.5% for Group B; therefore we can say the lower the dose, the higher the recurrence rate [26,27]. This study showed, the recurrence rate more among >50 years of age, but Anguria et al showed the reverse [28].
Regarding stromalysis; it is incidence was stromalysis 0.71%, a mild scleral stromalysis confirmed in Group A only, who was 65 years old male and was high myopic, the p-value for stromalysis in relation to both gender and the age was p-value=0.07, nonsignificant, but the correlation with the groups were significant p-value=0.03, i.e. the longer the duration more chances for the stromalysis to happen but the shorter the duration is more protective, and it is well known that scleral thickness does not decrease with age, but regional decreases in the thickness occur in eyes with an elongated axial length that affects the posterior segment in the majority of cases, Shen L et al revealed in their study [26], the same result achieved in our study, therefore, more researches needed to figure out the real vulnerable age and or specific diseases to the stromalysis. In our study, a very low dose, (0.1 mg/ml washing) of MMC with graft caused a high recurrence rate; the same result had been shown in another study by Lotfy A et al [27].
Surgical intervention in the limbal area causes severe barrier function destruction. As repeated surgery adds another burden on limbus and cornea, therefore, repeated the surgical procedure in the same area for the same entity causes more anatomical and histological destructions which might cause more recurrent cases [13]. Regarding the period of follow up is not sufficient for stromalysis as it might happen decades after the MMC exposure but it gives a good idea regarding the recurrence rate; however, Fakhry MA study’s had shown similar results compared to our study [13], while, Gris and colleagues had no recurrences cases [29], but Akura and colleagues technique showed no recurrences during the period of 6 to 32 months [30]. Martins et al [7] highlighted that increasing the dose and duration of exposure of MMC decreases the recurrence but increases the complication rate. In the current study, the Group C, (0.2 mg/ml with just washing), is the best to follow and might become the standard procedure regarding the dose and duration of MMC, as no recurrence and stromalysis recorded. To prove that, a larger number and a longer period of the follow up needed for every single patient.
Conclusion
This study emphasizes that the shorter the duration (just washing) of MMC, the better the protection against scleral stromalysis, but the lower the dose, the higher the recurrence rate. Therefore, much more workup requires for setting up a customized surgical procedure, to achieve utmost preventive criteria for both recurrence and stromalysis.
Declarations
Funding: None
Conflict of interest: None declared
30948
References
- Detorakis ET, Spandidos DA (2009) Pathogenetic mechanisms and treatment options for ophthalmic pterygium: trends and perspectives (Review). Int J Mol Med 23: 439-447.
- Kim KW, Park SH, Kim JC (2016) Fibroblast biology in pterygia. Experimental eye research 142: 32-39.
- Ghoz N, Elalfy M, Said D, Dua H (2018) Healing of autologous conjunctival grafts in pterygium surgery. Acta ophthalmologica 96: e979-e88.
- Duke-Elder S (1965) System of ophthalmology. In: Diseases of the Outer Eye. Mosby, St. Louis.
- Taqi AA, Abdullah OO (2016) The Frequency of Pterygium and Dry Eye in Chronic Cement Exposure: A Clinical Case-Control Study. Int J Med Res Prof 2: 40-44.
- Martins TG, Costa AL, Alves MR, Chammas R, Schor P (2016) Mitomycin C in pterygium treatment. Int J Ophthalmol 9: 465-468.
- Bahar I, Kaiserman I, McAllum P, Rootman D, Slomovic A (2008) Subconjunctival bevacizumab injection for corneal neovascularization in recurrent pterygium. Curr Eye Res 33: 23-28.
- D'Ombrain A (1948) The Surgical Treatment of Pterygium. Br J Ophthalmol 32: 65-71.
- Mastropasqua L, Carpineto P, Ciancaglini M, Lobefalo L, Gallenga PE (1994) Effectiveness of intraoperative mitomycin C in the treatment of recurrent pterygium. Ophthalmologica 208: 247-249.
- Cheng HC, Tseng SH, Kao PL, Chen FK (2001) Low-dose intraoperative mitomycin C as chemoadjuvant for pterygium surgery. Cornea 20: 24-29.
- Robin AL, Ramakrishnan R, Krishnadas R, Smith SD, Katz JD, et al. (1997) A long-term dose-response study of mitomycin in glaucoma filtration surgery. Arch Ophthalmol 115: 969-974.
- Fakhry MA (2011) The use of mitomycin C with autologous limbal-conjunctival autograft transplantation for management of recurrent pterygium. Clin Ophthalmol 5: 123-127.
- Cao D, Chu WK, Ng TK, Yip YWY, Young AL, et al. (2018) Cellular Proliferation and Migration of Human Pterygium Cells: Mitomycin Versus Small-Molecule Inhibitors. Cornea 37: 760-766.
- Lindquist TP, Lee WB (2015) Mitomycin C-associated scleral stromalysis after pterygium surgery. Cornea 34: 398-401.
- Pritsos CA, Briggs LA, Gustafson DL (1997) A new cellular target for mitomycin C: a case for mitochondrial DNA. Oncol Res 9: 333-337.
- Yulish M, Khatib A, Pikkel J (2018) Systemic Absorption of Mitomycin-C When Used in Pterygium Surgery. Cornea 37: 746-747.
- Norliza WMW, Raihan IS, Azwa JA, Ibrahim M (2006) Scleral melting 16 years after pterygium excision with topical Mitomycin C adjuvant therapy. Contact lens & anterior eye 29: 165-167.
- Georgopoulos M, Vass C, Vatanparast Z (2002) Impact of irrigation in a new model for in vitro diffusion of mitomycin-C after episcleral application. Current eye research 25: 221-225.
- Lei G (1996) Surgery for pterygium using a conjunctival pedunculated flap slide. Br J Ophthalmol 80: 33-34.
- Mohammed I (2013) Pre- and intraoperative mitomycin C for recurrent pterygium associated with symblepharon. Clin Ophthalmol 7: 199-202.
- Lee JS, Shin MK, Park JH, Park YM, Song M (2015) Autologous advanced tenon grafting combined with conjunctival flap in scleromalacia after pterygium excision. J Ophthalmol 2015: 547276.
- Solomon A, Kaiserman I, Raiskup FD, Landau D, Frucht-Pery J (2004) Long-term effects of mitomycin C in pterygium surgery on scleral thickness and the conjunctival epithelium. Ophthalmology 111: 1522-1527.
- Ha SW, Park JH, Shin IH, Kim HK (2015) Clinical analysis of risk factors contributing to recurrence of pterygium after excision and graft surgery. Int J Ophthalmol 8: 522-527.
- Sebban A, Hirst LW (1991) Pterygium recurrence rate at the Princess Alexandra Hospital. Aust N Z J Ophthalmol 19: 203-206.
- Shen L, You QS, Xu X, Gao F, Zhang Z, et al. (2015) Scleral Thickness in Chinese Eyes. Invest Ophthalmol Vis Sci 56: 2720-2727.
- Lotfy A, Gad AAM, Abdelrahman A, Samir A, Abdulhalim BH (2018) Conjunctival Autograft Combined With Either Preoperative Mitomycin C Injection or Intraoperative Local Mitomycin C Over the Medial Rectus Muscle Tendon in Primary Pterygium Surgery. Eye Contact Lens 2: S192-S195.
- Anguria P, Ntuli S, Carmichael T (2014) Young patient's age determines pterygium recurrence after surgery. Afr Health Sci 14: 72-76.
- Gris O, Güell JL, del Campo Z (2000) Limbal-conjunctival autograft transplantation for the treatment of recurrent pterygium. Ophthalmology 107: 270-273.
- Akura J, Kaneda S, Matsuura K, Setogawa A, Takeda K, et al. (2001) Measures for preventing recurrence after pterygium surgery. Cornea 20: 703-707.







