D Mahendra* and Gunosindhu Chakraborthy
Department of Pharmaceutical Sciences, Parul Institute of Pharmacy & Research, P.O Limda, Vadodara, Gujarat State, India
*Corresponding Author:
D Mahendra
Department of Pharmaceutical Sciences
Parul Institute of Pharmacy & Research
P.O Limda
Vadodara
Gujarat State
India
E-mail: Mahendra888d@gmail.com
Received Date: March 03, 2021; Accepted Date: March 17, 2021; Published Date: March 24, 2021
Citation: Mahendra D, Chakraborthy G (2021) A Novel Bioanalytical RP-HPLC Method Development and Validation for Simultaneous Determination of Amlodipine Besylate on Human Plasma. Int J Drug Dev & Res Vol.13 No.2: 156.
Copyright: © 2020 Mahendra D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Bioanalytical method; Amlodipine besylate; Human plasma; RP-HPLC method; Validation
Introduction
Amlodipine besylate is chemically 3-Ethyl 5-methyl (4RS)-2- [(2-aminoethoxy) methyl]-4-(2 Chlorophenyl)-6-methyl-1,4- dihydropyridine-3,5-dicarboxylate benzene sulphonate (Figure 1) [1]. It is long-acting calcium channel blocker of dihydropyridine class used in the treatment of hypertension and angina pectoris [2]. Amlodipine relax the smooth muscle in the arterial wall, decreases total peripheral resistance thereby reduces blood pressure and increases blood flow to the heart muscle [1,2]. Amlodipine besylate is official in USP and IP [3].
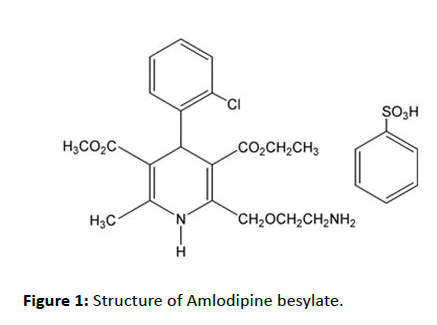
Figure 1: Structure of Amlodipine besylate.
Literature survey revealed that a wide variety of analytical techniques have been reported for the estimation of Amlodipine besylate either in combinations or individually which includes, UV Spectroscopy [1,4], RP-HPLC [5-7] and few bioanalytical techniques: LC-MS [8,9], LC/MS/MS [10]. Therefore, our aim to developa simple, precise and accurate bioanalytical method for estimation of amlodipine besylate by using RP-HPLC in human plasma and validate as per USFDA guidelines [11].
Materials and Methods
Chemicals and reagents
Amlodipine besylate were obtained from Lupin Ltd.Aurangabad, Maharashtra. Water is purified by water purification system for HPLCpure lab UHQ, ELGA, Mumbai. HPLC grade methanol and acetonitrilewere obtained from Merck Ltd, Mumbai, India. Human plasma was procured from SKN hospital, Pune. Analytical grade of chemicals were obtained from Loba chemie Pvt. Ltd Mumbai, India.
Instrumentation
Chromatographic separation of drug was performed on Shimadzu LC-2010 CHT equipped with LC solution software with UV detector and weighing was done on electronic balance Shimadzu ATY 224. Separation was carried out using “Waters” C18 column. Sample was prepared using REMI C24BL cooling centrifugation and REMI CM 101 cyclo mixer.
Standard stock solution
Amlodipine besylate API powder (100 mg) was accurately weighed and transferred to 100 ml volumetric flask. The drug was dissolved and diluted up to the mark with methanol. This solution besylate.
Preparation of mobile phase
Mobile phase was prepared by mixing 10 mM Potassium dihydrogen phosphate buffer (pH 6.4), Acetonitrile and Methanol in ratio 20:70:10 v/v/v and filtered membrane filter.
Disodium hydrogen phosphate dehydrate 1.78 g was weighed accurately, dissolved in 1000 ml of distilled water and pH was adjusted to 6.4 by using O-Phosphoric acid.
Preparation of sample
Protein precipitation technique: Frozen human plasma was thawed to ambient temperature and aliquots of 500 l plasma were taken in centrifugation tubes of 10 ml capacity with the help of micropipette and 300 ?l of stock solution was added and the plasma proteins were precipitated by using methanol. The tube was vortexed for 30 sec [12].Then the solution was centrifuged at 10000 RPM for 12 min. below the temperature 10°C. The supernatant liquid wastaken and evaporated in water bath. Then, the residue was dissolved in methanol and transfer to HPLC vials.
Method validation
The method performance was evaluated for accuracy, precision, linearity and stability during various stress conditions which include freeze thaw stability, stock solution and short term stability.
Linearity and range
Working solution of various concentrations was injected under the operating chromatographic condition and peak area of each drug were calculated at 238 nm. The calibration curves were constructed using simple linear regression between peak area and concentrations. The range of solution has been decided according to correlation coefficient of regression equation.
Accuracy
The accuracy of the method was performed by calculating % recovery for the different concentration levels of drug. The samples of three concentration levels prepared as LQC, MQC and HQC by standard addition method.
Precision
The precision of this method was evaluated by the % CV at different concentration levels corresponding to LQC, MQC and HQC. Intraday and interday precision was evaluated in 3 replicate batches of different concentrations (24, 36 and 48 ng/ ml).
Stability studies
The stability of Amlodipine besylate in solution and plasma sample was evaluated using two concentration level (LQC and HQC i.e., 24 and 48 ng/ml respectively). The stability of Amlodipine besylate was also evaluated in deep freezing at -20°C for 12 hrs. The plasma samples were kept at freezer and after stressed to three freeze thawing cycles (for 24 hrs per cycle). All samples described above were compared to freshly prepared Amlodipine besylate sample at the same concentration level [13,14].
Results and Discussion
Optimization of chromatographic conditions
The chromatographic conditions were optimized in order to provide good system suitability parameters. The mobile phase was selected on the basis of its polarity and different trials were taken. Acetonitrile was selected as an organic modifier, because lower column efficiency was observed using methanol. Finally, a mobile phase consisting Buffer (pH 6.4): Acetonitrile: Methanol (20:70:10, v/v/v) at a flow rate of 1 ml/min was selected. The retention time of Amlodipine besylate was found to be 4.1 min. The chromatogram of Amlodipine besylate obtained by optimized conditions is shown in Figures. The optimized chromatographic conditions and system suitability parameters are listed in Table 1.
| Sr. no. |
Condition/Parameter |
Details |
| 1 |
Column |
Waters C18 5 µm (4.6 × 250 mm) |
| 2 |
Mobile phase |
Buffer: ACN: Methanol (20:70:10 v/v/v) |
| 3 |
Flow rate |
1.0 ml/min. |
| 4 |
Column temperature |
28à¹ÂÂÂÂC |
| 5 |
Volume of injection |
20 µl |
| 6 |
Detection wavelength |
238 nm |
| 7 |
Theoretical plate |
2300 |
| 8 |
Retention time |
4.1 min. |
| 9 |
Tailing factor |
1.29 |
Table 1: Optimized chromatographic conditions and system suitability parameters.
Typical chromatograms of drug free human plasma and spiked drug-plasma of Amlodipine besylateare shown in Figures 2 and 3, respectively. The retention time of amlodipine besylate was found to be 4.1 min indicating this method is faster than other methods. The typical column efficiency expressed as the number of theoretical plates was found to be 2300 for Amlodipine besylate.
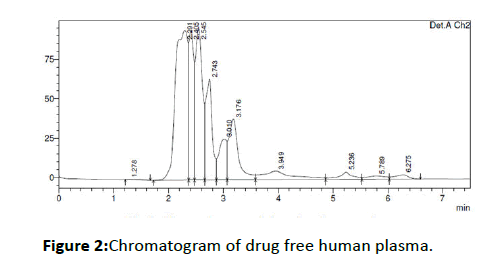
Figure 2: Chromatogram of drug free human plasma.
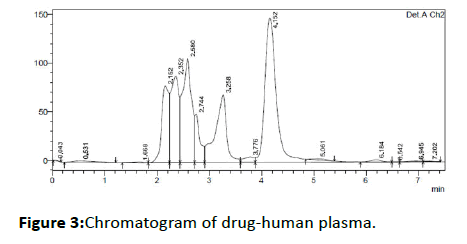
Figure 3: Chromatogram of drug-human plasma.
Linearity and range
The calibration curve was found to be linear in the range 24-48 ng/ml (R2=0.9913) and equation is y=mx+c, where y represents the area of Amlodipine besylate and x represents concentration of Amlodipine besylate in ng/ml (Figure 4).
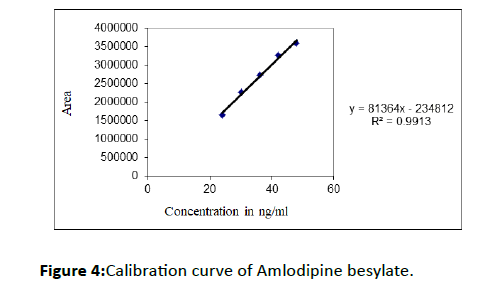
Figure 4: Calibration curve of Amlodipine besylate.
Accuracy
The mean % recovery of calculated concentrations for all quality control samples at LQC, MQC and HQC concentration levels are ranged from 100.41%-103.80%, which are within the acceptance criteria 85%-115% (Table 2).
| |
LQC |
MQC |
HQC |
| Mean (ng/ml) |
22.512 |
32.476 |
51.063 |
| S.D. |
1.3021 |
0.0849 |
0.7791 |
| % CV |
5.7828 |
0.2614 |
1.5234 |
| % Mean |
93.79 |
90.19 |
108.18 |
Table 2: Results of accuracy study.
Precision
The % CV of calculated concentrations for all quality control samples at LQC, MQC and HQC concentration levels are ranged from 1.478%-0.6472% for intraday and 3.586-1.6589 for interday precision, which is within acceptance criteria 15.00% (Table 3).
| Parameter |
LQC |
MQC |
HQC |
| Intraday |
S.D. |
0.2471 |
0.2099 |
0.7127 |
| % CV |
1.1338 |
0.6472 |
1.4786 |
| Interday |
S.D. |
0.8533 |
1.2114 |
0.69143 |
| % CV |
1.6589 |
3.5869 |
2.70504 |
Table 3: Results of intraday and interday precision.
Stability studies
The results of all stability studies are within acceptance criteria (Table 4). The results of freeze thaw stability suggested that Amlodipine besylate was stable in human plasma for at least 24 hrs. The results of short term stability studies indicated that the quality control samples were stable for 12 hours at -20°C. Similarly, the results of stock solution studies confirmed stability of stock solution.
| Stability |
Parameter |
Concentrations |
| Freeze and thaw |
|
LQC |
MQC |
| Mean |
22.18 |
47.96 |
| S.D. |
0.2879 |
0.646 |
| % CV |
1.2981 |
1.347 |
| % Mean stability |
92.41 |
99.91 |
| Short term stability |
Mean |
23.24 |
48.13 |
| S.D. |
2.7942 |
0.8791 |
| % CV |
12.02 |
1.82 |
| % Mean stability |
96.83 |
100.27 |
| Stock solution stability |
Mean |
21.48 |
45.01 |
| S.D. |
0.7794 |
0.0832 |
| % CV |
3.6288 |
0.1849 |
| % Mean stability |
89.5 |
93.77 |
Table 4: Results of stability studies.
Conclusion
The work describe in this paper deals with analysis of Amlodipine besylate using RP-HPLC method in human plasma. The precision and accuracy of the method met the acceptance criteria laid down in guideline for industry, Bioanalytical method validation, USFDA. Sufficient stability of both LQC and HQC was shown to allow for completion of sample analysis in clinical trials. From the results, we can conclude that developed method id simple, accurate, rapid and precise. Thus, it can be used for routine analysis of Amlodipine besylate in human plasma.
36271
References
- Jampana P, Varma B, Babu G, Surekha P, Praveen T, et al. (2014) Visible spectroscopic method for estimation of Amlodipine besylate in tablets. Int J Pharm Chem Biol Sci 4: 173-177.
- Rang H, Dale M, Ritter J (2003) Pharmacology. 5th ed Elsevier publication 282-283.
- Delhi N (2001) Ministry of Health and family welfare.Government of India 7-30.
- Topal B, Bozal B, Demircigil B, Uslu B, Ozekan S (2009) Electroanalytical studies and simultaneous determination of amlodipine besylate and atorvastatine calcium in binary mixtures using first derivative of the ratio-voltammetric methods. Wiley Intersci Electroanalysis 21: 2427-2439.
- Sudhakar M, Rao V, Devika S, Ramesh R (2010) A validated RP-HPLC method for simultaneous estimation of NebivololHydrochloride and S-Amlodipine besylate in tablet dosage forms. Int J Chem Pharm Sci 1: 28-33.
- Usha N, Satya B, Ankani N (2015) Method development and validation for the simultaneous estimation of Nebivolol Hydrochloride and Amlodipine besylate in tablet dosage forms by RP-HPLC. Int J Pharm Sci Drug Res 7: 110-115.
- Ahmed M, Manohara N, Ravi C (2012) RP-HPLC method development and validation for simultaneous estimation of Atorvastatin calcium and Amlodipine besylate. Int J ChemTech Res 4: 337-345.
- Streel B, Laine C, Zimmer C, Sibenaler R, CEccato A (2002) Enantiomeric determination of Amlodipine in human plasma by liquid chromatography coupled to tandem mass spectrometry. J Biochem Biophy methods 54: 357-368.
- Sarkar A, Das D, Selvan P, Gowda K, Mandal U, et al. (2008) Simultaneous determination of Metoprolol succinate and Amlodipine besylate in human plasma by liquid chromatography–tandem mass spectrometry method and its application in bioequivalence study. J Chromatography B 873: 77-85.
- Narapusetti A, Bethanabhatla S, Sockalingam A, Repaka N, Saritha V (2015) Simultaneous determination of rosuvastatin and amlodipine in human plasma using tandem mass spectrometry: Application to disposition kinetics. J Adv Res 6: 931-940.
- FDA (2013) Guidance for industry, Bioanalytical method validation, US Department of health and human services Food and Drug Administration 1: 1-23.
- Pushpalatha E, Sailaja B (2014) Biaanalytical method development and validation by HPLC: A review. J Med Pharm Innov 1: 1-9.
- Murugan S, Pravallika N, Sisrisha P, Chandrakala K (2013) A review on Bioanalytical method development and Validation by using LC-MS/MS. J Chem Pharm Sci 6: 41-45.
- Varghese A, Pandita N, Gaud R (2014) A novel, sensitive, bioanalytical method for estimation of Amlodipine besylate in rat plasma using flourescence detection by RP-HPLC. Int J Pharm Sci Res 5: 2813-2820.










