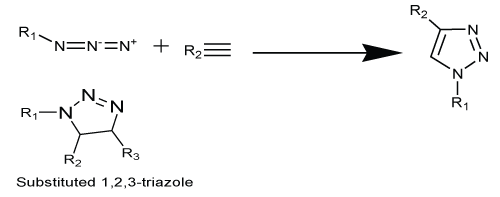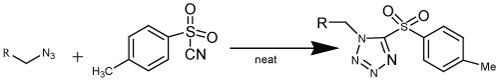Keywords
|
| Bioconjugation; Radiochemistry; Nanoscience; 1,3-dipolar cycloaddition |
Introduction
|
| The main approach of medicinal chemistry during the process of drug discovery or lead optimization is to synthesize compounds or libraries of compounds. Due to this reason, the loom of this concept in this field is attracted for rapid generation of new molecules. Click chemistry, a term coined by K. Barry Sharpless is a way to generate substances rapidly and reliably by joining small modular units together. Click chemistry is an approach to develop a set of powerful, selective, and modular building blocks, such as azide and alkyne that work for both small and large scales [1]. Its applications are increasingly in all aspects of drug discovery in medicinal chemistry, such as for generating lead compounds through combinatorial methods, in-situ applications for target templating, and in bioconjugation reactions. There are many reactions, which comprises the click universe due to its high reliability, specificity and selectivity. The Husigen 1,3-dipolar cycloaddition (Scheme 1) of alkynes to azides for 1,4-disubstituted-1,2,3- triazole copper catalyzed reaction is mild, efficient and powerful linking reaction requiring no protecting groups and no purification lead to high degree of dependability, complete specificity and biocompatibility of the reactants [2] (Scheme 1). |
| In history and even now a day lead discovery and optimization had aided by combinatorial methods and high throughput screening to generate library of test compounds for screening. However, due to unreliability and new discoveries revealed click chemistry as a modular for the synthesis of drug-like molecules that can accelerate the drug discovery process by utilizing a few practical and reliable reactions. It is a new type of chemistry that able to synthesize complex molecule in a efficient manner. It makes use of few chemical reactions for the synthesis and designing of new building blocks. There are two important features that makes click reaction as a novel approach for clinical and preclinical studies. First, in click reactions neither the reactant nor the product functional groups interact with functional biomolecules. Second, the reaction continues with ease under mild conditions, easy to perform, uses only readily available reagents, and is insensitive to oxygen and water. In fact, in several instances water is the ideal reaction solvent, providing the best yields and highest rates. Reaction work-up and purification uses benign solvents and avoids chromatography [3]. |
| It is a powerful and attractive technology alternative to conventional technology proven it to be highly fulfilled in different fields such as selectivity, stereo specificity, yield and biocompatibility and a novel approach for a wide range of application in upcoming discoveries. Many of the reactions was remain untouched due to different reasons one of the example of such kind of reaction is azidealkyne cycloaddition reaction discovered by Huisgen in 1963, which is ignored for past few years due to high temperatures and pressures and was revived by in 2001 by employing Cu(I) as a catalyst. They give the criteria for successful click chemistry by stating that “the reaction must be modular, wide in scope, give consistently high yields, generate only inoffensive byproducts that can be removed by non-chromatographic methods, and be stereo-specific (but not necessarily enantioselective). To fulfill this criteria, there should be a variety of starting material, easy to perform, insensitive to oxygen and water and use only readily available reagents and should proceed at room temperature. Reaction conditions, work-up and product isolation should be smooth and simple. Thus, this methodology allows rapid construction of molecules with efficiency, versatility and selectivity [3]. |
| Click reactions click on carbon-heteroatom bond formation but the reaction is irreversible. For lead discovery and optimization, click reaction provides a mean of rapid exploration of library of chemicals and for later, it helps in providing rapid SAR profiling through generation of number of molecules. The main characteristic of this reaction is to provide novel structure that might not resemble the existing pharmacophores. The research based on click chemistry is very fast because it avoids the screening of library of compounds [4-6]. Exharatingly it have been concluded that greater drug discovery can be achieved with limited reactions as it is not the number of reactions that makes an importance but it is the ability of reaction to compete with variations in nature of their components. Click chemistry was now being use increasingly in various fields of research ranging from lead discovery and optimization to tagging of biological systems, such as protein, nucleotides, and whole organism. The applications of this approach have been review in this article. |
| Nucleophilic ring opening and Husigen 1, 3-diploar cycloaddition are the best known and extensively studied click reaction till date. Azides and alkynes are the building blocks for the preparation of potential drug candidates. They are the least reactive functional group in organic chemistry and have been termed bioorthogonal because of their stability and inertness towards the functional groups found in biological molecules. This reaction has been termed the “cream of the crop” of click reactions [7]. |
| Drug discovery based on Natures secondary metabolites is very slow and complex synthesis and thereby, click chemistry provides faster lead discovery and optimization. This concept of Click Chemistry has been twisted into convenient, resourceful and reliable two-step coupling procedures of two molecules A and B [1,8], that are widely used in biosciences [9-11], drug discovery [12] and material science [1,13]. Today, there are many researches going on based on click reactions due to its wide scope, modularity and simplicity. |
Universe of Click Chemistry
|
| Different click reactions applied in different field of science and yielded extremely reliable process for the synthesis of building block and compound libraries. The various reactions is classified as: |
| 1. Cycloaddition reaction of such as 1,3-dipolar cycloaddition reaction [14] and hetero Diels Alder reaction [15,16]. |
| 2. Nucleophilic substitution reaction specifically rings opening reaction of strained heterocyclic electrophiles such as epoxides, aziridines, azridiniumions, and episulfoniumions [5]. |
| 3. Carbonyl chemistry of the non-aldol type such as formation of ureas, thioureas, aromatic heterocyles, oxime ethers, hydrazones and amides. |
| 4. Addition to carbon-carbon multiple bonds particularly oxidation reaction such as epoxidation [10], hydroxylation, dihydroxylation [17], aziridination [18] and sulfenyl halide [19] addition also Michael addition of Nu-H reactants. |
| 5. Thiol-ene clicks reactions. |
| 6. Azide-Phosphine (Staudinger ligation) reaction. |
Principles of Click Chemistry
|
|
1,3-Dipolar cycloaddition reaction of azides and alkynes
|
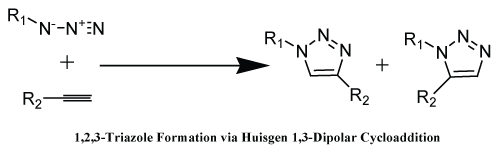
| In floral of click reactions the most accepted reaction till date, which was found to be 1,3-dipolar cycloaddition reaction also known as Husigen cycloaddition between an azide and a terminal alkynes affording 1,2,3-triazole (Scheme 2). Dimorth in early 1900 but the generality, first reported the formation of triazoles with cycloaddition reaction of azide and acetylene and mechanism of these cycloaddition is not fully realized till 1960’s. The reactions were discovering at the beginning of 20th century but the prospective of this reaction were unveiled in 1960 by Husigen. Due to some drawbacks of this reaction such as formation of mixture of 1, 4 and 1, 5-regiomers, which were uneasy to separate using chromatographic methods and transformation, requires heating and long reaction time for completion [20] therefore, in 2002, two groups Sharpless and Meldal independently reported that copper salts were able to accelerate this reaction upto 10 million times (Scheme 2). |
|
1(a) Copper catalyzed Azide Alkyne cycloaddition (CuAAC)
|
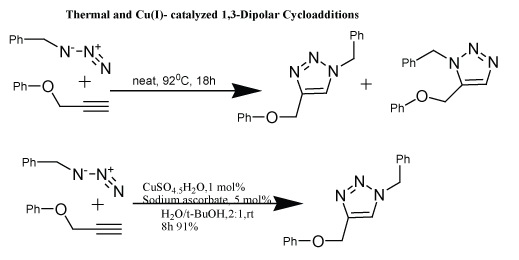
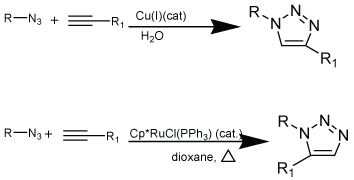
| Copper catalyzed alkyne-azide reaction (Scheme 3) features an enormous rate acceleration of 107 to 108 compared to uncatalyzed reaction. More importantly, this reaction succeeds over a broad temperature range, directs the formation of only one of the two regiomers, mainly the 1,4-regiomers insensitive to aqueous conditions and pH range over 4-12 and tolerates a broad range of functional groups. No chromatography is required for the separation or isolation of products. The standard catalytic system uses copper (II) salts or copper (I) salt in the presence of reducing agent e.g., sodium ascorbate or metallic copper prevents the formation of oxidative homocoupling products [21]. A mixture of tert-butanol and water was use as a solvent because it rectified the use of base to form copper acetylide species. The reactions where aqueous conditions cannot be used, organic solvents e.g., THF, toluene, DCM in the presence of stochiometric amount of copper (I) salts e.g., CuI [14], Cu(CH3CN)4PF6 [15], CuBr(PPh3)4 or CuIP(OEt)3 [22,23] and an excess of a base, usually a tertiary amine (e.g., TEA, DIPEA) can be used. Till date research is going on for the improvement of better copper-based catalyzed reaction, e.g., copperin- charcoal have been developed as simple, inexpensive and efficient heterogeneous catalyst for triazole formation. This development is proving advantageous as it can easily remove by filtration and can reuse in another cycloaddition without losing its potency. This discovery led to renaissance of Husigen cycloaddition in synthetic chemistry including polymer chemistry and biochemistry [24]. |
| Although the copper found to be efficient, catalyst but still further investigations are proceeding in order to get the potential products. Therefore, a number of additives have been investigated which increases the efficiency of reaction and reduction of copper concentration such as (tris-(benzyl-triazolylmethyl) amine) (TBTA) [25], triethylamine hydrochloride [26] and the water-soluble sulfonated bathopenantroline. They found to be very efficient as was found in bioconjugation of expensive and precious substrates [27] (Scheme 3). |
|
1(b) Ruthenium catalyzed alkyne- azide cycloaddition
|
| In the search of more valuable catalyst the ruthenium catalyzed cycloaddition reaction found to be more fruitful as compared to copper catalyzed reaction as it can yield 1, 5-disubstituted 1, 2, 3-traizoles regioselectively. The classical Husigen 1, 3-dipolar cycloaddition often gives mixtures of regiomers, whereas the copper catalyzed reaction allows the synthesis of 1, 4-disubstituted regiomers selectively. However, later developed ruthenium-catalyzed reaction gives the opposite regioselectivity with the formation of 1, 5-disubstituted triazoles (Scheme 4) [28]. The reaction of 1, 3-dipolar cycloaddition between alkynes and azide using the Cp*RuCl (PPh3)2 catalyst was reported in 2005 [29]. Another catalyst was pentamethylcyclopentane diol ruthenium chloride complexes, which also has the capability to catalyze the cycloaddition of azides and alkynes regioselectively leading to 1, 5-disubstituted 1, 2, 3-traizoles. It allows the reaction between internal alkynes and appears to proceed via oxidative coupling of azide and alkyne to give a six-membered ruthenacycle followed by reductive elimination to form triazole product. However, some other methods have also been reported for the synthesis of 1, 5-disubstituted triazoles products by the reaction of bromomagnesium acetylenes and organic azides. Substituted trimethylsilyl acetylenes have also been reported to direct the cycloaddition affording the 1, 5-regioisomers. It found that trimethylsilyl group probably controls the selectivity by a combination of 2 factors: the ability to stabilize a positive charge on acetylene at β-carbon position in transition state and stearic hindrance that prevents the reverse orientation. Density functional calculations support this mechanism and shows that reductive elimination is a ratedetermining step [30]. Therefore, these catalyzed reactions comply fully with definition of click chemistry and put focus on azide-alkyne cycloaddition as a prototype click reaction (Scheme 4). |
|
1(c) Toulenesulfonyl cyanide as catalyst
|
| Toulenesulfonyl cyanide has been use in the presence of different unhindered azide yield 1, 5-disubstituite tetrazole in good yield (Scheme 5). The substituted tetrazole can be functionalizing by the replacement of sulfonyl group with various nucleophiles. The reaction was expand by the use of acyl cyanides, cyanoformates and cyanoformides with azide substrates generating acyltetrazoles e.g., reaction of benzoyl cyanide with various azides at 120°C gives cycloaddition product regioselectively in high yield [31] (Scheme 5). |
|
Non transition metal click reaction
|
| Copper makes the reaction efficient but some instances reported in-vitro copper-induced degradation of viruses or oligonucleotides and in-vivo copper ions found to be highly toxic for living life form. In 2008, Dondoni reported metal free cyclo addition reaction and their correlation with click chemistry including Diels-Alder reactions, and thio-alkene radical addition reaction [32] Scheme 6. |
|
Non-azide 1, 3-cycloaddition reaction as click reaction
|
| Many of the alkynes engaged in non-azide 1, 3-cycloaddition reaction with electron deficient cases are usually being the most reactive. Interestingly, these reactions yield five-membered heterocycles. The sequence started with addition of hydrazine to aziridinium intermediate generated from (a) The resulting cyclic hydrazide (b) then undergoes condensation with aromatic aldehydes to give azomethine ylides c which react with a variety of components to give cycloadducts. These reactions provide the opportunities to create unique combinatorial libraries with different array of functionalities [33]. |
|
Nucleophilic ring opening
|
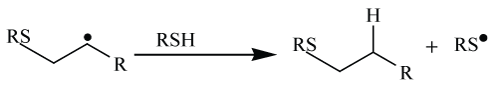
| The ring opening of three-membered heterocycles is generally facilitated by the release of strain [34] and so these compounds have been termed as “spring loaded electrophiles” [35]. Nucleophilic opening of epoxides, aziridines (Scheme 7), cyclic sulfates, and episulfonium and aziridinium ions can lead to wide variety of library of compounds. However, epoxides and aziridines are the most common substrates for ring opening and their specificity can be useful for formation of large variety of compounds. Regioselective opening of aziridines is primarily substrate controlled by the opening of unsymmetrical aziridines using TMS azide [36]. Benzyl substitution is more reactive than primary and secondary-carbon substitution, which obeys normal SN2 behavior. However, library of compound have been synthesize through variation of the nitrogen substituent (Scheme 7). |
| Regioselective opening of oxirane ring (Scheme 8) can be continuing in the same way as that of nucleophilic opening of diepoxide with benzylamine. Therefore, nucleophilic ring opening reactions of oxiranes and aziridines can be yigh yielding, stereospecific, and regioselective fulfilling the criteria of click reactions [37] (Scheme 8). |
|
Triazole ring as bioesters
|
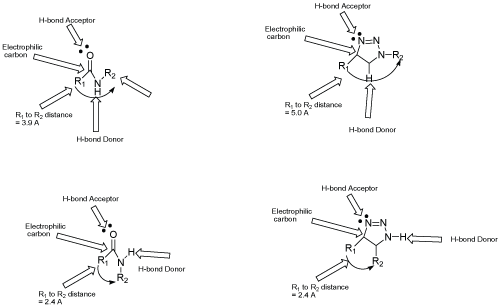
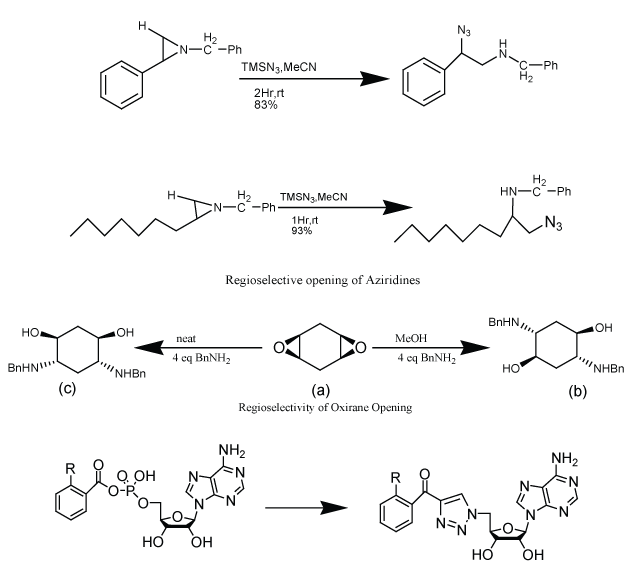
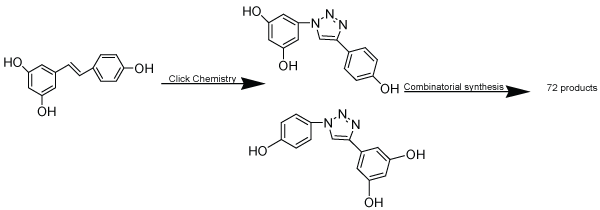
| Bioesters are the substituent or groups that have similar physical or chemical properties and hence similar biological action. As CuAAC has made possible the insertion of triazole moiety relatively easy, so biosteric potential of this moiety is important to establish to broaden the use of the reaction in improvement of target compounds. Biosteric replacement may help to decrease toxicity or to change activity spectra. The 1, 4-disubstituted triazole moiety mimics Z-amide bond as the carbonyl oxygen of amide bond is similar to the 3-nitrogen of the moiety, C (5)-H bond can act as a hydrogen donor and carbonyl carbon is electronically similar to amide N-H bond (Figure 1). Since, some properties such as dipole moment, hydrogen bonding donor and acceptor are more marked than amide bond with enhanced peptide mimicry. The difference between two exist in the distance between the substituent linked by two atoms in amide and three atoms in a triazole ring, with an overall increase of about 1.1 Å. However, 1, 5-substitution pattern mimics E-bond. Therefore, it has suggested that triazole ring has use as bioester of amide bond [38]. Including this, it has also been suggested that the triazole moiety may act as bioesters of acyl-phosphate and trans olefionic group. The substitution of trans olefinic group with a triazole moiety in resveratrol proved to be more rewarding (Scheme 8). Resveratrol has been shown to have a number of potentially interesting biological activities but its usefulness is limited because its shows its properties at high micromolar concentration, making it difficult to predict which are the targets responsible for single effects. So, an approach of click chemistry has utilized to synthesize triazole analogues via click chemistry. This applicability was not limited to resveratrol but also possible to maintain in esterogenic activity in diethylstilbesterol analogues [39,40]. |
Applications of Click chemistry
|
|
Click in oligonucleotide lipids and sugars
|
| Click chemistry had a large impact on lipid and carbohydrate chemistry and an efficient strategy for the synthesis of oligonucleotides for applications such as labeled carbohydrate ONs, fluorescent ONs, and multi modified ONs [41]. Many instances have been existed where click triazole formation has generated to improve the biological profile of this biomolecules. The cyclic decapeptide an antibiotic have coupled to different sugar moieties to improve its safety profile. Two of the synthesized triazole grafted glycopeptides showed six fold better therapeutic index compared to tyrocidine. |
| The 1, 3-dipolar cycloaddition reaction has become an efficient method and found its application in preparation of high order molecular conjugates, such as glycoprotein, neoglycoconjugates [42], protein oligonucleotides [43] and DNA-peptide conjugates [44]. Wang et al. has prepared flourscent labeled oligonucleotide found to be an efficient primer with high specificity in a Sanger dioxy DNAsequencing reaction to produce DNA-sequencing fragments [45]. Similarly, Baccaro et al. carried out synthesis of two-azide modified analogs of thymidine and incorporated them into DNA by using primer extension reaction and polymerase chain reaction (PCR) conjugated to Biotin by using Staudinger ligation and found it to be less sterically hindered [46]. Staudinger ligation reaction was use to remodel cell surfaces in mice by Prescher et al. Mice were injected with an azide carbohydrate derivative (Man-NAz) and then with a phosphinederived peptide conjugate and the reaction progress was monitored by flow cytometry [47]. |
| CuAAC chemistry has implied to synthesize amino acidsaccharide by using carbohydrate and amino acid derived azides and alkynes as building blocks. The idea behind this strategy was the finding that oligosaccharides covalently attached top proteins facilitate the development of vaccines against pathogenic bacteria. Danishefsky et al. combined three-tumor associated carbohydrate antigens on a single molecule polypeptide scaffold by using strategy of multiple click triazole formation. This linkage of sugars to poorly soluble drugs via click catalyzed reaction might be exploited to improve pharmacokinetic profile e.g., ferrocene derivatives that have antimalarial activity solubility profile was improved by coupling it to sugars using click chemistry [3]. |
|
Click in bioconjugation
|
| Another classical extension of this click chemistry is in the development of bifunctional molecules. Bioconjugation covers a wide range of science between molecular biology and chemistry. It involves the attachment of synthetic labels to bimolecular building blocks such as fusing two or more proteins together or linking a carbohydrate with a peptide [48]. The challenge for this reaction is the ability to perform both in vitro as well as in vivo experiments. Tornoe was the first who demonstrated the use of click chemistry in bioconjugation for the preparation of peptidotriazole solid-state synthesis and the idea behind this was to set up an efficient synthetic method to prepare triazole derivatives for potential biologic targets [49]. Click chemistry continues to attract attention for the labeling of proteins and living organism. One of the examples was demonstrated by Wang when a fluorescent dye was attached to cow pea mosaic virus. Another instance was established by Link modified Eschericia coli with an azide-bearing outer membrane protein C (OmpC) followed by biotinylated by reacting with a biotin alkyne derivative under copper-catalyzed click chemistry conditions [50]. Deiters et al. developed a method to genetic encode proteins of Saccharomyces cerevisiae with azide- or acetylenebased synthetic amino acids [51]. |
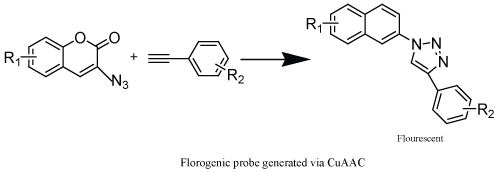
| The application of CuAAC to generate fluorogenic compounds (Scheme 9) have broadly evaluated, as it is a powerful method to track biomolecules in the cell. Examples include fluorescent DNA probes an important tool has generated by this method [52]. |
| The approach of click chemistry represents a new bioconjugation strategy that can be used to conveniently various ligands to the surface of performed liposomes. The CuAAC under mild condition can be considered to be efficient and chemoselective reaction in this strategy. Frisch et al. demonstrated the application of click reaction to the conjugation, in a single step of unprotected alpha-1-thiomannosyl ligands, functional with azide group to liposomes containing a terminal alkyne-functionalized lipid anchor. The use of water-soluble Cu (I) chelator, act as a catalyst such as phenathrlinedisulfonate gives an excellent yield. Limitation of CuAAC reaction in this conjugation is restricted to liposomes made of saturated phospholipids [53]. |
|
Polymer therapeutics and polymer chemistry
|
| Polymer therapeutics is an approach to treat various diseases. In this aspect polymer is a broad term include polymeric drug, polymer-drug conjugates, polymer protein, polymer-protein conjugates, polymeric micelles, and multi-component polyplexes and they all utilizes the property of water-soluble polymers for improved drug, protein, or gene delivery [54]. Biocompatible polymers such as PEG(Polyethylene glycol), if conjugated with proteins and peptides has proved to be very efficacy in increasing bioavailability and so it can be said that these polymers play an important role in drug delivery and formulations [55,56]. Yet, polymer chemistry is required to meet the demand for versatile pharmaceutical properties. Schlaad et al. reported an excellent work on the polymer chemistry. |
| Many of the important reactions such as 1,4-benzethiol and 1,4-diethynylbenzene in the synthesis of block copolymer, linear multifunctional copolymer, dendrimer, polymer network, and polymer analogs have been described in previously published articles. So, it is revealed that this new “tool in the box” may prove as one of the new stratagem of polymer therapeutic development. |
|
Click reactions on linear polymers
|
| Three main types of controlled radical polymerization are: |
| 1. Atom transfer radical polymerization (ATRP). |
| 2. Stable free radical polymerization (SFRP). |
| 3. Nitroxide mediated polymerization (NMP). |
| 4. Reversible addition fragmentation chain transfer (RAFT). |
| The above mentioned methods have been extensively used in the synthesis of Block copolymers which have many pharmaceutical applications including in the formulations of various nanocapsules, nanospheres, polymersomes [57]. As click chemistry gives efficient reaction product and had a tolerance to variety of chemicals therefore, it has been used to form block copolymers from homopolymers with azide and alkyne end fuctionalities. Van amp et al. amphiphilic copolymer structures by combination of ATRP and HDC reaction. Wherein, Opsteen et al. carried out synthesis of polystyrene (PS), poly (tert-butyl acrylate) (PtBA), poly (methyl acrylate) (PMA) block copolymers using click chemistry [58]. |
| This combination of polymer chemistry and click chemistry proved to be very advantageous as it gives lead to synthesis of different polymeric materials which was previously inaccessible to syntheise with traditional methods. Other complex polymer structures are also accessible to synthesize with this technique which proved to be very efficient as polymer therapeutics. Example continues as amphiphilic block copolymers provided application for the synthesis of polymeric micelles as delivery vehicle for therapeutics, imaging or diagnostic agents [59-61]. |
Activity Based Protein Profiling (ABPP)
|
| Approach of click chemistry has applied in other field of drug discovery such as target identification by activity based protein profiling and to understand the functions of proteins in their natural setting diphenylphosphaneincluding their regulation. This strategy would allow the visualization of proteins expressed at low level and the information would indicate the activity more than of abundance. This technique utilizes active-site directed chemical probes with broad target selectivity to label active proteins with various enzyme classes and allows the discovery of new targets [62]. These sites directed probes are composed of two different elements; the molecule that brings selectively to the binding and the label that have visualized and contains a functional group that covalently react with specific classes of enzymes such as serine hydrolases. These two components has been pre-assembled and added to cell extracts, and, indeed, that have done to profile a number of enzymes [63]. Previously, ABPP conducted in vitro because the bulky chemical tags inhibited cellular uptake and caused the enzyme to profile outside their natural biological environment. However, the discovery of coppercatalysed click reaction provided the enzyme to be profiled in-vivo. The use of a phenyl sulfonate ester reactive group allowed the in-vivo profiling of glutathione S-transferase, aldehydes dehydrogenases, and enoyl CoA hydratases [64]. The small azide-groups are easily take by the cells and covalently labels active proteins. Addition of the alkyne cycloaddition reaction under copper catalysis after lysing the cells tags the enzyme for detection and isolation. Therefore, the use of click chemistry in ABPP resulted in the isolation of several enzymes in breast cancer cell lines [65]. |
|
Click in peptide chemistry and cyclisation
|
| There are many articles defining the application of click chemistry in peptide synthesis. The clickable functional groups are easy to incorporate in peptides and are stable to oxidative conditions. Due to relative planarity, strong dipole moment, and hydrogen bonding ability of triazole it bear its physicochemical resemblance to the amide bond. Therefore, the triazole linkage has found its applicability in the field of peptide science [66]. Meldal et al. [67] has demonstrated the compatibility of protected amino acid side chain with CuAAC reaction. A few numbers of amino acids has assembled on a solid phase resin and then attached different combination of protected amino acids (AA3) modified with alkyne group. The resulting peptides were detached off the resin to give triazole containing amino acid in high yields. Various protecting group found to be stable and compatible to click reactions [68]. Click chemistry found its application in cyclisation of peptides to increase its potency and in-vivo half-life by locking its confirmation. Click reaction was found to be exploited in a number of different peptide cyclization reactions such as the preparation of novel heterodetic cyclopeptides by an intramolecular side-to-side chain click reaction, forming a 1,4-disubstituted [1,2,3] triazolyl-containing bridge; Cu(I)- and Ru (II)-mediated “click” cyclization of tripeptides [69-71] (Scheme 10). |
|
Target Guided Synthesis and in-situ click chemistry
|
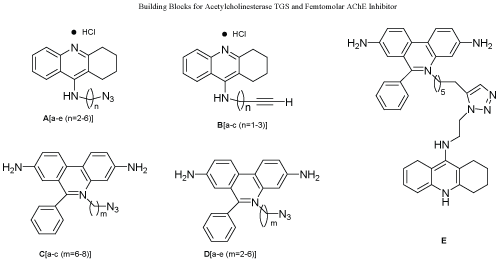
| Kinetic target guided synthesis (TGS) and in-situ click chemistry are among unconventional drug strategies having the potential to develop the libraries of compounds. TGS is the use of small molecules building blocks that was assembles by specifically targeted enzymes to synthesize their own inhibitors. Only building blocks that interact with active site of protein will be in close proximity to interact with one another and from potent inhibitors [72]. Mocks discovery of a dramatic rate acceleration of the azide-alkyne cycloaddition by sequestering the two components inside a host structure, prompted Sharpless et al. to investigate a paradigm for drug discovery, which was dependent on irreversible target guided synthesis of high affinity inhibitors from reagents that are inert under physiological conditions. Installation of the azide –alkyne within organic building blocks allowed the use of Husigen [3+2] cycloaddition to discover a Femtomolar inhibitor of acetylcholinesterase (AChE) [73]. Their inhibitors have been employing for a century in variety of therapeutic regimen to investigate the role of acetylcholine in neurotransmission. Acetylcholine esterase hydrolyses acetylcholine, a neurotransmitter, and plays an important role in the central nervous system. The active site is located at the base of a narrow 20 Å gorge lined with aromatic amino acids and second binding site at the rim of the gorge near to the enzyme surface [74]. The design of the building block based on the tacrine and phenanthridinium, two known specific site ligands. This cycloaddition reaction is opted in this study due to several reasons i.e., reaction is bioorthogonal, and is extremely slow at room temperature. Out of all possible azide-alkyne cycloaddition products, only the 1,5-traizole was formed from the combination of A(a) and D(e) (TZ2PA6 syn 6 triazole) in the presence of AChE. This inhibitor with a 100-fold greater affinity and a subpicomolar constant dissociation constant has potency greater than all known non-covalent organic AChE inhibitors [75,76] (Scheme 11). |
|
Click in radiopharmaceuticals
|
| Radiochemistry is no exception, as the canonical Cu(I)- catalyzed azide-alkyne cycloaddition, strain-promoted azide-alkyne cycloaddition, inverse electron demand Diels-Alder reaction, and other types of bioorthogonal click ligations have had a significant impact on the synthesis and development of radiopharmaceuticals. Click chemistry had a vast application in the preparation of imaging agents for SPECT and PET, including small molecules, peptides, and proteins labeled with radionuclides such as 18F, 64Cu, 111In, and 99mTc. CuAAC has been used in click chemistry for labelling both peptidioc and small molecule reaction tracers bearing the positron emiiting radio halogen 18F (half-life, 109.8 min) using 18F-labeled alkyne- or azide-bearing building blocks [77]. Various researchers have shown that this reaction of CuAAC is a great approach for the construction of radiotracers. Few examples include Gaeta et al. produce a high-affinity, 18F-labeled subtype A g-aminobutyric acid receptor ligand in 7% uncorrected yield over 2 steps and a specific activity of 0.9 GBq/mmol using the ligation between 2-18F fluoroethylazide and a diphenylphosphane bearing precursor [78]. In similar work, Carroll et al. reacts thioesterbased phosphane precursors and 2-18F-fluoroethylazide to create the 18F-labeled products in greater than 95% radiochemical yield for the single ligation step [59]. Pretze et al. in contrast, have moved the 18F to the phosphane, enabling the radiolabeling of an array of azidefunctionalized targets. In their work, an 18F-fluoroacyl-functionalized phosphane was synthesized and reacted with azide-containing model compounds to produce 18F-labeled products in 30%-35% decaycorrected radiochemical yields in 1 pot over 2 steps. Finally, the Staudinger ligation have recently been applied to pre-targeted PET imaging by Vugts et al. who ultimately concluded, however, that this reaction is not suitable for in vivo pretargeting, likely because of the relatively slow kinetics of the ligation and the in vivo oxidation of the phosphane-based radioligand [64]. |
Conclusion
|
| From the above review it was concluded that click chemistry was considered as a stringent and powerful tool and an important aid to medicinal chemist that define a set of dependable transformations that can be employed to design and synthesise novel compounds. Although variations of the reaction exist, and probably better reactions will described and applied in the future, the general principle of efficiency, versatility and selectivity will maintained if not improved. For example, there is the need to synthesize NH-1, 2, 3-triazole derivatives using the CuAAC approach, to expand the versatility of this reaction, as click chemistry with sodium azide was not optimize. The bioisosteric potential of the triazole ring and Huisgen 1, 3-dipolar cycloaddition cycloaddition is an ideal condition. Finally, it is crucial to highlight that click chemistry has been shown to be able to help bridge the gap between chemistry and biology, becoming a true interdisciplinary reaction which can be utilized in future prospects for the construction of novel pharmacophores. Indeed, click chemistry can directly link chemistry to biology (such as in the ABPP assay) and can use biology for creating tailored synthesis. |
Future Prospects and Application of Click Chemistry
|
| Click chemistry is a stringent criterion that used to construct novel pharmacophores to facilitate and in many other applications in the future. Strategies using click chemistry have shown significant advantages over traditional synthetic techniques for the modular, rapid, clean and efficient synthesis of various biomolecules. Click chemistry was fulfill with many conditions such as insensitivity to oxygen, inhibition and high yields with basic purification procedures can lead to photochemical and thermal initiation. It has many other wider applications such as synthesis of 1,4-substituted triazoles, modification of peptide function with triazoles, modification of natural products and pharmaceuticals, drug discovery, macrocyclizations using Cu(I) catalyzed triazole couplings, modification of DNA and nucleotides by triazole ligation, supramolecular chemistry: calixarenes, rotaxanes, and catenanes, dendrimer design, carbohydrate clusters and carbohydrate conjugation by Cu(1) catalyzed triazole ligation reactions, polymers, material science, nanotechnology 21, and Bioconjugation, for example, azidocoumarin. The versatility of the reaction can be expand by synthesis of NH-1,2,3-triazole derivatives. This reaction of click chemistry made the use of azidomethylpivalate to the desired derivative of NH-1, 2, 3-triazole that is amenable for further derivatization. β-tosylethylazide can react with alkynes to give the desired product. It is conclude that click chemistry has high potential if exploited appropriately. It links various types of chemistry with biology and can tailor various useful synthesis in future. |
Figures at a glance
|
 |
| Figure 1 |
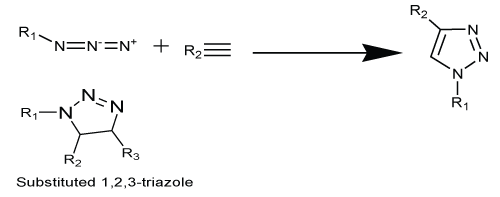 |
 |
 |
 |
| Scheme 1 |
Scheme 2 |
Scheme 3 |
Scheme 4 |
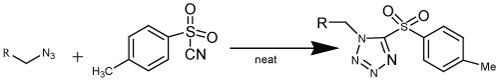 |
 |
 |
 |
| Scheme 5 |
Scheme 6 |
Scheme 7 |
Scheme 8 |
 |
 |
 |
| Scheme 9 |
Scheme 10 |
Scheme 11 |
|