Keywords
|
| Chronic graft versus host disease, Peyronie’s disease |
Introduction
|
| Chronic graft versus host disease (GvHD) can involve any organ system, including skin and soft tissue, lungs, eyes, liver and the gastrointestinal tract. Involvement of the genitalia may occur in both the sexes, and has been well described in women [1]. In contrast, chronic GvHD involving the male genitalia is rare, and most descriptions relate to the inflammatory/ sclerotic complications of the penile skin and glans leading to balanoposthitis, lichen sclerosis, and phimosis [2,3]. |
| Peyronie’s disease (PD), first described by Francois Gigot de la Peyronie in 1743 [4], affects about 0.3% to 8.9% of men between 40 to 60 years of age [5]. It is an acquired, localized fibrotic disorder of the tunica albuginea, which leads to penile deformity, pain, and eventually to erectile dysfunction. There has been only one case of PD reportedly associated with allogeneic hematopoietic stem cell transplant [6]. |
Case Presentation
|
| A 52 year old African-American male, diagnosed with Acute Myeloid Leukemia (AML) French American British class M0 (normal cytogenetics) in June 2008. He was treated with “7+3”induction chemotherapy, followed by consolidation chemotherapy with high dose Ara-C in July 2008. Subsequently, in first complete remission, he underwent fully myeloablative conditioning with 1200 cGy of total body irradiation; 125 mg/m2 of Fludarabine, and 120 mg/kg of Cytoxan. This was followed by a human leucocyte antigen (HLA) matched, ex-vivo T lymphocyte depleted (selective allodepletion) sibling allogeneic peripheral blood stem cell transplant in September 2008. |
| His post-transplant course was complicated by acute GvHD of the skin and mucosa; bronchiolitis obliterans; and renal insufficiency. Mucocutaneous GvHD manifestations were not found in sun exposed areas, instead the mucosa and the lower extremities were affected. Poor post-transplant immune reconstitution led to various infectious complications and prolonged hospitalizations including Methicillin resistant Staphylococcus aureus (MRSA) pneumonia requiring intubation, several episodes of skin and soft tissue MRSA infection, and Pseudomonas infection. |
| He received a low dose Donor Lymphocyte Infusion (DLI) in March 2010 to promote immune reconstitution. He achieved full donor myeloid and lymphoid chimerism in September 2010. After DLI, he developed acute GvHD of skin and gastrointestinal (with persistent perirectal ulceration), requiring prolonged systemic immunosuppressive therapy with steroids, calcineurin inhibitors, MMF, ultra-low dose interleukin-2 (IL2), rituximab and sirolimus over the past seven years. Acute GvHD of the skin progressed to chronic sclerotic type GvHD in December 2011 with subsequent improvement. The chronic GvHD is now controlled with low dose Sirolimus and intermittent steroid pulses as needed. Other chronic GvHD/steroid related comorbidities include chronic kidney disease, bilateral aseptic necrosis of femoral head, and bilateral steroid induced cataracts. |
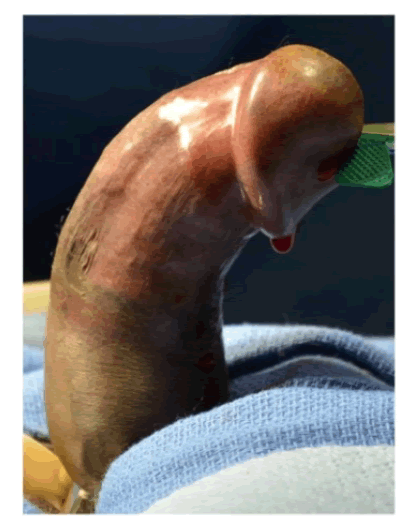
| Within about one year of ultralow dose IL2 administration, he started to develop progressive dorsal curvature of the penis with erections (Figure 1A). There was progressive worsening of the curvature over the first 12-months. At the time of presentation, he noted that his curvature had been stable for at least 8 months by his recall. There was no history of overt trauma or injury to the external genitalia. He had no difficulty with urination, achieving erections, libido, orgasm, rigidity or duration of erections. The erections were not painful, and he felt that without the curvature, his erections would be sufficient for penetrative intercourse. However, the almost 90 degrees of dorsal curvature with erection prevented intercourse. Genitourinary examination revealed a non-tender well-defined plaque 1-1.5 cm in width x 2-3 cm in length on the dorsal aspect of the penis. Color Doppler Duplex ultrasound evaluation of the erect penis revealed a 75-degree curvature and appropriate hemodynamic response to prostaglandin injection. |
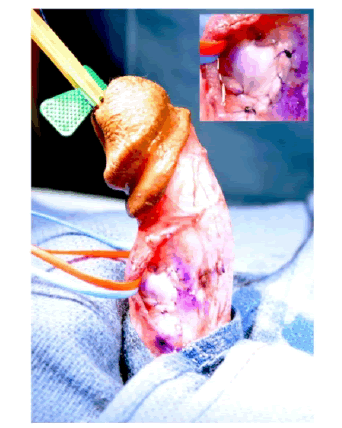
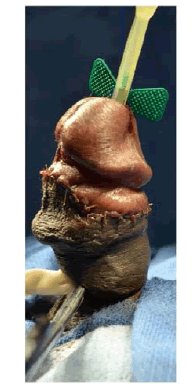
| The patient was counseled on his treatment options and elected to undergo incision and grafting. In July 2014, he underwent successful incision and grafting of the penile plaque (Figure 1B and 1C). A circumcision incision was made and the penis degloved. 2 para-urethral incisions were made ventrally through Buck’s fascia, and the neurovascular bundle elevated. An artificial erection was induced and the maximum point of curvature identified. The plaque was incised horizontally at this point in a ‘double-Y’ fashion and the edges of the tunica albuginea lifted off the underlying erectile tissue to create a rectangular defect. The edges were measured and a corresponding rectangular graft of Tutoplast® cadaveric pericardium was used to cover this defect. |
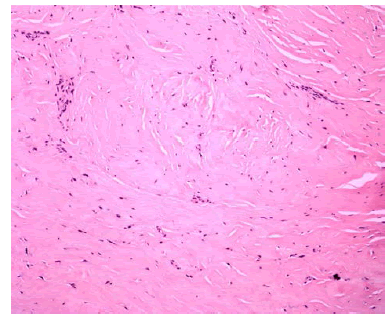
| Tunica albuginea biopsy revealed dense fibrous tissue consistent with penile fibromatosis (Figure 2). There was no significant residual curvature and is now able to engage in intercourse. |
Discussion
|
| The natural history of PD is divided into an acute (or inflammatory) phase and a chronic (stable) phase. During the former, there may be penile pain, even when flaccid, and there are often dynamic changes of penile malformation. During the latter, pain resolves and the malformation stabilizes in its characteristics, but spontaneous resolution does not usually occur. It can be a physically and psychologically devastating condition to the patient. |
| The pathogenesis of PD is unknown, but is likely to be multifactorial, with interplay between genetic predisposition, trauma, and tissue ischemia. PD has been associated with diabetes, hypertension and medications like beta blockers, HLADW2, HLA-A1, and Dupuytren’s contracture. |
| Our patient actually noted onset of his curvature within 12 months of administration of IL-2. A direct cause-and-effect relationship may be impossible to prove, but there is clearly a strong temporal association. He did not have any traumatic inciting factor and denied any significant underlying erectile dysfunction. Another plausible explanation is that a mild trauma or micro trauma that would not be noxious in an otherwise healthy patient was sufficient stimulus in this inflammatory environment for Peyronie's plaque formation. The synergistic impact of micro-trauma with some auto-immune predisposition to Peyronie’s disease is a known entity in diabetics. The added immune dysregulation of chronic GvHD and IL-2 administration may have made him more susceptible to the process of scarring characteristic of Peyronie’s, and this entire constellation of factors may have ultimately lead to his development of a Peyronie’s plaque and penile curvature. |
| Many oral therapies have been tried during the acute phase including vitamin E, pentoxyphilline, potassium amino benzoate, tamoxifen, colchicine, carnitine and Co-Q-10, with varying success at stopping progression of curvature and even lower success with reversing it. In the stable phase, there are non-surgical options such as Xiaflex, a recently FDA approved clostridial collagenase, and verapamil injections. Surgical treatments remain the gold standard and include penile plication, incision or excision and grafting, and penile prosthesis [7]. |
Conclusion
|
| In conclusion, while it is not possible to prove a direct cause and effect relationship between chronic GvHD and PD, it appears very likely that the disease and the treatments may be predisposing or inciting factors. Increasing awareness is required to diagnose PD in the post-transplant population especially given the rapidly growing number of transplants [8]. Patients are often embarrassed to report sexual dysfunction. Underreporting related to embarrassment, erectile dysfunction, and aging in males, parallels many of the factors challenging identification and treatment of vaginal chronic GvHD in females [9]. Specific history taking is the key to identifying PD. |
Acknowledgment
|
| This work was supported by the intramural research program of the NHLBI, NIH. (NIH Grant Number - Z99 HL999999). |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1a |
Figure 1b |
Figure 1c |
Figure 2 |
|
| |
| |
| |









