Abstract
The present work is aimed to develop a stable formulation of preferred combination of two Antibiotics Amoxycillin and Potassium Clavulanate by using various disintegrants.They are Prepared by direct compression method using different disintegrant i.e croscarmellose Sodium, cross povidone, Maize Starch and sodium starch Glycolate. Aspartame as sweetener and strawberry flavours were used to increase palability. The powder blends were subject to various characterstics test such as bulk density, tapped Density, Haushners ratio and combressibility index. These tablets were evaluated for hardness, friability, disintegration time and wetting time and in vitro drug release. Amoxycillin and Potassium clavulanate dispersible tablets were found to be of good quality fulfilling all the requirements for dispersible tablets.The result indicated that concentration of cross povidone, cross carmellose sodium, sodium starch glycolate and maize starch significantly affected the release property of the drug. Cross carmellose sodium showed high disintegration time as compared to batches prepared from maize starch, sodium starch glycolate and cross povidone. The optimized batch tablets were packed in Alu Alu pack and performed stability studies at 40. C/75% RH. There is no change in the physiochemical properties of the tablet during the stability period. Hence the developed dispersible tablet could be found suitable alternate dosage form.
Keywords
Amoxycillin; Potassium clavulanate; Sodium starch glycollate, Cross povidone
Introduction
Oral route of administration is the most important method of administering drugs for systemic effects. Many pharmaceutical dosages are administered in the form of tablets, hard gelatin capsules, granules, powders, and liquids. Many patients, particularly pediatric and geriatric and bed ridden patients have difficulty in swallowing or chewing solid dosage forms. This problem is also applicable to active working or travelling people who do not have ready access to water. Recent advances in Novel Drug Delivery Systems (NDDS) aim to develop fast dissolving/disintegrating tablets to improve patience compliance [1]. Dispersible tablets (DTs) Dissolve or disintegrate in saliva within a minute without the need of water or chewing.
Advantages of dispersible tablets include convenience of administration, patient compliance, rapid onset of action, increased bioavailability, accurate dosing as compared to liquids, good stability, ability to provide advantages of liquid medication in the form of solid preparation, ideal for pediatric anti geriatric patient and rapid dissolution/absorption of the drug. Some drugs are absorbed from the oral cavity (mouth, pharynx and esophagus) as the saliva passes down into the stomach [2]. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablet dosage form. Dispersible tablet also beneficial for schizophrenic, parkinsonism or developmentally disabled patients with persistent nausea, those with conditions of motion sickness, sudden episodes of allergic attack or coughing, and patients who do not have ready access to water.
The various technologies used to prepare DTs include conventional methods like direct compression, wet granulation, and moulding, spray drying, freeze drying and sublimation. Direct compression represents a simple and cost effective tablet manufacturing technique. The basic approach used in the development of the dispersible tablets is the use of Super disintegrates. Superdisintegrants facilitate the break upon disintegration of tablet content into smaller particles that can dissolve more rapidly than conventional dosage form. The commonly used superdisintegrants are Croscarmellose sodium, Crospovidone, Kollidon CLM and sodium Starch glycolate [3].
Excipients used for preparing of tablet
Excipients balance the properties of the actives in the release of dosage forms. This demands a thorough understanding of the chemistry of these excipients to prevent interaction with the actives. Determining the cost of these ingredients is another issue that needs to be addressed by formulators. The role of excipients is important in the formulation of fast-melting tablets. These inactive food-grade ingredients, when incorporated in the formulation, impart the desired organoleptic properties and product efficacy. Excipients are general and can be used for a broad range of actives, except some actives that require masking agents [4].
Bulking materials
Bulking materials are significant in the formulation of fast-melting tablets. The material contributes functions of a diluent, filler and cost reducer. Bulking agents improve the textural characteristics that in turn enhance the disintegration in the mouth, besides; adding bulk also reduces the concentration of the active in the composition. The recommended bulking agents for this delivery system should be more sugar-based such as mannitol, polydextrose, lactitol, dcl (direct compressible lactose) and starch hydrolystate for higher aqueous solubility and good sensory perception. Mannitol in particular has high aqueous solubility and good sensory perception. Bulking agents are added in the range of 10 percent to about 90 percent by weight of the final composition [5].
Emulsifying agents
Emulsifying agents are important excipients for formulating immediate release tablets they aid in rapid disintegration and drug release. In addition, incorporating emulsifying agents is useful in stabilizing the immiscible blends and enhancing bioavailability. A wide range of emulsifiers is recommended for fast-tablet formulation, including alkyl sulfates, propylene glycol esters, lecithin, sucrose esters and others. These agents can be incorporated in the range of 0.05 percent to about 15 percent by weight of the final composition [6].
Lubricants
Lubricants, though not essential excipients, can further assist in making these tablets more palatable after they disintegrate in the mouth. Lubricants remove grittiness and assist in the drug transport mechanism from the mouth down into the stomach [7].
Flavors and sweeteners
Flavors and taste-masking agents make the products more palatable and pleasing for patients. The addition of these ingredients assists in overcoming bitterness and undesirable tastes of some active ingredients. Both natural and synthetic flavors can be used to improve the organoleptic characteristic of fast-melting tablets.
Super disintegrates
A disintegrant is an excipient, which is added to a tablet or capsule blend to aid in the breakup of the compacted mass when it is put into a fluid environment.
Excipients used for preparation of dispersible tablet
Super disintegrates
It increases the rate of disintegration and dissolution. For the success of orally disintegrating tablet, the tablet having quick dissolving property which is achieved by super disintegrants. Examples are- Crospovidone, MCC, Sodium starch glycolate, CMC, Carboxy methyl cellulose and modified corn starch [8].
Sweeteners and sugar Based excipients
Sugar based excipient act as bulking agents. They exhibit high aqueous solubility and sweetness and impart taste masking property. Examples are- Aspartame, Sugar derivative, Dextrose, Fructose, Mannitol, Sorbitol, Maltose etc.
Flavors
It increases patient compliance and acceptability. Examples are-Vanilla, Citrus oil, Fruit essence, Eucalyptus oil, Clove oil, Peppermint oil etc.
Surface Active Agents
It reduces interfacial tension and thus enhances solubilization of Dispersible tablets .Examples are-Sodium lauryl sulfate, Sodium doecyl sulfate, Polyoxyethylene sorbitan fatty acid esters, Polyoxyethylene steartes etc.
Binder
It maintains integrity of dosage form. Examples are-PVP, Polyvinyl alchol, Hydroxy propyl methylcellulose.
Color
It enhances appearance and organoleptic properties of dosage form.
Examples are-Sunset yellow, Red iron oxide, Amaranth
Lubricants
It helps reduces friction and wear by introducing a lubricating film. Examples are- Stearic acid, Magnesium stearate, Zinc stearte, Talc, Polyethylene glycol, Liquid paraffin, Colloidal silicon-di-oxide etc [9].
Fillers
It enhances bulk of dosage form. Examples are-Mannitol, Sorbitol, Xylitol, Calcium carbonate, Magnesium carbonate, Calcium sulfate, Magnesium trisilicate etc.
Materials and Methods
| S.no |
Equipments |
Manufacturer |
| 1 |
Tablet compression machine-27 station(single Rotary) |
CIP Machinery |
| 2 |
Die Punch |
CIP Machinery |
| 3 |
Planetary Mixer |
Kenwood |
| 4 |
Hot air oven |
Lab India |
| 5 |
Dissolution apparatus (usp) |
Electro lab |
| 6 |
Electromagnetic sieve shaker( ESM-8) |
Electro lab |
| 7 |
Tablet Hardness tester (8M) |
Dr.Schleuniger, pharmaton USA |
| 8 |
PH meter |
Lab India |
| 9 |
Reverse phase High Pressure Liquid chromatography (HPLC) |
Shimadzu |
| 10 |
Electronic Weighing Balance |
Essec Teraoka Lid(Japan) |
| 11 |
Digital high precision balance (single pan) |
Mettler- Toledo(Switzerland) |
| 12 |
Disintegration tester |
Electro Lab |
| 13 |
Roche Friabilator USP |
Electro Lab |
| 14 |
Mechanical stirrer |
Remi Motors, Bombay |
| 15 |
Tabbed density tester |
Electro Lab |
| 16 |
Bulk density apparatus |
Electro Lab |
| 17 |
Stability chambers |
Thermo lab, Mumbai |
| 18 |
Humidity Chamber HTC 3003 |
Thermo lab |
Table 1: list of instruments and manufacturer.
| S.no |
Materials name |
Company name |
| 1 |
Amoxicillin trihydrate (powder) BP |
DSM Anti Infectives India Ltd. India |
| 2 |
Colloidal silicon dioxide (Aerosil) USP/BP |
Degussa, Germany |
| 3 |
Maize starch USP/BP |
Maize Products, Ahemedabad, India |
| 4 |
Potassium Clavulanate + Avicel blend (1: 1) BP |
Fermic, S.a.de c.v, Mexico D.F / LEK Pharmaceuticals, Germany |
| 5 |
Microcrystalline cellulose (Avicel pH 101) USP/BP |
FMC Biopolymer, Ireland. |
| 6 |
Powdarome Strawberry Premium flavor IH |
Firmenich. |
| 7 |
Yellow oxide of irons |
Roha Dyes & Chemicals. |
| 8 |
Magnesium stearate USP/BP |
Nitika Chemicals, Nagpur |
| 9 |
Sodium starch Glycolate |
Signet pharma agencies, mumbai |
| 10 |
Crospovidone |
ISP Technologies, USA |
| 11 |
Croscarmellose Sodium |
Signet Pharma Agencies Mumbai. |
| 12 |
Aspartame |
Firmenich |
Table 2: Drug excipients and the manufacturer.
Experimental work
Calibration curve of Amoxicillin Trihydrate
100 mg of amoxicillin trihydrate was accurately weighed and dissolved in 25 ml of methanol in 100 ml volumetric flask and volume was made up to the mark using methanol, to make (1000 µg/ml) standard stock solution. Then 2 ml stock solution was taken in another 100 ml volumetric flask and further diluted in 100 ml of methanol to make (20 µg/ml) standard stock solution, then final concentration were prepared with water. The absorbance of standard solution was determined using UV/VIS spectrophotometer at 220 nm.
Calibration curve of diluted potassium clavulanate
Accurately weighed 100 mg Diluted Potassium Clavulanate was transferred into 100ml volumetric flask and dissolved in small quantity of methanol and the volume was made up with water to give a stock solution of concentration of 1mg?ml. further dilutions were made in the range of 2-10 mcg/ml with water and absorbance was measured at 220 nm.
Pre-formulation studies
IR Spectroscopic analysis
The IR absorption spectra of the pure drug and with different excipients were taken in the range of 4000-400 cm-1 using KBR disc method. Triturate 1-2 mg of the substance to be examined with 300-400 mg, specified quantity; of finely powered and dried Potassium bromide .These quantities are usually sufficient to give a disc of 10- 15 mm diameter and spectrum of suitable intensity by a hydraulic press. The Infrared spectrum of Amoxicillin and Potassium Clavulanate was recorded by using FT-IR spectroscopy and observed for characteristic peaks of drug.
Method of preparation
Formulation compositions
In the present research work, a dispersible tablet of Amoxicillin and Potassium Clavulanate was formulated using direct compression method. Wet granulation method was not used because this formulation is highly sensitive to moisture and temperature conditions (11). Therefore Direct compression method was used for the manufacture of Amoxicillin and Potassium Clavulanate dispersible tablets.
Sixteen formulations were prepared by direct compression method using different disintegrates in various ratios of from F1 to F4 using maize starch as disintegrate in the ratios of 5,10,12.5 and 15, and F5 to F8 using CCS as disintegrates in the ratios of 5,10, 12.5 and 15, and F9 to F12 using Crospovidone as disintegrant in the ratios of 5,10,12.5 and 15, and F13 to F16 using sodium starch glycolate as disintegrant in the ratios of 5,10,12.5 and 15. All the formulation and composition was shown in Table No:7 [10].
The Commonly used sweetening agents are Aspartame, Sugar derivative, Dextrose, Fructose, Mannitol, Sorbitol, Maltose etc. In this present study Aspartame is used as sweetening agent.
The Commonly used Flavoring agents are Vanila, Citrus oil, Fruit essence, Eucalyptus oil, clove oil, Peppermint oil, strawberry flavor. In this present study Strawberry flavor is used as a flavoring agent.
| Ingredients |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
F10 |
| Amoxicillin |
234.3 |
234.3 |
234.3 |
234.3 |
234.3 |
234.3 |
234.3 |
234.3 |
234.3 |
234.3 |
| Potassium Clavulanate |
75.7 |
75.7 |
75.7 |
75.7 |
75.7 |
75.7 |
75.7 |
75.7 |
75.7 |
75.7 |
| MCC AVICEL PH 101 |
149 |
144 |
141.5 |
139 |
149 |
144 |
141.5 |
139 |
149 |
144 |
| Maize starch |
5 |
10 |
12.5 |
15 |
- |
- |
- |
- |
- |
- |
| Croscarmellose sodium |
- |
- |
- |
- |
5 |
10 |
12.5 |
15 |
- |
- |
| Crospovidone |
- |
- |
- |
- |
- |
- |
- |
- |
5 |
10 |
| SSG |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Aerosil |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Aspartame |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
| Strawberry |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
| Magnesium stearate |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
| Yellow oxide of Iron |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
| Total Weight |
500 |
500 |
500 |
500 |
500 |
500 |
500 |
500 |
500 |
500 |
| F1–F3: Maize starch, F4-F6: Croscarmellose sodium, F7–F9: Crospovidone F10-F12: Sodium starch glycolate (SSG) |
Table 3: Composition of formulations (F1-F10).
Evaluation of blend
The evaluation of blend was done by the following parameters
Angle of repose
The frictional force in a loose powder can be measured by the angle of repose. Angle of Repose is the maximum angle between the surface of a pile of powder and horizontal plane. It is usually determined by fixed funnel method and is the measure of the flow ability of powder/granules.
A funnel with 10 mm inner diameter of stem was fixed at a height of 2 cm over the platform. About 10 gm of sample was slowly passed along the wall of the funnel till the tip of the pile formed and touches the steam of the funnel. A rough circle was drawn around the pile base and the radius of the powder cone was measured48.
Angle of repose was calculated from the average radius using the following
Formula
Where,
θ=Tan-1 (h/r)
θ=Angle of repose h=Height of the pile
r=Average radius of the powder cone
| Angle of repose |
Type of flow |
| < 25 |
Excellent |
| 25 – 30 |
Good |
| 30 – 40 |
Passable |
| > 40 |
Very Poor |
Table 4: Limitation of angle of repose.
Higher the angle of repose the rougher and more irregular is the surface of the particles.
Bulk density
Bulk density of a compound varies substantially with the method of crystallization, milling or formulation. Usually, bulk density is of great importance when none considers the size of a high-dose drug product or homogeneity of a low-dose formulation. Apparent bulk density (g/ml) of all types of drug were determined by pouring preserved (40-mesh) gently 25 gm of sample through a glass funnel into a 100 ml graduated cylinder. Bulk density was calculated.
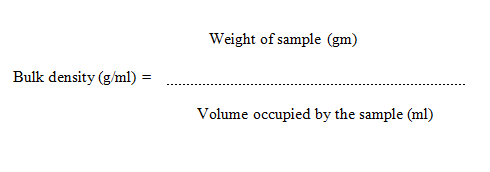
The Bulk Characterization is done in Electrolab-Tap Density Tester by method
USP-I.
Tapped density
Tapped densities of all types of granules were determined by pouring gently 25 gm of sample through a glass funnel into a 100 ml graduated cylinder. The cylinder was tapped from height of 2 inches until a constant volume was obtained.
Volume occupied by the sample after tapping were recorded and tapped density was calculated.

Percentage compressibility
Compressibility is the ability of powder to decrease in volume under pressure
.Compressibility is a measure that is obtained from density determinations. It is also one of the simple methods to evaluate flow property of powder by comparing the bulk density and tapped density. A useful empirical guide is given by the Carr’s compressibility or compressibility index. Compressibility measures gives idea about flow property of the granules as per Carr’s index which is as follow
| Compressibility |
Flow description |
| 5–15 |
Excellent |
| 12 –16 |
Good |
| 18–21 |
Fair |
| 23–35 |
Poor |
| 35–38 |
Very poor |
| > 40 |
Extremely poor |
Table 5: Limitation of percentage compressibility index.
Hausner ratio
It provides an indication of the degree of densification which could result from vibration of the feed hopper.
Bulk density
Hausner ratio =Tapped density
Lower Hausner ratio−better flowability
Higher Hausner ratio−poor flow ability
| Hausner’s ratio |
Type of flow |
| <1.25 |
Good flow |
| 1.25 – 1.5 |
Moderate |
| >1.5 |
Poor flow |
Table 6: Limitation of Hausner’s ratio.
Manufacturing procedure
• Amoxicillin Trihydrate Was Shifted (Electrolab) Through≠ 20 Mesh
• Potassium Clavulanate, disintegrant all were passed through ≠40 mesh. Both blends (Kenwood planetary mixer) were mixed.
• Mixed blend was sifted through sieve no ≠24 mesh.
• Remaining amount of aspartame, flavor and talc were shifted through ≠40mesh and colour shifted through ≠60 mesh.
• Magnesium stearate was sifted through ≠ 60mesh and mixed with the powder blend
• Blend was compressed to prepare tablets.
Evaluation of tablets
IPQC tests
After Compression all the tablets should be checked for the physical appearance and removal of any obvious defective tablets.
All the tablets should be inspected only on the tablet inspection belt attached with metal detector. Record the weight of good tablets and rejection for each batch.
Shape of tablets
Randomly picked tablets from each formulation were examined for the shape of the tablets.
Weight variation
The test ensures that all the tablets in each batch are of same potency, within reasonable limits. Each tablet in the batch should be uniform in weight and weight variation if any, should be generally within ± 10% for tablets weighing 130 mg or less, ± 7.5% for tablets weighing more than 130 mg and up to 324 mg and ± 5% for tablets weighing 325 mg or more. According to the official test, 20 tablets were weighed individually and collectively. Average weight per tablet was calculated from the collective weight. Then the weights of the individual tablets were compared with the average weight to determine weight variation.
Hardness test
Tablets require a certain amount of strength, or resistance to friability, to withstand the mechanical shocks of handling in manufacture, packaging, and shipping. The strength of the tablet was determined by Dr Schleuniger pharmaton apparatus. The force of fracture was recorded.
Friability
Friability test was performed to assess the effect of friction and shock which may often cause tablets to chip, cap or break. It generally reflects poor cohesion of tablet ingredients. Weighed tablets sample was placed in the chamber and the friabilator was operated for 100 revolutions at 25 RPM and the tablets were weighed again. Compressed tablets should not lose more than 1% of their weight.
Tablet thickness
Variation in the tablet thickness may cause problems in counting and packaging in addition to weight variation beyond the permissible limits. Tablet thickness should be controlled within a ± 3% of a standard value. Tablet thickness was measured by Vernier calipers.
Disintegration test
The disintegration time was determined by using USP Tablet disintegration test apparatus using 900 ml of distilled water without disk. Time taken by tablets (Sec) for complete disintegration of the tablets until no mass remaining in apparatus was measured.
Uniformity of dispersion test
The fineness of dispersion test was done by place 2 tablets in 100 ml of water and stir until completely dispersed. A smooth dispersion is produced, which passes through a sieve no. #25.
Wetting time
The wetting time and capillarity of oral dispersible tablets were measured by a conventional method. Tablet was placed in a petri dish containing 10 ml water at room temperature and the times for complete wetting of tablets were recorded.
References
- Remington: the science and practice of pharmacy, 20th Edition 1:858-885
- Aulton M, Pharmaceutics: the science of dosage form design 304-321
- Leon Lachman, Herbert A, Liberman, Joseph L. Kanig. The Theory and practice of Industrial Pharmacy, 3rd Edition 293-345
- Ansel H,Allen L,Jr. Popovich N, Ansel’s Pharmaceutical dosage forms and drug delivery systems, 8th edition 227-259
- Lachman L, Liberman L,Schwartz J, Pharmaceutical Dosage forms: tablets, 2nd Edition, I:88-121
- Aulton M. Pharmaceutics : The science of dosage form design; international student edition; 304-321,347-368
- Aulton ME, wells TI (1988)”Pharmaceutics: The Science of Dosage form design”, London, England: Churchill Livingstone133
- Parikh D, Drugs and pharmaceutical sciences: Handbook of pharmaceutical granulation technology 81:151-227
- Keith Marshall Dr. Section 3- Compression/compaction. 350-360
- Shiromani PK (2006)“Tabletting Tips” in Pharma & Bio ingredient Published in January








