Review Article - (2024) Volume 16, Issue 3
A Review on Bioactive Compounds from Plants Available in South India with Uroprotective and Nephroprotective Activity
T. Usha Kiran Reddy1,2*,
Annegowda H. V1,
Maged Alkanad1,
A Anish Kumar2 and
P Harshavardhan2
1Department of Pharmacognosy, Sri Adhichunchagiri College of Pharmacy, Adichunchangiri University, Karnataka, India
2Department of Pharmaceutical Sciences, SV University, Tirupathi, India
*Correspondence:
T. Usha Kiran Reddy, Department of Pharmacognosy, Sri Adhichunchagiri College of Pharmacy, Adichunchangiri University, Karnataka,
India,
Tel: 9100243580,
Email:
Received: 17-May-2024, Manuscript No. IJDDR-24-14672;
Editor assigned: 20-May-2024, Pre QC No. IJDDR-24-14672 (PQ);
Reviewed: 03-Jun-2024, QC No. IJDDR-24-14672;
Revised: 11-Jun-2024, Manuscript No. IJDDR-24-14672 (R);
Published:
19-Jun-2024
Abstract
Nephrotoxicity and urotoxicity are two menacing entities that can wreak havoc on renal function and patient well-being, making them a pressing concern in the realms of clinical medicine and drug research. The review examines the underlying causes and mechanisms of these conditions, including drug-induced nephrotoxicity and the effects of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), antibiotics, and chemotherapeutic agents. In vivo and in vitro methods for studying these outcomes are also explored. The advantages and limitations of different experimental models for studying nephrotoxicity are discussed, such as rodents, zebrafish, and organ-on-a-chip technologies. Additionally, botanical extracts such as Punica granatum and Curcuma longa are considered for their potential therapeutic benefits in managing nephrotoxicity. These extracts have shown antioxidative, anti-inflammatory, and immunomodulatory properties that make them promising candidates for future research. This review aims to encourage and inspire further research and innovation in the field of nephrotoxicity and urotoxicity management, by highlighting the potential of botanical extracts and the importance of utilizing experimental models for studying these conditions.
Keywords
Nephrotoxicity; Urotoxicity; Herbal extract; Drug
induced nephrotoxicity
Introduction
Nephron is the most fundamental unit of kidney, both
structurally and functionally at microscopic level. The
constitution is comprised of a renal corpuscle and a renal tubule.
The renal corpuscle is comprised of a glomerulus, which has a
bundle of capillaries, and Bowman's capsule, shaped like a cup.
The renal tubule originates from the renal capsule. The capsule
and tubule exhibit interconnectivity and are comprised of
epithelial cells featuring a central cavity. An individual with good
health possesses approximately 1 to 1.5 million nephrons in
each of their kidneys [1].
As the fluid from the capsule descends into the tubule, it
undergoes processing by the epithelial cells that lines the
tubule. This processing involves reabsorption of water and the
exchange of substances, being added, and removed. The
exchange occurs initially with the interstitial fluid outside the
tubules and subsequently with the plasma in the adjacent peri-tubular capillaries through the endothelial cells lining that
capillary. This physiological mechanism maintains homeostasis
of body fluids and various biochemical’s. Upon reaching the
terminus of the tubule, the residual liquid, known as urine, is
expelled. This substance is comprised of water, metabolic by-products, and harmful substances, as documented by Stevens,
et al. [2].
The four mechanisms employed for the generation and
manipulation of the filtrate (which leads to the conversion of
blood into urine) are filtration, reabsorption, secretion, and
excretion [1,3]. Filtration or ultrafiltration takes place within the
glomerulus and is primarily a passive process that relies on the
intra-capillary blood pressure. Approximately 20% of the plasma
undergoes filtration during its transit through the glomerular
capillaries, while the remaining 80% proceeds into the
peritubular capillaries. Typically, the sole constituents of the
blood that do not undergo filtration into Bowman's capsule are
blood proteins, erythrocytes, leukocytes, and thrombocytes.
Daily, the glomeruli of an adult individual receive a volume of
fluid exceeding 150 litres, out of which 99% of the water content
is subjected to reabsorption. Reabsorption takes place within
the renal tubules and can be passive, resulting from diffusion, or
active resulting from pumping against a concentration gradient.
The process of secretion is also observed in the tubules and
collecting duct and it is an active phenomenon. The substances
that are subjected to reabsorption comprises of water, sodium
chloride, glucose, amino acids, lactate, magnesium, calcium
phosphate, uric acid, and bicarbonate. The excretory process
involves the secretion of various substances such as urea,
creatinine, potassium, hydrogen, and uric acid. Several
hormones regulate the reabsorption or secretion rate in the
tubules to maintain homeostasis. These hormones include anti-diuretic hormone (water), aldosterone (sodium, potassium),
parathyroid hormone (calcium, phosphate), atrial natriuretic peptide (sodium), and brain natriuretic peptide (sodium). The
renal medulla employs a counter current system to produce a
hypertonic interstitium, facilitating the retrieval of solute-free
water from the nephron and its subsequent return to the venous
vasculature as needed.
In this context, we have conducted a review on nephro/urotoxicity and the effect of plants that exhibit nephroprotective
properties against pre-renal, intrinsic, and post-renal diseases.
The utilization of nephroprotective plants as a supplementary
pharmacological intervention was supported by scientific
evidence that demonstrated their effectiveness in mitigating the
pathophysiology’s that result in kidney injuries (Figure 1).

Figure 1: Graphical abstract.
Literature Review
Nephro/urotoxicity
Nephro/urotoxicity is characterized by the swift decline in renal
function resulting from the toxic influence of pharmaceuticals and
chemicals. There exist diverse manifestations, and certain
pharmaceuticals might impact the renal system through multiple
mechanisms. Nephrotoxic substances exhibit nephrotoxicity. It is
important to differentiate nephrotoxicity from medications that
are primarily excreted through the kidneys and require dosage
adjustment in the presence of renal impairment, such as
heparin. The renal toxicity of the majority of medications is
more pronounced in individuals who are already experiencing
renal dysfunction. Several prevalent clinical manifestations of
anticancer drugs on the kidneys are Acute Kidney Disease (AKI)
caused by tubular necrosis, glomerulopathy-induced
proteinuria, hypertension, electrolyte disturbance-induced
tubulopathies, and Chronic Kidney Disease (CKD) (Figure 2) [4].
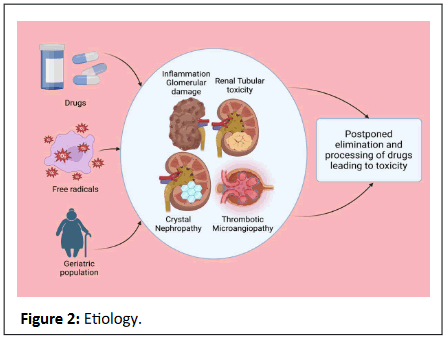
Figure 2: Etiology.
Approximately 20% of instances of nephrotoxicity are drug-induced. This proportion is amplified in geriatric populations due
to prolonged lifespans and the use of multiple medications.
Frequent risk factors associated with drug-induced nephrotoxicity
in patients include underlying renal insufficiency (GFR<60 mL per
minute per 1.73 m²), age over 60 years, hypertension, congestive
heart failure, diabetes, and volume depletion [5].
Sex and genetic variability are frequently implicated in the
predisposition to drug-induced nephrotoxicity, with males
exhibiting greater susceptibility than females due to hormonal
disparities. In addition, it has been observed that dehydration,
heart failure, and hepatic insufficiency are among the
contributing factors that elevate the susceptibility to drug-induced renal damage [6].
There exist various mechanisms of nephrotoxicity, such as
renal tubular toxicity, inflammation, glomerular damage, crystal
nephropathy, and thrombotic microangiopathy, as reported by
Al-Kuraishy, et al. [7].
Moreover, impairment of the kidneys leads to postponed
elimination and processing of drugs and widespread toxicity.
Consequently, numerous medications may necessitate dosage
modification in the setting of renal insufficiency [5].
Drug induced nephro-uro toxicity
The selective endocytosis and accumulation of aminoglycosides via the multi-ligand receptor megalin causes nephrotoxicity,
which specifically affects the proximal tubule epithelial cells. The
induction of oxidative stress by toxic agents and drugs can
potentially damage the tubular transport system, resulting in
tubular mitochondrial damage. Aminoglycoside, amphotericin B,
and antivirals like adefovir and foscarnet are known to cause
tubular damage, as per the research conducted by Vormann, et
al., which has been tabulated in Table 1 [8].
| Nephrotoxic mechanism |
Drugs involved |
References |
| Selective endocytosis and accumulation |
Aminoglycosides |
Vormann, et al. |
| Tubular damage |
Aminoglycoside, Amphotericin B, Adefovir, Foscarnet |
Vormann, et al. |
| Tubular damage |
Antiretroviral drugs, Cisplatin |
Qu, et al. |
| Decrease in intra-glomerular pressure and glomerular filtration rate |
Diclofenac, Valsartan, Captopril |
Sudjarwo, et al. |
| Vasoconstriction of the afferent arteriole |
Cyclosporine, Tacrolimus |
Sudjarwo, et al. |
| Interstitial nephritis |
Allopurinol, Rifampicin, Sulfonamide, Lansoprazole, Quinolones, Cyclosporine, Chinese Herbal Medicine, NSAID's (over 1 gram/day for 2 years) |
Suzuki, et al. |
| Glomerulonephritis |
Gold, Interferon, NSAIDs, Lithium, Hydralazine, Pamidronate |
Frazier, et al. |
| Crystal formation in renal tubules |
Sulfonamides, Ampicillin, Acyclovir, Ciprofloxacin, Methotrexate, Triamterene |
Pawar, et al. |
| Drug-induced microangiopathy |
Ticlopidine, Cyclosporine, Quinine |
Brocklebank, et al. |
Table 1: Drugs involved in nephrotoxic mechanism.
The compact nature of renal drug transporters plays a
significant role in the heightened susceptibility of proximal renal
tubules to toxic substances like antiretroviral drugs and cisplatin,
as per the findings of Qu, et al. [9].
Nonsteroidal anti-inflammatory drugs such as diclofenac,
angiotensin receptor blockers like valsartan, and angiotensin-converting enzyme inhibitors like captopril have been observed
to cause significant decline in intraglomerular pressure and
glomerular filtration rate. Furthermore, it has been observed
that cyclosporine and tacrolimus induce vasoconstriction of the
afferent arteriole in a dose-dependent manner, as reported by
Sudjarwo, et al. [10].
An allergic reaction to certain medications such as allopurinol,
rifampicin, sulfonamide, lansoprazole, and quinolones may
result in interstitial nephritis. Several pharmaceutical agents
have been identified to potentially induce chronic interstitial
nephritis, such as cyclosporine, Chinese herbal medicine, and
nonsteroidal anti-inflammatory drugs administered at a dosage
exceeding one gram per day for a duration of 2 years. The timely
identification and acknowledgement of this ailment's onset and
preliminary stages is imperative, as it has the potential to
advance into a state of renal failure [11].
Glomerulonephritis is an inflammatory condition of the
glomeruli that can be triggered by various nephrotoxic agents
such as gold, interferon, NSAIDs, lithium, hydralazine, and
pamidronate [12].
Numerous pharmaceutical agents generate crystals that
exhibit poor solubility in urine and subsequently deposit within
the distal renal tubules, thereby eliciting an interstitial response
and obstructing the tubular lumen. The crystal formation is
frequently observed in sulfonamides, ampicillin, acyclovir,
ciprofloxacin, methotrexate, and triamterene drugs, as reported
by Pawar, et al. [13]. Microangiopathy induced by drugs is a
result of an immune response triggered by drugs that causes
thrombotic thrombocytopenic purpura and platelet activations.
This eventually leads to endothelial cytotoxicity, as observed in
various medications like ticlopidine, cyclosporine, and quinine
[14].
Treatment
The renal dysfunctions caused by nephrotoxic agents are
mostly reversible. Therefore, the primary approach in this
condition is to cease and desist the causative agents. Several
nephrotoxic agents have been observed to elevate blood urea
and serum creatinine levels. However, it has been noted that
trimethoprim and cimetidine may cause an increase in serum
creatinine levels prior to the onset of nephrotoxic effects. This
could be attributed to their competition with creatinine during
renal tubular secretion, as suggested by Elsby, et al. [15].
The assessment of renal function is better accomplished by
utilizing serum creatinine as a marker, as it remains unaffected
by dietary factors, unlike blood urea. Therefore, an increase of
50% or greater in baseline creatinine levels by 2 mg/dL is
considered an early indication of acute kidney failure.
Additionally, it is necessary to perform a thorough assessment
of the patient's medications in order to determine the
nephrotoxic agent responsible for the adverse effects [16].
Intravenous administration of normal saline or hypertonic
saline, with or without mannitol supplementation, is employed
to mitigate the toxic effects of the drug through induced
diuresis. Magnesium supplementation is administered to
alleviate the condition of hypomagnesemia. Amifostine, a
glutathione analog and free radical scavenger, is known to prevent nephrotoxicity by being taken up by healthy cells and
mitigating the effects of cisplatin.
It is noteworthy that the irreversible nephrotoxic
consequences of ifosfamide in children have been associated
with the concurrent usage of ifosfamide and cisplatin.
Supplementation of vitamin D is imperative in the paediatric
population that is affected [17].
Mesna is utilized as a prophylactic measure against the
development of cyclophosphamide-induced nephrotoxicity
owing to its capacity to bind toxic metabolites with sulfhydryl
groups. While mesna has been found effective in preventing
haemorrhagic cystitis and bladder cancer resulting from
cyclophosphamide, its efficacy is limited in cases of renal injury
caused by ifosfamide. As ifosfamide is transported into proximal
tubular cells via OCT2, we are currently exploring the potential
of cimetidine as a preventative measure by acting as a
competitive inhibitor to OCT2, as suggested by Ciarimboli, et al.
[18].
The efficacy of glucocorticoid therapy, such as prednisone, in
treating interstitial nephritis caused by bortezomib is still
uncertain. However, some studies have indicated that kidney
function may improve with this treatment [19].
Hypomagnesemia is the primary nephrotoxic effect observed
in association with cetuximab. The observed phenomenon can
be attributed to the reliance of magnesium on EGF signalling at
the basolateral membrane to facilitate absorption in the distal
convoluted tubule. The administration of intravenous
magnesium, in addition to calcium and potassium, is typically
necessary for the management of hypomagnesemia, as stated
by Schrag, et al. [20].
The discontinuation of vemurafenib leads to reversible renal
damage, as reported by Hurabielle [21]. The renal toxicity linked
with IL-2 is ascribed to pre-renal azotaemia due to hypoperfusion
and hypotension, as reported by Webb [22]. The cessation of IL-2
administration resulted in the manifestation of typical levels of
urinary protein and serum creatinine. The reversible
nephrotoxicity caused by IL-2 can be effectively managed by
promptly administering fluid boluses upon the onset of oliguria, as
reported by Guleria, et al. [23].
Drug interactions
The significance of meticulous medication management in
clinical practice is exemplified by the drug-drug interactions that
occur between antibiotics such as aminoglycosides, vancomycin,
and quinolones, and nonsteroidal anti-inflammatory agents. The
interactions may result in escalated nephrotoxicity or elevated
susceptibility to seizures, thereby requiring meticulous
observation and dosage modifications to mitigate unfavourable
outcomes. Scientists must maintain an elevated level of
vigilance, especially when dealing with susceptible groups like
premature neonates or individuals with a past medical history of
kidney impairment or neurological conditions. By keeping
abreast of possible drug interactions and implementing suitable
measures to handle them, scientists can enhance patient
outcomes and guarantee the safety and effectiveness of their
therapies and these were tabulated in Table 2.
| Drug or drug class |
Potential renal adverse effects |
Proposed measures |
| Aminoglycosides (when combined with NSAID's) |
Increased nephrotoxicity |
Monitor patients for signs of increased nephrotoxicity |
| Aminoglycosides and vancomycin |
Increased nephrotoxicity |
Monitor aminoglycoside serum levels and adjust dosage accordingly |
| NSAID's and quinolones |
Increased risk of seizures |
Consider increased risk of seizures when co-prescribing, especially in those with impaired renal function, history of seizures, or high dosages/serum levels |
| NSAID's |
Chronic renal dysfunction, acute interstitial nephritis |
Monitor renal function, limit duration of use |
| Opioids |
Albuminuria, alterations in renal parameters |
Use caution with prolonged use, consider alternative pain management |
| Statins |
Acute kidney injury |
Monitor renal function, consider alternative therapies if necessary |
| Fibrates |
Transient elevation in serum creatinine levels |
Monitor renal function, adjust dosage as needed |
| Proton Pump Inhibitors (PPIs) |
Allergic interstitial nephritis, acute and chronic renal failure, end-stage renal disease |
Use caution when prescribing, consider alternative therapies in those with renal dysfunction |
| Metformin |
Accumulation in those with renal dysfunction |
Monitor renal function, adjust dosage as needed, consider alternative antidiabetic therapies if necessary |
| Aminoglycosides |
Renal toxicity |
Monitor renal function and drug administration |
| Fluoroquinolones |
Allergic interstitial nephritis |
Use caution when prescribing, monitor renal function |
| Tetracyclines (specifically doxycycline) |
Potential role in reducing proteinuria in diabetic nephropathy |
Monitor renal function, adjust dosage as needed |
| Vancomycin |
Nephrotoxicity |
Monitor vancomycin levels and renal function |
Table 2: Class of drugs with adverse effects leading to nephrotoxicity.
The administration of NSAID's has been observed to decrease
the excretion of aminoglycosides, a class of antibiotics utilized in
the treatment of gram-negative bacterial infections. This
interaction could potentially elevate the likelihood of
nephrotoxic consequences linked with aminoglycosides. It is
recommended that healthcare professionals observe patients
for indications of heightened nephrotoxicity upon commencing
or elevating the dosage of a nonsteroidal anti-inflammatory
drug while the patient is undergoing aminoglycoside treatment.
This holds significant importance for premature neonates as per
Zarfin, et al., study conducted in 1985 [24].
The co-administration of aminoglycosides with vancomycin, a
potent antibiotic utilized to treat severe bacterial infections, may
exacerbate the nephrotoxic impact. The co-administration of
these pharmaceuticals may result in heightened nephrotoxicity.
It is imperative for healthcare professionals to diligently observe
aminoglycoside serum levels and modify the dosage accordingly
when co-administered with vancomycin. It is advisable to
monitor for indications of elevated nephrotoxicity as per the
findings of Farber, et al. [25].
Nonsteroidal Anti-Inflammatory Drugs (NSAID's) have the
potential to augment the neuroexcitatory and/or seizure-potentiating properties of quinolones, a group of antibiotics
utilized for the management of diverse bacterial infections.
Furthermore, Nonsteroidal Anti-Inflammatory Drugs (NSAID's)
have the potential to elevate the serum levels of quinolone
antibiotics. The concomitant use of NSAID's and quinolone
antibiotics is associated with an elevated likelihood of
experiencing seizures. It is recommended that scientists
consider the increased likelihood of seizures when co-prescribing NSAID's and quinolones. Other variables that could
potentially elevate the likelihood of this interaction encompass
impaired renal function, a past medical history of seizures or
other neurological maladies, and elevated dosages/serum levels
of either compound [26].
Comprehending the potential renal adverse effects of diverse
drug categories, such as analgesics, lipid-lowering agents, proton
pump inhibitors, hypoglycaemic agents, and antimicrobial
agents, is of utmost importance for healthcare professionals.
Through meticulous observation and diligent surveillance of
renal function throughout the course of therapy, scientific
practitioners can mitigate the potential for renal impairment
and guarantee the safety and effectiveness of their
interventions. The optimal management of patients involves
weighing the therapeutic advantages of medication against the
possible negative consequences, while also considering
alternative treatment options when appropriate. In the end,
raising awareness about the renal complications caused by drugs
aids in enhancing patient outcomes and encourages the use of
safe and efficient pharmacotherapy.
Nonsteroidal Anti-Inflammatory Drugs (NSAID's) are a
commonly prescribed group of analgesics that have the
potential to induce chronic renal dysfunction with prolonged
use. Acute interstitial nephritis represents a plausible
unfavourable outcome in acute scenarios. It is imperative for
scientists to observe renal function and restrict the duration of
NSAID administration to mitigate the likelihood of renal
impairment.
The precise mechanisms underlying the various impacts of
opioids on renal function are yet to be fully elucidated.
Prolonged utilization of opioids has been correlated with the
manifestation of albuminuria and alterations in renal
parameters. As scientists, we should exercise prudence while
prescribing opioids for prolonged durations and explore
alternative pain management techniques whenever feasible.
Statins belong to a pharmacological category utilized for the
purpose of reducing cholesterol levels. However, it has been
observed that they can pose a potential threat to the occurrence
of acute kidney injury, especially in the elderly population. It is
recommended that scientists closely monitor the renal function
of individuals who are taking statins and explore alternative
therapies for reducing lipid levels if needed. Fibrates belong to a
distinct category of drugs that are effective in reducing lipid
levels and have the potential to induce a transient elevation in
serum creatinine levels. The monitoring of renal function and appropriate adjustment of fibrate dosing is crucial in order to
mitigate the potential for renal damage. Proton Pump Inhibitors
(PPI's) are frequently recommended by medical professionals to
decrease the secretion of gastric acid. Nevertheless, these have
been linked to drug-triggered allergic interstitial nephritis, acute
renal failure, chronic renal failure, and end-stage renal disease.
Medical professionals ought to exercise prudence while
recommending PPIs and contemplate substitutive therapies for
patients with documented renal dysfunction.
Metformin is a commonly prescribed medication for diabetes
management that has the potential to accumulate in individuals
with renal dysfunction. In these instances, it is imperative to
modify metformin dosages to mitigate additional renal
impairment. It is recommended that scientists closely monitor
the renal function of individuals who are taking metformin and
explore alternative antidiabetic therapies if deemed necessary.
Aminoglycosides belong to a category of antimicrobial agents
that are recognized for their capability to cause renal toxicity.
Renal toxicity may manifest even following a solitary
administration; however, renal performance typically
recuperates within a period of 20 days post cessation. Prudent
surveillance of renal function and aminoglycoside administration
is imperative to mitigate the possibility of renal impairment.
Fluoroquinolones belong to the class of antibiotics that have the
potential to induce allergic interstitial nephritis, which is a type
III hypersensitivity reaction. The majority of instances are
resolved within a time limit of one week to two months
following cessation. It is advisable for scientists to exercise
prudence while recommending fluoroquinolones and closely
observe renal function. Tetracyclines function as inhibitors of
protein synthesis, and certain types, such as doxycycline, may
have a potential role in mitigating proteinuria among individuals
with diabetic nephropathy. It is imperative to observe the renal
function of individuals undergoing tetracycline treatment and
make necessary dosage modifications. Vancomycin is a
compound that inhibits the synthesis of cell walls and has been
linked to the occurrence of nephrotoxicity. This matter presents
various quandaries for scientists who must consider the
advantages of administering vancomycin therapy versus the
likelihood of renal impairment. The surveillance of vancomycin
levels and renal function is of utmost importance in these
instances [27].
Models to investigate nephro/urotoxicity
In-vivo: The utilization of animal models has been broadly
employed to comprehend pathogenesis and fundamental
mechanisms of renal disease. Mice and rats are frequently
utilized in research to investigate nephrotoxicity and therapeutic
targets, as well as to discover novel biomarkers. This is primarily
due to their ease of breeding and relatively low cost of housing
and maintenance. However, limited progress has been made in
comprehending the mechanisms of nephrotoxicity or in
discovering novel biomarkers or a set of biomarkers. Based on
our investigation, primates have also been utilized in the study.
Nevertheless, irrespective of the dimensions and species of the
creatures, the findings did not exhibit a reliable ability to detect
unfavourable impacts in Homo sapiens [28]. The scientific inquiry
has encompassed models such as ischemia-reperfusion, drug-induced nephrotoxicity (cisplatin, gentamicin, aristolochic acid,
folic acid), glycerol-induced nephrotoxicity, and warfarin-related
nephrotoxicity.
The ischemia-reperfusion model is a widely used experimental
techs model is cantered on the proximal tubule and endothelium,
emulating the pathophysiology of acute nephrotoxicity in humans.
The methodology entails the occlusion of the renal artery for a
period of 30-45 minutes, succeeded by a reperfusion duration of
24-48 hours. The primary benefit of this model lies in its ability to
mimic the pathology akin to the human ailment, rendering it a
valuable tool for investigating the fundamental mechanisms and
potential therapeutic targets. Nevertheless, a noteworthy
constraint is the necessity for surgical intervention, which may
give rise to inconsistencies and intricacies [29].
Models of nephrotoxicity induced by drugs
Mechanism of nephrotoxicity induced by drugs has been
tabulated in Table 3.
| Model |
Inducing agent/method |
Animal |
Key features |
| Cisplatin |
Cisplatin |
Rodents |
Proximal tubule-specific; mimics human pathology and drug doses |
| Gentamicin |
Gentamicin |
Rodents |
Affects proximal tubule and glomerulus; induces chronic kidney disease |
| Aristolochic acid |
Aristolochic acid |
Rodents |
Proximal tubule-specific; induces rapidly progressing chronic kidney disease |
| Folate |
Folic acid |
Rodents |
Proximal tubule-specific; mimics acute nephrotoxicity induced by elevated folate intake |
| Glycerol-induced nephrotoxicity |
Glycerol |
Rodents |
Proximal tubule-specific; mimics acute nephrotoxicity associated with rhabdomyolysis |
| Warfarin-induced nephrotoxicity |
Warfarin |
Rodents |
Proximal tubule and glomerulus-specific; mimics sudden nephrotoxicity induced by anticoagulant agents |
| 5/6 Nephrectomy |
Surgical procedure |
Rodents |
Induces chronic kidney disease; resembles human condition |
| Streptozotocin-induced diabetic nephropathy |
Streptozotocin |
Rodents |
Replicates diabetic nephropathy; various genetically modified models available |
| Hypertensive nephropathy |
Uninephrectomy, Ang II |
Rodents |
Mimics hypertensive nephropathy; angiotensin II infusion model also available |
| Adriamycin or puromycin-induced glomerular injury |
Adriamycin, Puromycin |
Rodents |
Replicates acquired glomerular injury; valuable for studying focal segmental glomerulosclerosis |
| IgA nephropathy |
IgA deposits |
Rodents |
Models available but with limitations in replicating human disease progression |
| Polycystic Kidney Disease (PKD) |
Genetically modified |
Rodents |
Various genetically modified models available; mimic certain aspects of PKD |
| Chronic tubulointerstitial nephritis |
Dose-dependent |
Rodents |
Model for chronic tubulointerstitial nephritis; replicates CKD-associated changes |
| Inherited glomerular injury |
Genetically modified |
Rodents |
Models available for studying hereditary glomerular diseases |
| Calcium oxalate stone formation |
Naturally occurring |
Canines, felines |
Utilizes naturally occurring animal models; pathophysiology not fully understood |
Table 3: Inducing models with targets.
Cisplatin: Cisplatin, a chemotherapeutic agent that is
extensively used and has been found to cause nephrotoxicity.
This experimental design specifically focuses on the proximal
tubule and entails administering a solitary Intraperitoneal (IP)
injection of cisplatin at a dosage ranging from 6-20 mg/kg,
succeeded by a 72-hour monitoring phase. The model exhibits
advantageous features such as similarity to the pathology,
timing, and drug doses observed in human diseases.
Nevertheless, there is no immediate clinical correlation as the
observed nephrotoxicity is a result of drug-induced factors
rather than an inherent renal process [30].
Gentamicin: The aminoglycoside antibiotic known as
Gentamicin has been observed to induce nephrotoxicity by
affecting both the proximal tubule and glomerulus. This
experimental design entails administering gentamicin through
intraperitoneal injections in a sequential manner, with varying
dosages of 40-200 mg per kg per day over a period of 4-10 days.
The primary benefit of this model is its ability to induce Chronic
Kidney Disease (CKD) at a rapid pace. However, it does not
completely replicate clinical diseases observed in humans,
thereby restricting its utility in investigating human
nephrotoxicity [31].
Aristolochic acid: The naturally occurring compound
Aristolochic acid, which is present in specific plants, has been
associated with nephrotoxicity. This experimental design
specifically focuses on the proximal tubule and entails
administering aristolochic acid via intraperitoneal injections at a
dosage of 5 mg per kg per day over the course of five
consecutive days. Analogous to the gentamicin model, this
particular model has the potential to elicit swiftly advancing
chronic kidney disease. Nevertheless, it exhibits a deficiency in
clinical correlation, thereby constraining its pertinence to human
pathology.
Folate: At elevated levels of folate intake, acute nephrotoxicity
may occur. This experimental design specifically focuses on the
proximal tubule and entails administering a solitary
intraperitoneal injection of folic acid at a concentration of 250
mg/kg, followed by a monitoring period of 24-48 hours. The
benefit of this model lies in the resemblance of the pathology
findings to acute nephrotoxicity in humans induced by other
factors. Nevertheless, it lacks a straightforward clinical
correlation, since the observed nephrotoxicity is a result of the
elevated dosage of folic acid rather than an inherent renal
mechanism [32].
The glycerol-induced nephrotoxicity model is designed to
target the proximal tubule. It requires a single I.M injection of 8
mL/kg of 50% glycerol, followed by a 24–48 hours observation
period. The principal benefit of this model lies in its clinical
significance, as it bears resemblance to acute nephrotoxicity
associated with rhabdomyolysis in humans. Nevertheless, this
model exhibits certain constraints. The research emphasizes the
manifestation of symptoms and the general process of
nephrotoxicity, rather than delving into the specific underlying
mechanisms. Furthermore, it is noteworthy that the extent of
nephrotoxicity induced by glycerol may exhibit variations from
the human condition, thereby affecting the translational
potential of the results [28].
The proposed model aims to study the nephrotoxic effects of
warfarin. The model specifically focuses on the proximal tubule
and glomerulus. It involves performing a 5/6 nephrectomy
procedure and administering warfarin for eight consecutive
days. This particular model proves to be beneficial in the
examination of sudden nephrotoxicity instigated by
anticoagulant agents such as warfarin. Nevertheless, it cannot
be ensured that the observed nephrotoxicity will adhere to the
identical mechanism as human cases, and the surgical procedure
of 5/6 nephrectomy brings in supplementary variables and
complexities [33].
It is imperative to acknowledge that a solitary model cannot
encompass all facets of acute nephrotoxicity or chronic kidney
disease in humans. Rather than relying on a single model or
approach, it is advisable for scientists to utilize multiple models
or complementary methods to corroborate their discoveries and
guarantee the applicability of their outcomes to human subjects.
Chronic Kidney Disease (CKD) is a situation characterized by a
gradual and permanent decline in kidney function. Various
animal models have been established to investigate the
progression from Acute Kidney Injury (AKI) to Chronic Kidney
Disease (CKD), such as the 5/6 nephrectomy model in rodents.
This experimental design entails the excision of 66.67% of the
renal tissue, resulting in a decline in renal capacity and the
emergence of Chronic Kidney Disease (CKD) at the right time.
This model is deemed analogous to the human condition;
however, it has certain constraints such as the elevated mortality
rate of the second surgery, particularly in mice, and necessitates
technical proficiency. In addition, it has been observed that
various strains of mice exhibit varying degrees of responsiveness
to renal mass reduction in terms of chronic kidney disease
progression. Moreover, the quantity of kidney tissue available
for analysis following the surgical procedure is limited and may
be subject to distortion, as per the findings of Ortiz, et al. [27].
Diabetic nephropathy is a type of kidney disease that occurs
in people with diabetes. It is caused by damage to the small
blood vessels in the kidneys, which can lead to kidney failure if
left untreated. Symptoms of diabetic nephropathy include
protein in the urine, high blood pressure, and swelling in the legs
and feet. Treatment options include controlling blood sugar
levels, managing blood pressure, and taking medications to
protect the kidneys.
Diabetes-induced kidney disease, well known as diabetic
nephropathy, a prevalent complication of diabetes and a primary
contributor to Chronic Kidney Disease (CKD) on a global scale.
Numerous animal models have been established for the
investigation of diabetic nephropathy, such as streptozotocin-
induced diabetic rats and mice, NOD mice, BB-DP rats, ob/ob
mice, db/db mice, STZ-eNOS-/-mice, and db/db-eNOS/mice.
These genetically modified models are readily available for
commercial use, and they offer a valuable tool for investigating
the underlying mechanisms of diabetic nephropathy. However,
none of the numerous animal models flawlessly imitate the
human ailment. Furthermore, certain strains exhibit infertility,
and the models induce slight albuminuria without any reduction
in GFR, as reported by Kitada, et al. [34].
Hypertensive nephropathy is a renal disorder that arises as a
result of chronic high blood pressure. Hypertensive nephropathy
is a prevalent etiology of Chronic Kidney Disease (CKD). Various
animal models have been established to investigate
hypertensive nephropathy, such as the model involving
uninephrectomy in Spontaneously Hypertensive Rats (SHR) and
models involving infusion of angiotensin II. These models exhibit
high relevance to hypertensive nephropathy and serve as
valuable tools for investigating the impact of angiotensin II on
renal function. The SHR rodents exhibit higher resistance
towards streptozotocin-induced diabetes. Additionally, inducing
significant kidney injury necessitates uninephrectomy.
Moreover, there is no gradual decline in glomerular filtration
rate, and elevated dosages or extended durations of exposure
result in heightened serum creatinine levels. These models can
be costly and have the potential to induce prolonged stress in
animals, as noted by Ruiz-Ortega, et al. [35].
Acquired glomerular injury is a pathological condition
affecting the glomeruli, which are the tiny filters in the kidneys
responsible for removing waste products from the blood. The
etiology of Chronic Kidney Disease (CKD) often involves acquired
glomerular injury, which encompasses Focal Segmental Glomerulosclerosis (FSGS) as a frequent pathology. The
Adriamycin or puromycin models are frequently employed in
scientific research to investigate acquired glomerular injury,
particularly in rats and mice. These models replicate acute
glomerular injury and are valuable for investigating the
mechanisms of FSGS. Typically, these models exhibit insufficient
replication of the gradual advancement of the human ailment,
and there exists no clinical application of efficacious NOD.
IgA nephropathy is a renal disorder characterized by the
deposition of Immunoglobulin A (IgA) in the glomerular
mycangium, leading to inflammation and damage to the kidneys.
IgA nephropathy is the prevailing primary glomerulonephritis
globally. Various animal models have been established for the
investigation of IgA nephropathy, such as the ddY mouse, HIGA
mice, and Uteroblobin-deficient mice. The models utilized
successfully replicate human pathology; however, the
progression of the disease is mild and does not advance towards
end-stage renal disease, nor does it result in a gradual decline of
the glomerular filtration rate. Furthermore, the efficacious
models presented by Ortiz, et al., have yet to be translated into
clinical practice [27].
Polycystic Kidney Disease (PKD) is a genetic disorder
characterized by the formation of multiple cysts in the kidneys.
Polycystic Kidney Disease (PKD) is an inherited condition that
manifests as the formation of numerous cysts filled with fluid in
the kidney. The utilization of genetically modified mouse models
is a prevalent approach in the investigation of PKD. These
models serve as valuable tools for investigating the underlying
mechanisms of PKD and identifying potential therapeutic
targets. The outcomes have also led to the attainment of
regulatory authorization for Tolvaptan in Japan concerning
autosomal dominant polycystic kidney disease in humans.
However, these murine models typically exhibit a limited range
of human phenotypes, and ARPKD murine models often exhibit
a limited range of human phenotypes. Furthermore, the
exorbitant expense poses a constraint as per the findings of
Ortiz, et al. [27].
Chronic tubulointerstitial nephritis is a medical condition
characterized by inflammation and damage to the tubules and
interstitial tissue of the kidneys. Chronic tubulointerstitial
nephritis is a prevalent etiology of chronic kidney disease. The
utilization of a dose-dependent reduction in glomerular filtration
rate serves as a common model for chronic tubulointerstitial
nephritis in scientific research. The model exhibits potential
reversibility in rats and effectively replicates extrarenal
complications, function, and molecular changes associated with
CKD. Nevertheless, male rats with adenine-induced kidney
disease exhibited a more pronounced deterioration, and the
animal model lacks human specificity [36].
Inherited glomerular injury encompasses a range of
conditions such as PKD, Alport syndrome, and other hereditary
glomerular diseases. Various genetically modified mouse models
are frequently utilized to investigate hereditary glomerular
damage. These models include the Pkd1 or Pkd2 gene-engineered mouse model, Col4a43 gene knockout mouse, and
Alport syndrome mouse model. These models successfully
replicate characteristics of the human ailment, such as gradual
decline in glomerular filtration rate, presence of protein in urine,
and eventual renal malfunction. Nonetheless, the translation of
genetic discoveries into enhanced patient outcomes poses a
challenge, and the exorbitant expenses involved serve as a
constraint. These models have led to clinical recommendations.
In addition to the species, canines and felines have also been
proposed as potential subjects for investigating calcium oxalate
stone formation, given the potential advantages of utilizing a
naturally occurring animal model. Although this approach may
serve as a promising model, the pathophysiological and
etiological mechanisms underlying calcium oxalate
nephrolithiasis remain incomplete, as per the findings of O’Kell,
et al. [37].
The primary focus of these models should be on molecular
mechanisms. This is because comprehending these mechanisms
can aid in the development of therapeutic interventions and
clinical diagnoses, and also facilitate the identification of novel
biomarkers for nephrotoxicity. Therefore, it is also our scientific
belief that novel approaches are crucial for progress in this field.
Furthermore, alternative methodologies, as discussed in this
analysis, are significant instruments that can alter the
nephropathy paradigm and revolutionize the process of
designing novel, potent, and less hazardous compounds.
In-vitro models
Our search results indicate that the quantity of in vivo investigations has increased commensurately with that of in
vitro studies. However, these models have exhibited constraints
in producing insights into nephrotoxic mechanisms in human
beings. In contrast, there is a growing need for novel techniques
that enhance, minimize, and substitute the utilization of
animals. The utilization of cell culture methodologies is highly
pertinent in conducting in vitro investigations on nephrotoxicity.
The quantity of investigations utilizing animal models and in
vitro assessments to evaluate renal toxicity has increased over
the past two decades. Despite the heightened employment of
both methodologies, no documented findings have yielded a
more comprehensive comprehension of the mechanisms
underlying nephrotoxicity.
Through the creation of human induced Pluripotent Stem
Cells (hiPSCs) derived from individuals afflicted with a specific
genetic ailment, such as degenerative conditions and
malignancies, it is conceivable to investigate personalized
disease mechanisms and conduct drug screening in vitro,
obviating the need for animal models. Significantly, recent
developments in genome editing have presented novel
methodologies for simulating genetic kidney disorders through
the utilization of human Pluripotent Stem Cells (hPSCs) in vitro,
as reported by Dakhore, et al. [38].
The screening of nephrotoxicity in vitro models typically
involves the utilization of primary human renal proximal tubule
cells, which are commonly obtained from cadaveric specimens
and cultured in a two-dimensional format. Nevertheless,
primary cells exhibit substantial interdonor variability, possess
restricted expansion potential, and are susceptible to
dedifferentiation and transporter expression loss [39].
The challenge of preserving transporter and metabolic activity
in primary cells and cell lines, as well as achieving transporter
function levels that match those observed in vivo, is due to the
utilization of static 2D culture configurations. The in vitro assays
for renal transport or toxicity typically involve the utilization of
primary proximal tubule cells or cell lines that are cultured in a
monolayer on a permeable support system, such as a Transwell
insert. This system is coated with an extracellular matrix, as
described by Wilmer, et al. [40].
Renal cells derived from pluripotent stem cells and
reprogrammed cells have garnered significant attention due to
their capacity to differentiate into fully functional renal cells.
These pluripotent cells possess the ability to differentiate into
any cell lineage within the human anatomy and can be
conveniently amplified [41].
By utilizing a combination of growth factors and small
molecules that simulate the natural progression of embryonic
kidney development, renal cells have been successfully
generated in vitro from both embryonic stem cells and human
induced Pluripotent Stem Cells (hiPSCs) [42].
The cell lines utilized in this study were obtained from various
origins including human foreskin, human dermal fibroblasts,
human pluripotent stem cells, and human female fibroblasts.
The cell lines were differentiated using various inducing factors
such as activin A, BMP7, CHIR, FGF2, RA, and FGF9.
The cellular lineage originates from human foreskin and
human dermal fibroblasts (α), and the differentiation
methodology employed is IMa, which incorporates CHIR, FGF2,
and RA. The cellular mechanism in response to nephrotoxicity
for this particular cell line remains undescribed as per the
findings of Lam, et al. [40].
Likewise, the cellular lineage obtained from a human
pluripotent stem cell. The differentiation protocol is IMa, which
employs activin A, BMP7, and CHIR. The response of this cell line
to nephrotoxicity is characterized as AQP1c and LTLc, as
reported by Mae, et al. [43]. The cell line specified has been
obtained from human pluripotent stem cells. The differentiation
process employed includes the utilization of BMP4 and bFGF in
the IM protocol, and RA, activin A, and BMP2 in the UB protocol,
as documented by Xia, et al. [44]. The human dermal fibroblasts
utilized in the experiment were obtained from the RIKEN
bioresource centre. The differentiation process employed was
IM, which necessitated the application of activin A, BMP4, CHIR,
RA, and bFGF. The cellular response to nephrotoxicity is
explicated as being modulated by SALL1 and Cadherin 6,
according to Taguchi, et al's findings [45].
The cell line mentioned is obtained from human BJ foreskin
fibroblasts, human HDFα dermal fibroblasts, human LR5-iPSCs,
and human fibroblast hfib2-iPS4 and hfib2-iPS5 iPSCs. The
employed differentiation protocol is IMa, which entails the
utilization of CHIR, and MM and nephrogenesis, which
necessitate the use of B27 supplement. The cellular response to
nephrotoxicity is elucidated by the organoids that exhibit KIM1
expression upon exposure to cisplatin and gentamycin
treatment, along with Megalin and Cubilin, as reported by
Freedman, et al. [46].
The human female fibroblasts were utilized to derive the cell
line, and the differentiation process employed the IM protocol.
This protocol entails the utilization of CHIR, FGF9, and heparin.
Additionally, the MM and nephrogenesis stages were
incorporated, which involved using CHIR pulse and FGF9 for the
initial five days, followed by FGF9 for the subsequent 12-25 days.
The cellular response to nephrotoxicity involves the activation of
caspase 3 in LTL+ECAD+PTECs within organoids following
exposure to cisplatin and Cubilin, as reported by Takasato, et al.
[47].
The human foreskin fibroblasts utilized in this study were
obtained from the WiCell Research Institute. The differentiation
process involved the utilization of BMP2, BMP7, and B27
supplement, and the IMa and MM protocols were employed to
generate HPTC-like cells. The cellular response to nephrotoxicity
is characterized by the expression of NF-κB, γH2AX, and 4-HNE in
2D cells upon exposure to cisplatin and aristolochic acid.
Additionally, the cell line exhibits the presence of various
transporters including GLUT1c, SGLTc, AQP1c, OAT3c, PEPT1c,
and ATPasec [48].
The human dermal fibroblasts utilized in this study were
obtained from Invitrogen C-013-5C. The differentiation process
employed the IM protocol, which entails the administration of
CHIR, Noggin, and activin A. Additionally, the MM and
nephrogenesis stages involved the application of FGF9 and CHIR
for the initial 14 days, followed by a growth factor-free period
for the subsequent 14 days. The cellular response to
nephrotoxicity was observed in organoids that expressed KIM1
upon treatment with cisplatin and gentamycin, and γH2AX
expression was observed upon treatment with cisplatin, as
reported by Morizane, et al. [49].
Organoids
The nephrons exhibit intricate three-dimensional architecture.
Therefore, in summary, in order to reconstruct these anatomical
features outside of the organism, it is necessary to establish
three-dimensional cultivation methodologies. Organoids are
three-dimensional tissue structures that closely resemble the
structural and functional characteristics of in vivo organs when
cultured in laboratory plates. Organoids derived from human
Pluripotent Stem Cells (hPSCs) present a promising avenue for
investigating the underlying mechanisms of inherited kidney
disorders in humans. This approach could potentially be
extended to more prevalent diseases and aid in the
development of novel drug therapies utilizing human tissue.
Such methodology may enhance the translatability of research
findings to human subjects. To optimize this methodology, it is
imperative to thoroughly consider differentiation protocols,
genetic background, and epigenetic variation when examining
disease phenotypes in kidney organoids.
The objective of human Pluripotent Stem Cell (hPSC) research
is to restore renal function. The kidneys are intricate organs that
facilitate blood filtration, and the unit responsible for re-absorbing urine is crucial for maintaining their function and
overall balance within the body. The utilization of organoids to
produce operational bioengineered kidney tissues poses several
challenges. The induction of vascularization in kidney organoids must be conducted in a systematic manner to ensure proper
arterial blood flow and venous drainage. This phenomenon poses
as one of the significant obstacles concerning vascularization, as
stated by Morizane, et al. [50].
Kidney-on-a-chip
Renal cells are seeded in a microfluidic device that enables
the flow of media across the cell surface and/or cell surfaces,
thereby creating kidneys-on-a-chip. In a pioneering investigation
of this technology, primary human proximal tubule cells were
cultivated on 2D chips featuring apical and basolateral media
compartments. The study revealed that the flow of fluid across
the apical surface triggered MDR1 expression and function and
led to the development of tight junctions and columnar
morphology [51].
Biofunctionalized hollow fibers
In 2015, a study was conducted which demonstrated that
proximal tubule epithelial cells with conditional immortality
could be utilized to seed the exterior surface of hollow fibres
coated with an extracellular matrix. This resulted in the creation
of biofunctionalized hollow fibres that possessed a monolayer of
cells. The fibres on which the proximal tubule cells were grown
exhibited enhanced formation of tight junctions and OAT1
expression in comparison to the cells cultured in 2D, even in the
absence of flow through the fibres. This indicates that the 3D
culture alone led to an improvement in the expression of these
markers, as per the findings of Jansen, et al. [52].
Herbs as neuroprotective
Renal disorders can be tackled at various stages based on the
physiological pathway of the underlying etiology.
Pharmacological interventions are available for both pre-renal
and post-renal diseases. However, it is noteworthy that most of
the drugs utilized in the treatment of these conditions are
associated with unfavourable outcomes and may even result in
intrinsic renal impairment. Included in the list are Non-Steroidal
Anti-Inflammatory Agents (NSAIDs), proton pump inhibitors,
antibiotics, as well as chemotherapeutic agents. The potential
nephrotoxicity of drugs used to treat pre-renal and post-renal
diseases, as well as other medical conditions, is now recognized
as a significant risk factor for both acute and chronic kidney
disorders has been tabulated in Table 4. To mitigate the negative
impacts of medication, various alternatives have been explored
for the treatment of these pathologies, as noted by Petejova, et
al. [53].
| S. no |
Plant (part used-family) |
Type of extract |
Dose |
Mechanism |
References |
| 1 |
Descurainia sophia (seed-Brassicaceae) |
Ethyl alcohol extract |
50, 100, 200, and 300 mg/kg |
Reduce inflammation, swelling and necrosis |
Moshaie-Nezhad P, et al. |
| 2 |
Eurycoma longifolia (root-Simaroubaceae) |
Standardized aqueous extract |
100, 200, and 400 mg/kg |
Improves biomarkers of kidney function, and histopathology changes |
Chinnappan SM, et al. |
| 3 |
Theobroma cacao (fruit-Malvaceae) |
Hydroalcoholic extract of natural Forastero cocoa |
10% of diet |
Decrease glucose levels |
Alvarez-Cilleros, et al. |
| 4 |
Caffea arabica (fruit-Rubiaceae) |
Aqueous extract |
1000 mg/kg |
Raise catalase levels |
Boonphang O, et al. |
| 5 |
Eysenhardtia polystachya (bark-Fabaceae) |
Methanolic extract (flavonoids) |
20 mg/kg |
Decrease oxidative stress |
Perez-Gutierrez RM, et al. |
| 6 |
Anchomanes difformis (leaf-Araceae) |
Aqueous extract |
200 mg and 400 mg/kg |
Induce dissociation of Nrf2/keap, activating Nrf2, reduced oxidative stress |
Alabi TD, et al. |
| 7 |
Hibiscus sabdariffa (flower-Malvaceae) |
Aqueous extract |
2% in drinking water |
Increase the antioxidant systems including non-enzymatic and enzymatic effect |
Rodriguez-Fierros FL, et al. |
| 8 |
Passiflora spp. (peel-Passifloraceae) |
Methanolic extract |
250, 500 mg/kg |
Keep urea and creatinine at normal levels |
Nerdy N, et al. |
| 9 |
Euphorbia paralias (Aerial parts-Euphorbiaceae) |
Methanolic extract |
100, 200 mg/kg |
Reduce levels of urea and creatinine |
Al-Yousef HM, et al. |
| 10 |
Pistacia atlantica (leaf - Anacardiaceae) |
Hydroethanolic extract |
200, 400, and 800 mg/kg |
Decrease levels of urea, creatinine, and uric acid |
Heidarian E, et al. |
| 11 |
Costusafer (leaf- Costaceae) |
Aqueous leaf extract |
375, 750 and 1125 mg/kg |
Decrease serum potassium and BUN levels |
Ezejiofor AN, et al. |
Table 4: Herbs and extracts used to treat nephron/urotoxicity.
Discussion
Throughout history, plants have been utilized as remedies for
a multitude of illnesses, including those related to pre, intra, and
post-renal factors. The secondary metabolites of plants are
responsible for their medicinal properties, as they can provide
protection against pathogens and offer significant physiological
advantages that aid in the prevention of certain illnesses [54].
Moreover, botanical nephroprotective agents alleviate
phenomena such as interstitial nephritis, modified intra-glomerular hemodynamic, tubular necrosis, or glomerulonephritis
[55]. Prior research has extensively examined the utilization of
plants and phytochemicals as nephroprotective agents, yielding
valuable insights into the mechanisms by which extracts or
individual compounds modulate molecular pathways to
ameliorate kidney ailments [56].
The limited utilization of medicinal plants worldwide,
particularly in countries where modern medicines are prevalent,
can be attributed to the insufficient comprehension of their
mechanism of actions. The utilization of botanicals as a
fundamental element of harmonizing and alternative medicine
has garnered renewed concentration. As a noteworthy
achievement, the conventional Chinese medicine, which
primarily relies on botanical sources, has gained worldwide
recognition, and is extensively employed across all tiers of the
healthcare system in China. The administration and academic
establishments have already put in place protocols for endorsing
and utilizing plant-based remedies. Likewise, Ayurveda and
other traditional medicines of India have demonstrated their
efficacy in treating various ailments and hold significant value in
the Indian healthcare industry's economy [57].
Combined approach to treat urotoxicity
In the field of renal pathophysiology, it has been
demonstrated by Moorthi, et al. [58]. That a plant based diet can
be advantageous, particularly for patients with mild proteinuria
and diabetic nephropathy. Similarly, herbal formulations have
been found to decrease the need for dialysis by addressing the
underlying causes and modifying symptoms of renal failure.
Additionally, they can aid in alleviating the adverse effects of
dialysis [59]. Hence, the contemporary methodology of utilizing
flora as an adjunctive therapy confers a dual advantage; they aid
in combating the ailment and simultaneously function as agents
that protect the kidneys. The synergistic integration of Western
and Indigenous medicinal practices presents novel inquiries
regarding the therapeutic applications of botanicals and natural
substances. The efficacy of Indian and Chinese medicines is
widely acknowledged, as they have been found to have fewer
adverse effects when compared to contemporary medical
systems. Moreover, the utilization of plants in disease
management is being advocated as a viable and economical
substitute [60].
The Table 5 presents an analysis of the nephroprotective
properties of diverse botanical extracts, with a particular
emphasis on their potential to safeguard and enhance renal
function. The observed nephroprotective effects of these
extracts can be ascribed to their anti-oxidative, anti-inflammatory, and Reno protective mechanisms. The
subsequent sections will offer a comprehensive examination of
every plant extract, specifying the plant component employed
the extract type, the dosage, and the mechanism by which it
applies its nephroprotective properties.
| S. no |
Plant |
Mechanism |
Bioactive |
Model of study |
References |
| 1 |
Tinospora crispa |
Increase SOD activity |
Genkwanin |
Albino Wistar rats+CCl4 |
Rakib A, et al. |
| 2 |
Suaeda vermiculata |
Decrease AST and ALT |
Quercetin, quercetin-3-O-rutinoside |
Male Sprague Dawley rats+ CCl4 |
Mohammed SA, et al. |
| 3 |
Arbutus pavarii |
Exert bacteriostatic and bactericidal effect, against different methicillin-resistant Staphylococcus aureus strains |
Proanthocyanins, quercetin |
Disc diffusion assay |
Buzgaia N, et al. |
| 4 |
Punica granatum |
Improve kidney function biomarkers, exerted antioxidant activity |
Dulcitol, loganin, bergenin, quercitrin, cosmosin, folic acid, khayanthone |
Wistar rats+gentamicin |
Mestry SN, et al. |
| |
| 5 |
Cichorium intybus |
Improve the systolic function and increase the levels of LVEF and LVFS |
1,4-naphthalenedione, oleic acid, β-asarone, naphtho furanone |
Wistar albino rats+ISO-induced myocardial ischemia model |
Epure A, et al. |
| 6 |
Gardenia jasminoides |
Exert antioxidant activity and decreased CK-MB, AST, ALT, and MDA levels. |
Geniposide |
Wistar SHR, and Wistar Kyoto rats |
Hou Y, et al. |
| 7 |
Curcuma longa |
Reduce ROS, inflammation, and histopathology changes |
Curcumin |
C57BL/6J mice |
Guerrero-Hue M, et al. |
| Increase enzymatic antioxidant activity |
Wistar rats+doxorubicin |
Benzer F, et al. |
| 8 |
Passiflora spp. |
Keep urea and creatinine at normal levels |
Ellagic acid, Kaempferol and Quercetin glycosides |
Albino rats+paracetamol |
Alabi TD, et al. |
| 9 |
Cynanchum wilfordii |
via down regulation of androgen receptor 5α gene expression |
4-hydroxyacetophenone |
Male Sprague-Dawley rats+testosterone |
Lee G, et al. |
Table 5: Herbal isolated molecules used to treat nephro/urotoxicity.
The seeds of Descurainia sophia exhibit anti-inflammatory,
anti-swelling, and anti-necrotic properties. The administration of
ethyl alcohol extract at varying doses of 50, 100, 200, and 300
mg/kg has exhibited properties that protect the kidneys. The
anti-inflammatory and anti-necrotic properties of this extract
play a role in maintaining renal function, mitigating the
likelihood of complications associated with the kidneys.
Eurycoma longifolia is a plant species belonging to the family
Simaroubaceae. It is commonly known as Tongkat Ali and is
native to Southeast Asia. The plant has been traditionally used
for its medicinal properties, particularly as an aphrodisiac and to
treat various ailments such as fever, malaria, and high blood
pressure. Scientific studies have also shown that Eurycoma
longifolia contains compounds that may have potential benefits
for improving athletic performance, reducing stress, and
enhancing male fertility. Further research is needed to fully
understand the mechanisms of action and potential therapeutic
applications of this plant. standardized aqueous extract of the
root.
Eurycoma longifolia, an indigenous plant to Southeast Asia,
possesses a prolonged chronicle of utilization in customary
medicine. The standardized aqueous extract of the root has
demonstrated a significant improvement in kidney function
biomarkers and a reduction in histopathological alterations in the kidney at doses of 100, 200, and 400 mg/kg. The observed
nephroprotective effects may be ascribed to the extract's
capacity to augment renal function and regenerate impaired
renal tissue.
The hydroalcoholic extract obtained from the fruit of
Theobroma cacao, commonly known as natural Forastero cocoa,
has exhibited hypoglycemic effects upon being incorporated into
the diet at a concentration of 10%. The decrease in glucose
levels can aid in preserving renal function by hindering the onset
of diabetic nephropathy, a prevalent cause of renal failure
among diabetic patients.
The aqueous extract of Coffea arabica, a well-known beverage
commonly referred to as coffee, has been observed to elevate
catalase levels at a dosage of 1000 mg/kg. Catalase is an enzyme
with antioxidant properties that counteracts the deleterious
effects of reactive oxygen species. These species are known to
induce oxidative stress and inflict harm upon renal cells.
Through upregulating catalase expression, the aforementioned
extract confers nephroprotection by mitigating renal oxidative
stress.
The bark methanolic extract of Eysenhardtia polystachya, a
plant indigenous to Mexico, is abundant in flavonoids. This
particular extract, when administered at a dosage of 20 mg/kg,
has demonstrated a reduction in oxidative stress within the
renal system. Through the reduction of oxidative stress, the
extract facilitates the preservation of renal cells and sustains the
general renal function.
The aqueous leaf extract of Anchomanes difformis, a plant
species found in tropical regions, has been observed to trigger
the dissociation of Nrf2/keap, leading to the activation of Nrf2
and a subsequent decrease in oxidative stress. This effect has
been observed at doses of 200 mg and 400 mg/kg. The
activation of Nrf2, a transcription factor responsible for
regulating the expression of antioxidant enzymes, plays a crucial
role in bolstering the kidneys' defence against oxidative stress,
safeguarding kidney function.
The aqueous extract of Hibiscus sabdariffa, commonly
referred to as roselle, has been found to enhance non-enzymatic
and enzymatic antioxidant systems. This effect was observed
when the extract was administered at a concentration of 2% in
drinking water. The improved antioxidant mechanisms aid in
shielding the kidneys against oxidative stress-induced harm,
thereby conserving renal function.
The methanolic extract obtained from the aerial parts of Euphorbia paralias, a plant species found in coastal areas, has
demonstrated the ability to decrease urea and creatinine levels
in subjects administered with doses of 100 and 200 mg/kg. The
observed decrease in waste products suggests an enhancement
in renal performance, highlighting the potential
nephroprotective properties of the aforementioned botanical
extract.
The hydroethanolic extract of Pistacia atlantica leaves has
been found to exhibit potential in reducing levels of urea,
creatinine, and uric acid. The tree is indigenous to the
Mediterranean and Middle East regions. The administration of
doses of 200, 400, and 800 mg/kg has been observed to be
effective. The observed decrease in waste products indicates a
positive impact on renal function, underscoring the
nephroprotective properties of the aforementioned extract.
The aqueous leaf extract of Costus afer, an indigenous African
medicinal plant, has been observed to exhibit hypokalaemia and
hypoureaemic effects at varying doses of 375, 750, and 1125
mg/kg. A reduction in serum potassium and BUN levels is
indicative of enhanced renal function, as the kidneys exhibit
greater proficiency in eliminating these metabolic by-products
from the bloodstream.
The observed effects of Tinospora crispa indicate that it
enhances the activity of Superoxide Dismutase (SOD), thereby
exhibiting nephroprotective properties. The bioactivity observed
can be attributed to the presence of genkwanin, a compound
that is naturally occurring in the plant. The study investigated
the nephroprotective properties of Tinospora crispa in albino
Wistar rats that were exposed to Carbon Tetrachloride (CCl4).
Suaedavermiculata demonstrates nephroprotective
characteristics through the reduction of AST and ALT levels. The
bioactive compounds accountable for this phenomenon
comprise of quercetin and quercetin-3-O-rutinoside. The
observed nephroprotective activity was noted in male Sprague
Dawley rats that were subjected to CCl4 treatment.
While not possessing direct nephroprotective properties,
Arbutus pavarii exhibits bactericidal and bacteriostatic effects
against various strains of Methicillin-Resistant Staphylococcus
aureus (MRSA), which may have the potential to safeguard the
kidneys against damage caused by infections. The effect can be
attributed to the bioactive compounds namely
proanthocyanidins and quercetin. The investigation of Arbutus
pavarii's activity was conducted through the utilization of a disc
diffusion assay.
Punica granatum is a deciduous shrub or small tree belonging
to the family Lythraceae. It is widely cultivated for its edible
fruit, which is commonly known as pomegranate. The fruit is a
complex structure consisting of numerous seeds surrounded by
juicy arils, enclosed in a tough, leathery skin. Pomegranate has
been used for medicinal purposes for centuries and is believed
to have various health benefits due to its high content of
antioxidants and other bioactive compounds. Its phytochemical
composition and biological activities have been extensively
studied, and it is considered a promising source of natural
products for the development of new drugs and functional
foods. Punica granatum, scientifically referred to as such,
exhibits a positive impact on biomarkers associated with kidney
function and demonstrates antioxidant properties. The bioactive
constituents of the substance comprise dulcitol, loganin,
bergenin, quercetin, cosmosin, folic acid, and khayanthone. The
study investigated the potential nephroprotective properties of Punica granatum in Wistar rats that were administered with
gentamicin, a known nephrotoxic antibiotic.
Cichorium intybus, scientifically referred to as chicory,
enhances systolic function and elevates the levels of Left
Ventricular Ejection Fraction (LVEF) and Left Ventricular
Fractional Shortening (LVFS). The effect observed can be attributed to the bioactive compounds namely 1,4-naphthalenedione, oleic acid, β-asarone, and naphtho furanone.
The study investigated the effects of Cichorium intybus on
myocardial ischemia in Wistar albino rats induced by
Isoproterenol (ISO).
The antioxidant activity of Gardenia jasminoides is evidenced
by its ability to reduce the levels of Creatine Kinase-MB (CK-MB),
AST, ALT, and Malondialdehyde (MDA). The effects observed are
attributed to geniposide, a bioactive constituent present in the
plant. The study investigated the nephroprotective potential of Gardenia jasminoides in Wistar Spontaneously Hypertensive
Rats (SHR) and Wistar Kyoto rats.
Curcuma longa is a perennial plant belonging to the ginger
family, Zingiberaceae. It is commonly known as turmeric and is
widely used as a spice in many cuisines. Its active ingredient,
curcumin, has been extensively studied for its potential health
benefits. Turmeric, scientifically referred to as Curcuma longa,
has been found to possess the ability to decrease the levels of
Reactive Oxygen Species (ROS), inflammation, and
histopathological alterations in the kidneys. The bioactive
compound curcumin is renowned for its robust antioxidant and
anti-inflammatory properties. The study investigated the
nephroprotective properties of Curcuma longa in C57BL/6J
mice.
Passiflora spp., also referred to as passion fruit, aids in the
regulation of urea and creatinine levels, which are crucial
markers of renal function. The effect can be attributed to the
bioactive compounds such as ellagic acid, kaempferol, and
quercetin glycosides. The study investigated the
nephroprotective potential of Passiflora spp. in albino rats
subjected to paracetamol-induced renal toxicity, a well-known
adverse effect of the drug at high dosages. The administration of
250 and 500 mg/kg doses of the extract can aid in this regard.
Urea and creatinine are nitrogenous waste products that
undergo renal filtration, and their concentrations in the
bloodstream are reliable markers of renal performance. By
maintaining these levels within the normal range, the extract
demonstrates nephroprotective characteristics.
The nephroprotective effects of Cynanchum wilfordii are
observed through the downregulation of the androgen receptor
5α gene expression. The bioactive compound that is accountable
for this particular activity is 4-hydroxyacetophenone. The study
investigated the potential nephroprotective properties of Cynanchum wilfordii in male Sprague-Dawley rats subjected to
testosterone-induced renal damage.
Conclusion
In summary, the botanical extracts expounded upon in this
manuscript demonstrate renal safeguarding characteristics via
diverse pathways, encompassing antioxidative, anti-inflammatory, and nephroprotective effects. The bioactive
compounds that exhibit these effects exhibit potential
therapeutic significance in the prevention and management of
renal complications. Nevertheless, it is imperative to carry out
additional investigations to determine the most effective
dosages, safety profiles, and probable interactions with
medications or other supplements. It is imperative to seek
advice from a medical expert prior to integrating these botanical
extracts into an individual's dietary or supplementary routine.
The induction of nephrotoxicity is a major concern in drug
development, environmental exposure, and disease
management. As the scientific community's comprehension of
the molecular pathways involved in nephrotoxicity deepens,
there is an increasing demand for the identification of innovative
pharmacological compounds capable of safeguarding and
revitalizing renal function. The botanical extracts and biologically
active molecules mentioned in this manuscript present a
valuable reservoir of promising nephroprotective agents that
require additional scrutiny. The forthcoming outlooks in this
domain ought to concentrate on thorough investigation of the
mechanisms of action, safety profiles, and ideal dosages for the
botanical extracts and bioactive molecules possessing
nephroprotective characteristics. Moreover, it is imperative to
carry out meticulously planned clinical trials to authenticate the
therapeutic effectiveness of these extracts in human cohorts.
Further investigations should also examine probable interactions
with pharmaceuticals or other nutraceuticals, along with any
potential adverse reactions or contraindications. The integration
of plant extracts and their bioactive compounds into evidence
based, integrative strategies for kidney health holds promise for
enhancing patient outcomes and overall renal health. Sustained
investigation in this domain is imperative for unleashing the
complete potential of these auspicious therapeutic agents and
broadening our array of instruments to counteract renal
disorders and their correlated complications.
References
- Lote CJ (2012) Renal regulation of body fluid pH. Principles of renal physiology. 5th edition, Springer Science & Business Media 121-140
[Google Scholar]
- Stevens LA, Coresh J, Greene T, Levey AS (2006) Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med 354:2473-2483
[Crossref] [Google Scholar] [PubMed]
- Sands JM, Layton HE (2009) The physiology of urinary concentration: An update. Semin Nephrol 29:178-195
[Crossref] [Google Scholar] [PubMed]
- Perazella MA, Izzedine H (2015) New drug toxicities in the onco-nephrology world. Kidney Int 87:909-917
[Crossref] [Google Scholar] [PubMed]
- Naughton CA (2008) Drug-induced nephrotoxicity. Am Fam Physician 78:743-750
[Google Scholar] [PubMed]
- Prasaja Y, Sutandyo N, Andrajati R (2015) Incidence of cisplatin-induced nephrotoxicity and associated factors among cancer patients in Indonesia. Asian Pac J Cancer Prev 16:1117-1122
[Crossref] [Google Scholar] [PubMed]
- Al-Kuraishy HM, Al-Gareeb AI, Hussien NR (2019) Betterment of diclofenac induced nephrotoxicity by pentoxifylline through modulation of inflammatory biomarkers. Asian J Pharm Clin Res 12:433-437
[Google Scholar]
- Vormann MK, Gijzen L, Hutter S, Boot L, Nicolas A, et al. (2018) Nephrotoxicity and kidney transport assessment on 3D perfused proximal tubules. The AAPS J 20:1-11
[Crossref] [Google Scholar] [PubMed]
- Qu Y, An F, Luo Y, Lu Y, Liu T, et al. (2018) A nephron model for study of drug-induced acute kidney injury and assessment of drug-induced nephrotoxicity. Biomater 155:41-53
- Sudjarwo SA, Eraiko K, Sudjarwo GW (2019) The potency of chitosan-Pinus merkusii extract nanoparticle as the antioxidant and anti-caspase 3 on lead acetate-induced nephrotoxicity in rat. J Adv Pharm Res 10:27-32
[Crossref] [Google Scholar] [PubMed]
- Suzuki H, Yoshioka K, Miyano M, Maeda I, Yamagami K, et al. (2009) Tubulointerstitial Nephritis and Uveitis (TINU) syndrome caused by the Chinese herb “Goreisan”. Clin Exp Nephrol 13:73-76
[Crossref] [Google Scholar] [PubMed]
- Frazier KS, Obert LA (2018) Drug-induced glomerulonephritis: The spectre of biotherapeutic and antisense oligonucleotide immune activation in the kidney. Toxicol Pathol 46:904-917
- Pawar AT, Vyawahare NS (2015) Anti-urolithiatic activity of standardized extract of Biophytum sensitivum against zinc disc implantation induced urolithiasis in rats. J Adv Pharm Res 6:176-182
[Crossref] [Google Scholar] [PubMed]
- Brocklebank V, Wood KM, Kavanagh D (2018) Thrombotic microangiopathy and the kidney. Clinc J Am Soc Nephrol 13:300-317
[Crossref] [Google Scholar] [PubMed]
- Elsby R, Chidlaw S, Outteridge S, Pickering S, Radcliffe A, et al. (2017 ) Mechanistic in vitro studies confirm that inhibition of the renal apical efflux transporter Multidrug and Toxin Extrusion (MATE) 1, and not altered absorption, underlies the increased metformin exposure observed in clinical interactions with cimetidine, trimethoprim or pyrimethamine. Pharmacol Res Perspect 5:e00357
- Meijer-Schaap L, van Putten JW, Janssen WM (2018) Effects of crizotinib on creatinine clearance and renal hemodynamics. Lung Cancer 122:192-194
[Crossref] [Google Scholar] [PubMed]
- Loebstein R, Koren G (1998) Ifosfamide-induced nephrotoxicity in children: Critical review of predictive risk factors. Pediatrics 01:e8
[Google Scholar]
- Ciarimboli G, Holle SK, Vollenbrocker B, Hagos Y, Reuter S, et al. (2011) New clues for nephrotoxicity induced by ifosfamide: Preferential renal uptake via the human organic cation transporter 2. Mol Pharmaceutics 8:270-279
[Crossref] [Google Scholar] [PubMed]
- Izzedine H, Gueutin V, Gharbi C, Mateus C, Robert C, et al. (2014) Kidney injuries related to ipilimumab. Investigational New Drugs 32:769-773
[Crossref] [Google Scholar] [PubMed]
- Schrag D, Chung KY, Flombaum C, Saltz L (2005) Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Instit 97:1221-1224
[Crossref] [Google Scholar] [PubMed]
- Hurabielle C, Pillebout E, Stehle T, Pages C, Roux J, et al. (2016) Mechanisms underpinning increased plasma creatinine levels in patients receiving vemurafenib for advanced melanoma. PLoS One 11:e0149873
[Crossref] [Google Scholar] [PubMed]
- Webb DE, Austin 3rd HA, Belldegrun A, Vaughan E, Linehan WM, et al. (1988) Metabolic and renal effects of interleukin-2 immunotherapy for metastatic cancer. Clin Nephrol 30:141-145
[Google Scholar] [PubMed]
- Guleria AS, Yang JC, Topalian SL, Weber JS, Parkinson DR, et al. (1994) Renal dysfunction associated with the administration of high-dose interleukin-2 in 199 consecutive patients with metastatic melanoma or renal carcinoma. J Clin Oncol 12:2714-2722
- Zarfin Y, Koren G, Maresky D, Perlman M, MacLeod S (1985) Possible indomethacin-aminoglycoside interaction in preterm infants. J Pediatr 3:511-513
[Crossref] [Google Scholar] [PubMed]
- Farber BF, Moellering RC Jr (1983) Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chem 23:138-141
[Crossref] [Google Scholar] [PubMed]
- Morita H, Maemura K, Sakai Y, Kaneda Y (1988) A case of convulsion, loss of consciousness and subsequent acute renal failure caused by enoxacin and fenbufen. Nihon Naika Gakkai zasshi 77:744-745
[Crossref] [Google Scholar] [PubMed]
- Bamgbola O (2016) Review of vancomycin-induced renal toxicity: An update. Ther Adv Endocrinol Ametab 7:136-147
[Crossref] [Google Scholar] [PubMed]
- Camacho P, Fan H, Liu Z, He JQ (2016) Small mammalian animal models of heart disease. Am J Cardiovasc Dis 6:70
[Google Scholar] [PubMed]
- Ortiz A, Sanchez-Nino MD, Izquierdo MC, Martin-Cleary C, Garcia-Bermejo L, et al. (2015) Translational value of animal models of kidney failure. Eur J Pharmacol 759:205-220
- Bao YW, Yuan Y, Chen JH, Lin WQ (2018) Kidney disease models: tools to identify mechanisms and potential therapeutic targets. Zool Res 39:72
[Crossref] [Google Scholar] [PubMed]
- Hayward RS, Harding J, Molloy R, Land L, Longcroft-Neal K, et al. (2018) Adverse effects of a single dose of gentamicin in adults: A systematic review. Br J Clin Pharmacol 84:223-238
[Crossref] [Google Scholar] [PubMed]
- Khan KM, Jialal I (2018) Folic acid (folate) deficiency. StatPearls. 1
[Google Scholar] [PubMed]
- Brodsky SV, Nadasdy T, Rovin BH, Satoskar AA, Nadasdy GM, et al. (2011) Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 2:181-189
[Crossref] [Google Scholar] [PubMed]
- Kitada M, Ogura Y, Koya D (2016) Rodent models of diabetic nephropathy: Their utility and limitations. Int J Nephrol Renov Dis 14:279-290
[Crossref] [Google Scholar] [PubMed]
- Ruiz-Ortega M, Esteban V, Ruperez M, Sanchez-Lopez E, Rodriguez-Vita J, et al. (2006) Renal and vascular hypertension-induced inflammation: Role of angiotensin II. Curr Opin Nephrol Hyper 15:159-166
[Crossref] [Google Scholar] [PubMed]
- Joyce E, Glasner P, Ranganathan S, Swiatecka-Urban A (2017) Tubulointerstitial nephritis: Diagnosis, treatment, and monitoring. Pediatr Nephrol 32:577-587
[Crossref] [Google Scholar] [PubMed]
- O’Kell AL, Grant DC, Khan SR (2017) Pathogenesis of calcium oxalate urinary stone disease: Species comparison of humans, dogs, and cats. Urolithiasis 45:329-336
[Crossref] [Google Scholar] [PubMed]
- Dakhore S, Nayer B, Hasegawa K (2018) Human pluripotent stem cell culture: Current status, challenges, and advancement. Stem Cells Int 22:2018
[Crossref] [Google Scholar] [PubMed]
- Qi W, Johnson DW, Vesey DA, Pollock CA, Chen X (2007) Isolation, propagation and characterization of primary tubule cell culture from human kidney (methods in renal research). Nephrol 12:155-159
[Crossref] [Google Scholar] [PubMed]
- Wilmer MJ, Saleem MA, Masereeuw R, Ni L, van Der Velden TJ, et al. (2010) Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res 339:449-557
[Crossref] [Google Scholar] [PubMed]
- Ohnuki M, Takahashi K (2015) Present and future challenges of induced pluripotent stem cells. Philosophical transactions of the royal society B. Biol Sci 370:20140367
[Crossref] [Google Scholar] [PubMed]
- Lam AQ, Freedman BS, Morizane R, Lerou PH, Valerius MT, et al. (2014) Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. Am J Nephrol 25:1211-1225
[Crossref] [Google Scholar] [PubMed]
- Mae SI, Shono A, Shiota F, Yasuno T, Kajiwara M, et al. (2013) Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun 4:1367
[Crossref] [Google Scholar] [PubMed]
- Xia Y, Sancho-Martinez I, Nivet E, Esteban CR, Campistol JM, et al. (2014) The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor–like cells. Nat Protoc 9:2693-2704
[Crossref] [Google Scholar] [PubMed]
- Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, et al. (2014) Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell stem cell 14:53-67
[Crossref] [Google Scholar] [PubMed]
- Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, et al. (2015) Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6:8715
[Crossref] [Google Scholar] [PubMed]
- Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, et al. (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526:564-568
[Crossref] [Google Scholar] [PubMed]
- Kandasamy K, Chuah JK, Su R, Huang P, Eng KG, et al. (2015) Prediction of drug-induced nephrotoxicity and injury mechanisms with human induced pluripotent stem cell-derived cells and machine learning methods. Sci Rep 5:12337
[Crossref] [Google Scholar] [PubMed]
- Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, et al. (2015) Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33:1193-1200
[Crossref] [Google Scholar] [PubMed]
- Morizane R, Bonventre JV (2017) Kidney organoids: A translational journey. Trends Mol Med 23:246-263
[Crossref] [Google Scholar] [PubMed]
- Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung S, et al. (2013) Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol 5:1119-1129
[Crossref] [Google Scholar] [PubMed]
- Jansen J, de Napoli IE, Fedecostante M, Schophuizen CM, Chevtchik NV, et al. (2015) Human proximal tubule epithelial cells cultured on hollow fibers: Living membranes that actively transport organic cations. Sci Rep 5:16702
[Crossref] [Google Scholar] [PubMed]
- Petejova N, Martinek A, Zadrazil J, Kanova M, Klementa V, et al. (2020) Acute kidney injury in septic patients treated by selected nephrotoxic antibiotic agents-Pathophysiology and biomarkers—A review. Int J Mol Sci 21:7115
[Crossref] [Google Scholar] [PubMed]
- Isah T (2019) Stress and defense responses in plant secondary metabolites production. Biol Res 52
[Google Scholar]
- Sabiu S, O’Neill FH, Ashafa AO (2016) The purview of phytotherapy in the management of kidney disorders: A systematic review on Nigeria and South Africa. Afr J Tradit Complement Altern Med 13:38-47
[Crossref] [Google Scholar] [PubMed]
- Basist P, Parveen B, Zahiruddin S, Gautam G, Parveen R, et al. (2022) Potential nephroprotective phytochemicals: Mechanism and future prospects. J Ethnopharmacol 283:114743
[Crossref] [Google Scholar] [PubMed]
- Peterson CT, Denniston K, Chopra D (2017) Therapeutic uses of triphala in ayurvedic medicine. J Altern Complement Med 23:607-614
[Crossref] [Google Scholar] [PubMed]
- Moorthi RN, Vorland CJ, Gallant KM (2017) Diet and diabetic kidney disease: Plant versus animal protein. Curr Diab Rep 17:1-8
[Crossref] [Google Scholar] [PubMed]
- Ahmad QZ, Jahan N, Ahmad G. Tajuddin (2014) An appraisal of nephroprotection and the scope of natural products in combating renal disorders. J Nephrol Ther 4:170
- Al-Anbaki M, Cavin AL, Nogueira RC, Taslimi J, Ali H, et al. (2021) Hibiscus sabdariffa, a treatment for uncontrolled hypertension. Pilot Comp Interv. Plants 10:1018
[Crossref] [Google Scholar] [PubMed]
- Moshaie-Nezhad P, Bahari Z, Jangravi Z, Zarei SM, Iman M (2021) The effect of Descurainia sophia seed extract on nephrotoxicity markers induced by acetaminophen in mice. J Adv Med Biomed Res 29:139-144
[Google Scholar]
- Chinnappan SM, George A, Thaggikuppe P, Choudhary Y, Choudhary VK, et al. (2019) Nephroprotective effect of herbal extract Eurycoma longifolia on paracetamol-induced nephrotoxicity in rats. J Evid Based Complementary Altern Med
[Crossref] [Google Scholar] [PubMed]
- Alvarez-Cilleros D, Lopez-Oliva E, Goya L, Martin MA, Ramos S (2019) Cocoa intake attenuates renal injury in Zucker diabetic fatty rats by improving glucose homeostasis. Food Chem Toxicol 127:101-109
[Crossref] [Google Scholar] [PubMed]
- Boonphang O, Ontawong A, Pasachan T, Phatsara M, Duangjai A, et al. (2021) Antidiabetic and renoprotective effects of Coffea arabica pulp aqueous extract through preserving organic cation transport system mediated oxidative stress pathway in experimental type 2 diabetic rats. Mol 26:1907
[Crossref] [Google Scholar] [PubMed]
- Perez-Gutierrez RM, Garcia-Campoy AH, Muniz-Ramirez A (2016) Properties of flavonoids isolated from the bark of Eysenhardtia polystachya and their effect on oxidative stress in streptozotocin-induced diabetes mellitus in mice. Oxid Med Cell Longev
[Crossref] [Google Scholar] [PubMed]
- Alabi TD, Brooks NL, Oguntibeju OO (2021) Leaf extracts of Anchomanes difformis ameliorated kidney and pancreatic damage in type 2 diabetes. Plants 10:300
[Crossref] [Google Scholar] [PubMed]
- Rodriguez-Fierros FL, Guarner-Lans V, Soto ME, Manzano-Pech L, Diaz-Diaz E, et al. (2021) Modulation of renal function in a metabolic syndrome rat model by antioxidants in Hibiscus sabdariffa L. Mol 26:2074
[Crossref] [Google Scholar] [PubMed]
- Nerdy N, Ritarwan K (2019) Hepatoprotective activity and nephroprotective activity of peel extract from three varieties of the passion fruit (Passiflora sp.) in the albino rat. Open Access Maced J Med Sci 7:536
[Crossref] [Google Scholar] [PubMed]
- Al-Yousef HM, Alqahtani AS, Ghani AS, El-Toumy SA, El-Dougdoug WI, et al. (2021) Nephroprotective, cytotoxic and antioxidant activities of Euphorbia paralias. Saudi J Biol Sci 28:785-792
[Google Scholar]
- Heidarian E, Jafari-Dehkordi E, Valipour P, Ghatreh-Samani K, Ashrafi-Eshkaftaki L (2017) Nephroprotective and anti-inflammatory effects of Pistacia atlantica leaf hydroethanolic extract against gentamicin-induced nephrotoxicity in rats. J Diet Suppl 14:489-502
[Crossref] [Google Scholar] [PubMed]
- Ezejiofor AN, Udowelle NA, Orisakwe OE (2017) Nephroprotective and antioxidant effect of aqueous leaf extract of Costus afer Ker gawl on cyclosporin-a (Csa) induced nephrotoxicity. Clin Phytosci 1-7
[Google Scholar]
- Rakib A, Ahmed S, Islam MA, Haye A, Uddin SN, et al. (2020) Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Sci Nut 8:547-556
[Crossref] [Google Scholar] [PubMed]
- Mohammed SA, Khan RA, El-Readi MZ, Emwas AH, Sioud S, et al. (2020) Suaeda vermiculata aqueous-ethanolic extract based mitigation of ccl4-induced hepatotoxicity in rats, and hepg-2 and hepg-2/adr cell-lines-based cytotoxicity evaluations. Plants 9:1291
[Crossref] [Google Scholar] [PubMed]
- Buzgaia N, Awin T, Elabbar F, Abdusalam K, Lee SY, et al. Antibacterial activity of Arbutus pavarii pamp against Methicillin-Resistant Staphylococcus aureus (MRSA) and UHPLC-MS/MS profile of the bioactive fraction. Plants 9:1539
[Crossref] [Google Scholar] [PubMed]
- Mestry SN, Gawali NB, Pai SA, Gursahani MS, Dhodi JB, et al. (2020) Punica granatum improves renal function in gentamicin-induced nephropathy in rats via attenuation of oxidative stress. J Ayurveda Integr Med 11:16-23
[Crossref] [Google Scholar] [PubMed]
- Epure A, Parvu AE, Vlase L, Benedec D, Hanganu D, et al. (2020) Phytochemical profile, antioxidant, cardioprotective and nephroprotective activity of romanian chicory extract. Plants 10:64
[Crossref] [Google Scholar] [PubMed]
- Hou Y, Yuan P, Fu Y, Zhang Q, Gao L, et al. (2021) Geniposide from Gardenia jasminoides var. radicans Makino attenuates myocardial injury in spontaneously hypertensive rats via regulating apoptotic and energy metabolism signalling pathway. Drug Des Devel Ther 15:949-962
[Crossref] [Google Scholar] [PubMed]
- Guerrero-Hue M, Garcia-Caballero C, Palomino-Antolin A, Rubio-Navarro A, Vazquez-Carballo C, et al. (2019) Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J 33:8961-8975
[Crossref] [Google Scholar] [PubMed]
- Benzer F, Kandemir FM, Kucukler S, Comakli S, Caglayan C, et al. (2017) Chemoprotective effects of curcumin on doxorubicin-induced nephrotoxicity in wistar rats: By modulating inflammatory cytokines, apoptosis, oxidative stress and oxidative DNA damage. Arch Physiol Biochem 124:448-557
[Crossref] [Google Scholar] [PubMed]
- Lee G, Shin J, Choi H, Jo A, Pan S, et al. (2017) Cynanchum wilfordii ameliorates testosterone-induced benign prostatic hyperplasia by regulating 5α-reductase and androgen receptor activities in a rat model. Nutrients 9:1070
[Crossref] [Google Scholar] [PubMed]
Citation: Reddy UKT, Annegowda HV, Alkanad M, Kumar AA, Harshavardhan P (2024) A Review on Bioactive Compounds from Plants Available in
South India with Uroprotective and Nephroprotective Activity. Int J Drug Dev Res Vol:16 No:3








