Research - (2024) Volume 16, Issue 4
A study discovered the role of carbon dioxide in the pathogenesis of autoimmune disorders
Abdelrazak Mansour Ali1*,
Radwa Abdelrazak Ali2 and
Mohamed Abdeltawab Ibrahim3
1Professor of Pediatrics, International Center for Population Studies & Research, Al-Azhar University, Cairo, Egypt
2Department of Research, George Mason University, National Institute of Health, Fairfax, VA 22030, USA
3Ministry of Health, General Director of Marsa Alam Hospital, Quality Management Consultant, Egypt
*Correspondence:
Abdelrazak Mansour Ali, Professor of Pediatrics, International Center for Population Studies & Research, Al-Azhar University, Cairo,
Egypt,
Email:
Received: 11-Jun-2024, Manuscript No. ipaom-24-14924;
Editor assigned: 12-Jun-2024, Pre QC No. P-14924;
Reviewed: 19-Jun-2024, QC No. Q-14924;
Revised: 26-Jun-2024, Manuscript No. R-14924;
Published:
03-Jul-2024
Abstract
The incidence of autoimmune disorders has increased with rise of carbon dioxide since last century.
Objective: To determine whether CO2 is associated with autoimmune disorders.
Design: Case-control study at local tertiary hospitals in Egypt.
Method: A total of 150 cases of various autoimmune disorders, and 75 controls were 20 to 70 years of age. Exclusion criteria were neuromuscular disorder, critical, respiratory illness, and exposure to CO2, guided by criteria of National Institute for Occupational Safety. Cases recruited from November 2023 to March 2024, matched by age, sex, and other demographic variables. All participants were tested for blood gases. Certain cases were further tested to confirm autoimmune status. PaCO2 analysis performed using two methods of statistical significance to validate data.
Results: PaCO2 (Mean ± SD) was (48.18 ± 12.10) in autoimmune cases, compared to (42.63 ± 11.06) in control (p= 0.001), number (%) of cases with ↑PaCO2= 97(64.7%) for cases, and 30(40%) for control (RR=1.6167). OR (95% CI) = 2.7453 (1.552 to 4.857), p=0.0005.
Conclusion: Our study confirmed a correlation between CO2 and autoimmune disorders. The mechanism is a complex interplay between direct effect of CO2 on cell membrane, calcium “Ca2+ “homeostasis and signaling pathways. CO2 thermic effect increases mobility of antibodies to move away from antigens elicited their secretion, and then CO2 protonation increases electrostatic interactions between anionic cell membranes and positive charge of antibodies, thus orienting antibodies to host antigens initiating autoimmune reaction. The significant autoimmune phenomena in skin and musculoskeletal system are due to these tissues have more cells with negative charges. Pattern of gene expression to CO2 thermal effect imposes differences in mRNA gene translation with various phenotypic expression. CO2 directs pathways providing benefits to autoimmunity. A new autoimmune disorder treatment is proposed. The pattern of gene expression thermal effect imposes differences in mRNA gene translation. Membrane receptors absorb heat energy reemitted from CO2 would produce various oscillations of the electron cloud culminating in various phenotypic expression. CO2 directs pathways to benefit the autoimmune process. It is expectable that a new treatment of autoimmune disorders will prevail in the future.
Keywords
Cell, Calcium, Autoimmune, CO2, Ca2+
Abbreviations
PIP: Phosphatidylinositol 4-phosphate; ER/SR: Endoplasmic Reticulum/Sarcoplasmic Reticulum; TRPCs: Transient Receptor Potential Channels; cAMP: cyclic Adenosine Monophosphate; NFκB: Nuclear Factor Kappa Of B Cell; RA: Rheumatoid Arthritis; SLE: Systemic Lupus Erythematosus; IBD: Inflammatory Bowel Diseases; Ig V: Immunoglobulin Variable chain; Ag-Ab: Antigen Antibody; RGS: Regulator of G-Protein Signaling; APCs: Antigen Presenting Cells; MHC: Major Histocompatibility Complex; TCRs :T Cell Receptors; ITAMs: Immune-receptor Tyrosine-based-Activation-Motifs
Introduction
Despite global warming being implicated in diverse pathologies, currently no studies to explore defined carbon dioxide cellular thermal damage exist. Here, we present the novel study adopting a principle of thermo-acidic impact on individual cells and explored the mediated autoimmune disorders.
CO2 is a molecule composed of one carbon atom and two oxygen atoms, it absorbs infrared radiation in a unique manner due to its vibrational patterns of stretching and bending that can amplify absorption of radiation [1]. It is acidic, carried in blood in different forms, majority is converted to bicarbonate ions “HCO3 “by carbonic anhydrase, other forms bound to hemoglobin as carbamino compounds [2]. Hypercapnia is increased partial pressure of carbon dioxide (PaCO2) above 45 mm Hg, there are several mechanisms the body should moderate CO2 by acid-base buffering system. Retention of CO2 in blood is an important consequence of a handful of sleep disorders such as sleep apnea, and obesity [3]. Infrared pulses are absorbed by water to produce heating that reversibly alters electrical capacitance of plasma membranes, depolarizing target cells, and triggering thermosensitive ion channels forming membrane pores [4]. The pH of a liquid is determined by its hydrogen ion concentrations: the higher the concentration of hydrogen ions, the lower the pH value [5].
Calcium channels & homeostasis. Cells maintain precise intracellular Ca2+ via a complex system of Ca2+ channels, transporters, Ca2+ ATPases, and signaling effectors, including specific lipid kinases, and phosphatases. Excessive intracellular Ca2+ caused functional defects in subcellular organelles such as endoplasmic reticulum” ER”, lysosomes, and mitochondria [6].
Calcium is essential for intracellular processes, cell stored Ca2+ in high concentrations in (ER) or (SR) which serve as main storage site for calcium. Ca2+channels include all pore-forming, Ca2+ permeable proteins [7]. It is demonstrated that Ca2+ signals encode information in frequency, kinetics, amplitude, and spatial extent [8]. One of the most important types of calcium channels are voltage-gated channels which respond to change in voltage across cell membranes and driven by an electrochemical gradient. A particularly important calcium channel is Ca2+ ATPase at ER/SR membrane, called sarcoplasmic/endoplasmic reticulum Ca2+-ATPase [9]. When Ca2+ stores inside ER/SR becomes depleted, it will be replenished by special calcium channels called store-operated calcium channels, where low Ca2+ is sensed by a Stromal Interacting Molecule (STIMs). They interact with another protein called Orai1, referred to as a Calcium-Release Activated Channel (CRAC) [10]. STIMs act as sensors not only of decreased ER Ca2+ but also of temperature increases and acidosis. STIMs target proteins other than Orai channels, including voltage operated CaV1.2 channels, TRPCs, and SERCA Ca2+ pumps [11]. It was evidenced that altered Ca2+ in lymphocytes leads to autoimmune and immunodeficiency syndromes [12].
Ca2+ channels also generate signals that are spatial in nature and dependent on highly localized signaling structures. They may create elementary signals called calcium "puffs" or "sparks," to form organized complexes with proteins called G-protein coupled receptors or receptor tyrosine kinases [13]. Acidity can suppress anti-tumor T lymphocyte function by a significant upregulation of inhibitory immune checkpoints TIM-3, LAG-3, and CTLA-4 of T cells [14]. This acidosis-induced upregulation of immune checkpoints contributed to immune evasion and tumor progression. Extra tumoral acidity represented a mechanism of resistance to CTLA-4 inhibitors [15,16]. CTLA-4 is a critical negative regulator of autoimmune diseases [17]. The functional integrity of the CD28 molecule was necessary for CTLA-4 knockout to cause autoimmune diseases [18].
Ca2+ is an important cation able to function as a second messenger in different immune cells. Intracellular Ca2+ Signaling has been implicated in pathogenesis of autoimmune and congenital immunodeficiency disorders [19]. Dysregulated Ca2+ signaling is involved in the pathophysiology of autoimmune diseases [20].
Methods
For our knowledge, this is the first novel study to uncover role of CO2 in pathogenesis of autoimmune disorders. The study approval was received from administration of Hospitals in Cairo, Egypt. Cases were recruited from hospitals from November 2023 to March 2024. The index date for this study was established to comply with seasonality of autoimmune diseases [21]. A total of 150 cases (77 males and 73 females) from various autoimmune disorders compared to 75 controls (40 males and 35 females) from the same population and locality, matched to socio demographic characteristics were enrolled. Selected parameters including age, sex, urbanization, social status, and comorbidities showed close balance between two cohorts. All cases had been seen for examination, including vitals, weight, height, Body Mass Index (BMI), and demographic variables gathered by questionnaire including age, sex, occupation, smoking status, family history, and history of chronic respiratory illness or occupational exposure to CO2, guided by criteria of “National Institute for Occupational Safety and Health, August 1976”. Emphasis to exclude farmworkers, mining, and industries related to CO2 exposures was considered. Participants were free of neuromuscular disorder, critical illness, and multisystem organ failure. Participants were tested for arterial blood gases to determine partial arterial pressure of CO2 and stratified according to PaCO2 levels less than and greater than median. The considered standard range values of partial pressure of CO2 were between 35 to 45 mmHg. PaCO2 was analyzed using two methods of statistical significance to validate our findings; it included percentage of occurrence of events, and “mean, standard deviation” of values. If required, certain cases were tested to confirm diagnosis of autoimmune disease.
Results
Our findings derived from matched data, indicated that there is strong association between CO2 rise and increased autoimmune disorders.
Tab. 1. displays case categorization of participants included in the study. Notably, they are screened during selection to exclude underlying cardiovascular and pulmonary conditions that may predispose to increased CO2 levels.
| Case category |
Number of cases, n (%) |
Male/Female, (60/90) |
| RA |
40 (26.7) |
18/22 |
| SLE |
30 (20) |
5/25 |
| JDM |
24 (16) |
11/13 |
| IBD |
18 (12) |
9/9 |
| Celiac |
14 (9.3) |
6/8 |
| Vitiligo |
9 (6) |
5/4 |
| Psoriasis |
9 (6) |
4/5 |
| EGACU |
6 (4) |
2/4 |
RA= Rheumatoid Arthritis, SLE= Systemic lupus Erythematosus, JDM= Juvenile Diabetes Mellitus, IBD= Inflammatory Bowel Diseases, EGACU= Extra Gastric Autoimmune Chronic Urticaria.
Tab. 1. Distribution of diseases among cases.
Tab. 2. shows demographic characteristics and examination parameters of cases and controls.
| Variable |
Cases No. (%) (n =150), |
Controls No. (%) (n =75), |
Odds ratio (95% CI), P value |
| Sex (male) |
60 (40) |
32(42.7) |
0.8958- (0.511, 1.572), P=0.3507 |
| Age, mean (S.D.), years |
47.9 (14.5) |
50.1 (13.4) |
(-1.742 to 6.142), SE= 2.00, P= 0.273. |
| BMI, mean (S.D.), kg/m2 |
27.6 (5.1) |
26.1 (5.3) |
(-2.9401 to -0.0599), SE= 0.73, P= 0.0413 |
| Total annual outcome, $ |
- ≤ 12000.
- 12000 – 30000
- ≥ 30000
Current smoker |
87 (58)
47 (31.3)
16 (10.7) 34 (22.7) |
44 (58.7)
24 (32)
7 (9.3) 12(16) |
1.539 (0.744, 3.181), P= 0.1223 |
| Obesity total & Rheumatoid |
| Obesity=18 & 9 |
18 (12) 3(4) |
3(4) |
3.27(0.932, 11.486), P=0.032 |
| Overweight =19 & 5 |
19(12.7) |
5(6.7) |
2.03 (0.727, 5.670), P= 0.088 |
Obesity was defined as BMI ≥ 30 kg/m2, and overweight as BMI>25 and <30 kg/m2. As depicted in Tab. 2., positive associations of obesity with RA were observed in both sexes and in both seropositive and seronegative disease.
Tab. 2. Demographic characteristics and examination parameters of cases and controls.
Tab. 3. analyzed PaCO2 in cases and controls. To illustrate this finding more clearly, we tracked PaCO2 analysis to test data through two methods of statistical significance.
| Parameter |
Cases |
Control |
95% CI, SE, P value |
| PaCO2 levels (Mean ± SD) |
(48.18 ± 12.10) |
(42.63 ± 11.06) |
(-8.8288 to -2.2712), SE= 1.66. P=0.001 |
| No (%) of cases with ↑PaCO2, (RR & OR= 95% CI). |
97(64.7) |
30 (40) |
1.6167 & 2.7453 (1.552 to 4.857), P value =0.0005 |
PaCO2=Partial pressure of Carbon Dioxide, SD= Standard Deviation.
RR= Relative Risk, OR= Odds Ratio, CI= Confidence Interval.
To illustrate this finding more clearly, we tracked PaCO2 analysis to test data through two methods of statistical significance; Percentage of occurrence of events, and mean, standard deviation of the values.
Tab. 3. Analysis of PaCO2 in case and control groups.
Discussion
This research highlighted the important role of carbon-dioxide-mediated autoimmune disorders. We provide mechanistic insights to unveil the novel concept: “intracellular binding role” played by unique thermo-acidic CO2, and unique properties of Ca2+ channel signal sparks’ intensity. Contemplating a coordinated symmetry between CO2 and Ca2+ to get further insights into cellular response to micro-thermally altered proteins. This emerging concept, although highly versatile, but seems homogenous and acts in concert. The harmonically played mechanisms derive from unique characteristics of both CO2 and Ca2+. For instance, CO2 and Ca2+ acted as subcellular 2nd messengers, CO2 has unique thermo-acidic effect on all cells, likewise calcium channels play a critical role in a variety of physiological functions. The special channels (STIMs) act as sensors not only of decreased Ca2+ but also of temperature increases and acidosis. Park, et al. explained that the Orai1 mutations in autoimmunity result in decreased expression of several cytokines: IL-1, IL-4, IL-17, IFNγ and TNF alpha in CD4 and CD8 T lymphocytes [20]. Soboloff, et al. showed that STIMs target proteins other than Orai channels, including voltage operated channels and plasma membrane Ca2+ ATPase and SERCA Ca2+ [11]. We applied this approach to study the behavior of selected molecular protein receptors and channels in physiological context of living cells, with unprecedented detail, in sequence of elements mediating stress-induced autoimmune disorders. In view of these findings and evidence for stress-induced autoimmune disorders, we provide the first evidence linking CO2 and autoimmune disorders. Unexpectedly, our data revealed that increased PCO2 may be the etiologic pathogenic mechanism of autoimmune disorders. The mechanism is a complex interplay between direct CO2 local effect on cell membrane and whole cell homeostasis on one side, and Ca2+ homeostasis and signaling pathways on the other side. According to Phelan [22], CO2 acts as central signaling hub in gene transcription and cytokine regulation. By reemitting infrared, CO2 instantly causes rapid increase in cell temperature and excitability through electrostatic energy. Ahamad, et al. [23], indicated that Ca2+ entry increases activation of pathways involved nuclear factor” NFκB” translocation into nucleus to activate transcription of gene networks related to cell survival.
While it was previously believed that CO2 moves across biological membranes only by passive diffusion, we evidently introduce the novel concept of CO2 “drill-like” action and propose existence of other channels that enable CO2 to penetrate cell membranes acting as an electric drill, forcing Ca2+ to get inside cells against the concentration gradient. This increased intracellular calcium influx augments 2nd messenger signal that CO2 has generated by its immediate intracellular entry.
At cellular level, Mistrik, et al. reported that thermal damage primarily impairs proteins, causing their unfolding, aggregation, and denaturation [24]. Nadal, et al. added that adaptive responses to heat stress depend on intensity of micro-heat damage and involves an extensive reorganization of gene expression [25].
Heating reversibly alters electrical capacitance of plasma membranes, depolarizing the target cells, triggering thermosensitive ion channels, ultimately forming membrane pores, and increasing conductance which activate intracellular second messengers. Tsai, et al. assessed the role of infrared-stimulated and activated transmembrane ion channels which generate selective rechargeable electrolytic bio-battery with pathways including Ca2+, ATP and GTP, that provide energy for cellular reactions including signaling, and gene transcription [26].
Our findings show that CO2 imposed acidic milieu critical in regulating signaling cascade and Ca2+-dependent transcription factors, which regulate gene activity through three important pathways culminating in an autoimmune commence. CO2 diffusely penetrated cell membranes and maintained intracellular acidity as well. Thiel, et al. [27] has detailed these pathways. To respond to changes in microenvironment, cells need to switch genes on and off. One mechanism that links events at cell surface to gene expression in nucleus employs intracellular Ca2+. It seems clear CO2 effects on immune cell functions are dependent on pH changes and molecular CO2 sensing, which employ action potential generated ion channels.
Similarly, the mechanism of intracytoplasmic Ca2+-mediated-autoimmune disorders seems pivotal in generating cytokine pathways, and transcription factors which are key determinant in autoimmune disorders together with CO2. This is achieved through a communication network involving channels, transcription factors, and gene regulation. Thiel, et al. and Yeh, et al. confirmed our proposal [27,28]. Given that CO2 altering expression of genes of immunity, and Ca2+ influx activated NFAT, NF-κB, and c-fos factors which work in tandem to control cytokines, whose outcome is immune tolerance [20,28]. Guo, et al. explicated that oscillatory activation of NF-κB promotes transcription of inflammatory genes, whereas persistent activation reprograms epigenome to involve a broader range of genes [29].
In context of experiments examining impact of acidity on immune cell functions, it is important to consider cell type, receptors, and responses. The link established here between acidity stress signaling and immune functioning is consistent with Davern’s study, which documented that acidity suppresses anti-tumor T lymphocyte function by a significant upregulation of inhibitory immune checkpoints, CTLA-4 [14]. Navarro, and Sun, reported that addition of ICBs (Immune checkpoint blockade) to target ICs (Immune checkpoints) that were upregulated under severe acidity may be necessary to overcome treatment resistance [15,17]. These findings establish that acidosis-induced upregulation of immune checkpoints on T cells may potentially contribute to immune evasion, and upregulation of CTLA-4 which plays a key role in early development of autoreactive T-cells by skewing the balance between destructive T-effector cells and protective Treg cells [17,30]. Taken together and results of Peter, et al. [31] that clarified importance of CTLA-4 and PD-1/PD-L1 in protecting heart from autoimmune myocarditis, we conclude that CO2 can promote autoimmune diseases. This is consistent with many other studies which have demonstrated that CTLA-4 is a critical negative regulator of T cell activation and autoreactivity such as Hossen, et al. and Guo, et al. [29,30].
T cells role in immune homeostasis: Precise regulation of T cell activation is crucial for overall immune homeostasis. Microenvironmental cues and signaling pathways are required for T cell activation. In this context, CO2 can activate signaling pathways and transcriptional alterations leading autoreactive T cells to be in a state of activation and development of autoimmune diseases. This may occur by CO2 alone and/or combined CO2 Calcium circuit. The biological pathways most associated with differential gene expression may be compensatory reactions to limit injury from altered inflammatory activity. In line with our study, we conclude that transcriptional response to elevated CO2 may alter gene expression. Casalino, et al. found that hypercapnia downregulated expression of 183 genes, among these are genes linked to immune responses [32]. To further understand the mechanism, we proposed that acidic medium involves Histidine’s protonation resulting in imidazole ring ionization, and conformational changes promoting heterotrimeric G proteins resulting in increased production of intracellular 2nd messengers cAMP [33].
Our study was conducted on autoimmune cases, indicated that CO2 expressed its effect by multiple mechanisms and diverse actions such as, direct effect, Ca2+-induced effect, and pathways that finally impact overall autoimmune processes. To further understand these mechanisms and pathways we analyzed data from Marchesan, et al. to prove finding [34].
Significantly, CO2 provides constant physiological pH, through CO2-bicarbonate buffer system as it maintains optimum temperature in cell culture [35]. In other words, CO2 creates suitable conditions for biologic cellular processes including increased intracellular Ca2+ with subsequent activation of various transcription factors, receptors, and channels.
In summary, considering the CO2 role in cell culture, CO2 not only induced autoimmune process, but also maintained autoimmune disorder culminating in appearance of the disease. This explains why some serologic markers of autoimmune disorder can be identified long time before the disease manifest clinically.
CO2 impacted autoimmune antigen-antibody “Ag-Ab” reaction, Epitope-paratope: Our study investigated CO2 chemical effect on autoimmune interaction. The CO2 chemo-reflex is required for tonic drive underpinning electrostatic forces and paratope-epitope bonding. The chemical autoimmunity induced by chemical bonds is catalyzed by CO2 rise; In hope of gaining a better understanding of how CO2 impacted autoimmune reaction, we analyzed data from Quantum mechanics to understand another aspect of autoimmune reaction. It described; all chemical bonds are based on electrostatic forces. Ag-Ab reactions are stabilized at low temperature, as high temperature modulates their binding kinetics, diffusivity, and aggregation propensity. So, to dissociate antigen–antibody complexes formed, one would have to raise temperature to 56 â?? [36]. B-cells can neutralize pathogenic molecules by specifically targeting those using receptors on their surfaces. This is achieved via molecular interactions between paratope and epitope [37]. Antigens are molecular structures found on the surface of pathogens (bacteria, viruses, and other foreign substances), as well as on the surface of body cells. Antibodies or immunoglobulins are formed by “B-cells” in response to antigens. Binding of Ag-Ab reaction relies on specific interaction of amino acids at paratope-epitope interface. Akbar, et al. [38], reported that a fundamental premise for predictability of antibody-antigen binding is existence of paratope-epitope interaction motifs that are universally shared among antigen-antibody structures. Biochemical reaction between antibodies and specific antigens occurs when come closer to nanometers as they react in a ‘lock-and-key’ manner. When combination between lock and key is precise (in terms of geometry and chemical character), the goodness of fit is high, and the reaction will be stronger. The strength of bond between antigen and antibody known as “antibody affinity” depends on non-covalent bonds such as hydrogen bonds, and electrostatic forces. Predictors of antigen-binding affinity are critical for therapeutic interventions of antibodies since binding affinity between antibody and epitope occurs only if their structures are complementary [36,39]. Goodness of fit relies on time. Too short time means that antigen and antibody may not have had sufficient time to form a good reaction, prolonged time causes antigen–antibody complexes to dissociate. Other fitness factors include temperature, pH, and ionic strength. Low ionic strength solutions are commonly used to increase sensitivity of Ag-Ab reactions [36,40]. These observations support that local acidity could influence bioactivity and distribution of antibodies by weakening electrostatic interactions and/or hydrogen bonds, interfering with clinical efficacy of antibodies. Extreme pH values induce marked conformational changes in antibody molecule that probably destroy complementarity with antigen [40]. Moreover, decreased binding of antibody to antigen at acidic endosomal pH results in reduced recycling of antibody/antigen complexes and increased lysosomal trafficking of antigen for degradation, thus decreasing targeted antigen serum level [41]. Hironiwa confirmed that Ca2+ dependent antigen-binding antibody can dissociate its antigen in endosome and accelerate antigen clearance [42].
In summary, aside from factors that negatively influence affinity and efficacy of the antigen-antibody reaction, CO2 has been identified as ideal substance to inhibit antigen-antibody reactions at site of inflammation forcing antibodies to leave to nearby location away from local CO2 effect. Once antibodies found appropriate conditions for reaction, they become instantly available to interact with self-antigens and launching autoimmune process that depends on molecular complementarity between self-antigen and antibody. Low ionic strength in the new site (away from CO2) enhances self Ag-Ab reaction and increasing antigen-antibody association. Based on microenvironment enhanced autoimmune process, we speculate that specific Ag-Ab reaction is a pattern of biochemical reaction defined by cellular CO2 sensitivity and mediated by pH changes and other chemical factors.
Our work identified the first evidence linking CO2 influence on epitope-paratope reaction, broadly analogous with physicochemical features of antigen-antibody autoimmune responses. We concluded that information contained within antibody is potentially used to gain insight into physicochemical properties of the cognate epitope. In accord with this view, Boswell, et al. demonstrated a high correlation between corresponding structural properties of paratope and epitope [43]. Stank, et al. found that orientation of a single amino acid side chain in substrate binding pocket of the active site caused increased affinity [44]. Because an epitope corresponds to a small region (surface area of 4-6 amino acids), it is possible for different macromolecules to exhibit the same molecular identities and orientations. Antibodies secreted after binding to one epitope on an antigen may exhibit cross reactivity for similar epitopes on different antigens. Cross reactivity occurs when an antibody binds not to the antigen that elicited its synthesis and secretion, but to a different antigen such as that of nearby host antigens with nearly similar epitopes [45]. Thus, impelling antigen antibody reaction to be confined to host cells, culminating in established autoimmunity. This progressing autoimmune process is magnified by antibody kinetics which governs their binding to antigens and other cognate receptors. It is evidenced that electrostatic interactions between anionic cell membranes and positive surface charge of antibodies can influence tissue disposition kinetics in a manner independent of antigen recognition [43].
It is evident that CO2 thermic effect can increase mobile functional antibodies to move away from antigens that elicited their synthesis, then generalized protonation of CO2 increases electrostatic interactions between anionic cell membranes and positive surface charge of antibodies to be oriented to host antigens instead of antigens elicited their synthesis, thus initiating and boosting Ag-Ab autoimmune reaction. This notion was demonstrated from Reverberi, who described Ag-Ab reaction and hydrogen bonds are exothermic, and heat derives from energy released through a chemical bond formation. As temperature is a critical determinant in many physicochemical, and biological processes, such as diffusion, reaction equilibrium and kinetics, the initial high temperature can increase proportion of mobile functional antibodies [39,40]. According to these thermodynamic principles, we can explore CO2 thermic effect which catalyzes initiating autoimmune reaction, then after the reaction had been established, the total exothermic effects of both reaction and hydrogen bond cause heat energy release which speeds more antibodies to bind more host antigens. By time, autoimmune process would become organized and boosted by hypercapnic acidosis which impairs ability of lymphocytes to distinguish between self and non-self. This accords with Almanza [3].
In summary, CO2 catalyzed initiating, maintaining, and providing an optimum temperature and pH, mediated by receptors, proteins and signaling pathways.
Our results suggest that high CO2 is involved in autoimmune damage of certain tissues such as cartilage, and ligaments in certain cases (RA, SLE, diabetes), gastrointestinal tract (IBS, Celiac), and skin (Vitiligo, Urticaria). We suggest the reason behind selective damage of these tissues is due to electrostatic interactions between positive surface charge of antibodies and anionic cell membranes of negatively charged tissues in a manner that is independent of antigen recognition. Our suggestion may align with Veda, et al. and Leal, et al. who stated that human body contains a multitude of negatively-charged tissues including skin, joint, cartilage and ligaments, mucosa of GIT, and vitreous of eye, [46,47]. Furthermore, Nadal, et al. evidenced that pattern of gene expression in response to heat stress is affected by fine regulation of mRNA synthesis with diverse phenotypic expression [25]. Mistrik, et al. discussed the emerging field of plasmonic Nanoparticles (NPs) where absorption of light by NPs may affect plasmon resonance which depends on many parameters, including size, shape, and dielectric properties of NPs culminating in varied heat expression effects [24].
Carbon dioxide effect on immune cells and signal transduction: Different determinants have been reported to influence magnitude of autoimmune response, including inflammatory cytokines, costimulatory signals, second messengers and microenvironment. The interplay between (CO2, Ca2+) and other determinants governs the outcome of reaction. Studies found that increased CO2 pressure reduces pH, which increases extracellular adenosine concentration [48]. These signaling events contribute to a range of physiological responses, some of which are sustained and involve CO2-dependent suppression of NF-κ B-dependent immune regulatory cytokines involved in antibody class switching and immunological memory formation [22].
Antigen presentation & T cells: A critical element of immune function is the efficient interaction between activated APCs and T cells. Antigen presentation by “MHC” on APCs caused T cell receptor (TCR) internalization and gene rearrangement at endogenous TCRα locus [49]. A key question, which remains to be answered with respect to the MHC-TCR immune complexes binding, and selective orientation to host antigens instead of anti-microbial complexes binding. Obviously, change in ambient conditions caused by increased CO2 is reflected on binding topologies of TCR-MHC and the observed differences between autoimmune complexes and anti-microbial complexes as demonstrated by Wucherpfennig, et al. [50]. It is the CO2 thermo-acidic effect that creates curvature and fluidity of immune cell membranes with induction of topographic changes and mobilization of the membrane-linked molecules including TCR-MHC immune complexes.
Effect of CO2 on other immune cells: Inflammation negatively regulates memory precursor effector cell development (MPEC). Notably, we are aware that elevated CO2 generates anti-inflammatory effects via acidification. Elevated CO2 correlates with reduced monocyte and macrophage migration and inflammatory gene expression [51]. Several types of inflammatory molecules including IL-12, IFNγ, are known to inhibit acquisition of memory [52].
Antibody diversity and B cell: It is recognized that autophagy plays a key role in cell-type specific immune cell development and differentiation. Our study revealed the role of CO2 in driving B cell autoimmunity. This role is concluded from Soboloff, et al. and Raza, et al. By acting as sensors of temperature increases and acidosis, Ca2+ Channels STIM1/Orai1 can increase autophagy. Autophagy supports self-reactive B cells in subverting autoimmune checkpoints to present autoantigens to T-lymphocytes [11,53]. Additionally, autophagy enables B cells to present peptides derived from self–antigens to cognate T cells. So, CO2 can promote B cell autoimmunity through its acid-thermic effects on Ca2+ Channel STIM1/Orai1- autophagy pathway.
Role of antibody errors during random recombination of gene segments has been expertly reviewed. These errors are one of the sources of antibody diversity. B cells have unique property to somatically alter their immunoglobulin genes by recombination, hypermutation and class-switch recombination. Their mutations are initiated by Activation-Induced Cytidine Deaminase (AID), which deaminates cytosine [54].
Our results further attest to biological significance of a multifaceted immune-cellular response to thermal damage. Several studies yielded supporting findings to our results; Shiraz, et al. demonstrated that somatically mutated high-affinity autoantibodies are a hallmark of autoimmune diseases [55]. Jaiswal, et al. confirmed that somatic hypermutation introduces point mutations into immunoglobulin genes and initiated by cytosine deamination and completed by error-prone processing of resulting uracil by base excision repair factors [54]. Ehrlich, et al. found that DNA cytosine methylation and deamination were accelerated by heat [56]. Moreover, Sethi reported that acceleration of cytosine deamination is due to other factors such as protonation of free phosphate groups of cytosine [57]. Mikocziova, demonstrated that certain proteins can modulate B cell responses and MHC genes, [58]. Consequently, CO2 by its thermal and protonation abilities could thereby affect ability of B cells to respond to antigens with production of somatically mutated high-affinity autoantibodies through thermal effect of “CO2” on cytosine deamination. Also, CO2 protonation can dissociate phosphate groups to help attack cytosine amino group. CO2 induction of mutated antibodies may be evidenced by Casalino, et al. through hypercapnic downregulation of major genes linked to immune responses [35]. Interestingly, in line with our findings, Zhao, et al. found that CO2 can suppress catalytic antibody activated carbamate. Catalytic antibodies made it feasible to prevent emergence of autoimmunity [59]. Collectively, these data support our conclusion that high CO2 promotes emergence of autoimmune disorders in a pH-dependent manner.
In summary, multiple sites within the immune system can elicit adaptive responses to local CO2 concentrations. The evidence points to strong contribution of acid/pH CO2 sensing to modulate immune response. In this context, there is ample evidence that CO2 exerts changes on catalytic antibodies towards benefit of autoimmunity Fig. 1.
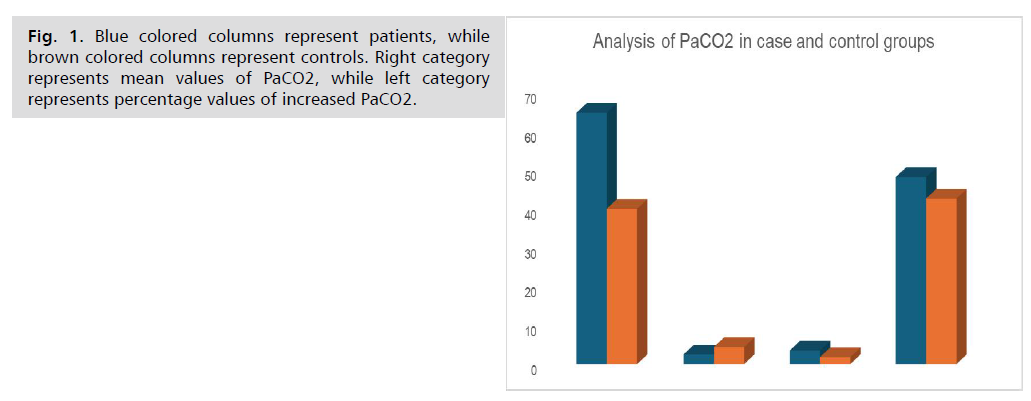
Fig. 1. Blue colored columns represent patients, while brown colored columns represent controls. Right category represents mean values of PaCO2, while left category represents percentage values of increased PaCO2.
CO2, T-cell activation, and function: Autoimmune induction by thermo-acidic effects unique to CO2, influences both TCR and BCR integrity and function of T and B cells respectively. The mechanism includes modulation of cellular cholesterol, and topological variations between TCRs and other membrane proteins which induce membrane bending to micro adhesion rings. CO2 modulates cellular cholesterol accumulation, and upregulation of lipid metabolism genes [60]. Robinson, et al. showed that cholesterol enriched in plasma membrane forms signaling platforms called lipid rafts, essential for T-cell activation and function. So, targeting lipid metabolism in T-cells is a promising autoimmune treatment [61]. TCR signal is initiated by phosphorylation of motifs (ITAMs) contained within cytoplasmic domains of the invariant subunits; ITAMs are critical to initiate signaling following ligand engagement [62]. Unexpectedly, Gaud, et al. found that T cells expressing TCRs containing inactivated CD3ζ ITAMs, exhibited reduced ability to discriminate between low- and high-affinity ligands [63].
Our work provides new evidence of dramatic change of subcellular surface receptor topology proteins in response to mechanical forces and thermally induced membrane fluctuation, which mediate tolerance to immune activation in response to heat. Al-Aghbar, et al. found that surface receptor topology can exert mechanical forces to induce curvature around engaged p MHC/TCR complexes to release CD3 which induced tolerance to immune activation [64]. It was seen that the rapid influx of Ca2+ may compete with negatively charged phospholipids, thus releasing CD3 ITAMs from plasma membrane [65].
In summary, CO2 modulates MHC/TCR complexes between the T cell-APC interface through; 1st. Thermally induced mechanically forced membrane fluctuation induced curvature around engaged p MHC/TCR complexes which releases CD3 cytoplasmic domains. Second, CO2 protonation of negatively charged phospholipids released CD3 ITAMs from membranes. The overall outcome may affect ligand affinity and discriminative ability between self and non-self-antigens. 3rd, CO2 thermal effect is linked to cellular stress signaling pathways, like heat shock proteins, with expected role in ribosomal DNA gene translation to protein due to subcellular mobilization [66]. 4th, acidic microenvironment has direct effect on shaping T-cell biology through affecting Ca2+ responses to TCR stimulation for acquisition of T cell full functional competence to avoid autoimmunity. The outcome would blunt immunity. 5th, Yang, et al. [67], discussed that low pH inhibits IFNγ → upregulated Th17 cells which are involved in pathogenesis of diverse autoimmune diseases.
In conclusion, CO2 plays a major role in orchestrating the immunologic response after a major insult of disturbed Ca2+ homeostasis.
Other studies have yielded contradictory findings to our conclusion. A meta-analysis stated that Methylenetetrahydrofolate reductase polymorphisms (MTHFR 677 C/T) were risk factor for autoimmunity [68]. The small number of studies included and heterogeneity among studies did affect their results. Sample size is still moderate, adding weak evidence of “MTHFR” effect on autoimmunity.
Our results explored surprising association between H. pylori infection and extra gastric autoimmune, chronic urticaria. We suggest that urease enzyme produced NH3 and CO2 from urea. Gradual build-up of CO2 develops extra gastric autoimmune diseases, including urticaria as evidenced by studies [69] which described that H. pylori elicit numerous adaptive mechanisms. Urease seems to be the most virulence factor essential for H. pylori colonization and survival.
Our study has several methodological strengths. 1st, study groups were obtained from hospitals, sample size was relatively large in terms of case control measures, and data results are reflective of target population, with a minimal likelihood of loss to follow-up cases, and recall bias. 2nd, we employed demographic matching of cases and controls to minimize potential confounders. 3rd, personal bias could not apply to blood work.
The CO2-induced gastrointestinal manifestations can be explained considering Abdelrazak’ studies, who discovered; H. pylori caused infantile colic, and demonstrated that H pylori LPS–activated TLR4 releases IL-8 from gastric epithelia, initiating inflammatory damage. The interaction between host immune factors and H. pylori virulence factors determines the outcome of H. pylori infection [69]. Furthermore, this course induces Ca2 influx which prolongs IL-8-induced CO2 signals [70].
Our results contradict molecular mimicry. Studies have conflicting opinions as large number of infectious agents may prevent autoimmune disease. Hygiene hypothesis theory breaches integrity of molecular mimicry [71]. Evidently, we propose that molecular mimicry is not a pathogenic mechanism of autoimmune diseases, rather, the specific inflammatory mediators associated with increased expression of autoreactivity. For instance, Komastu, et al. showed that Th1 CD4+ cells induced IL-12, are critical factors in viral immunity [72]. Callahan, et al. presented that during Campylobacter infection, dendritic cells and macrophages present processed C. jejuni antigens to T lymphocytes, which develop into Th1, Th17 CD4+ T lymphocytes, with secretion of IFN-γ and IL-17 [73], which are characterized by promoting post-inflammatory autoimmune Guillan-Barre syndrome. Furthermore, Soderholm, et al. reported that the Th1 and Th17 responses play significant roles in adaptive immunity to Group A Streptococcus pharyngitis [74], which causes rheumatic fever and rheumatic chorea.
Conclusion
The implications of this study are far-reaching, as it opens avenue of research in the pathogenesis, treatment, and prevention of autoimmune disorders. With the rapid accumulation of immunological knowledge, it will predict that improvement in the issues of selectivity, efficacy, and pharmacokinetics of the immune modulator drugs. We found that CO2 can induce initiation, stabilization, and maintenance of the autoimmune reaction.
- CO2 penetrates cell membranes to act as an electric drill with the result of forcing Ca2 to get inside the cells against their concentration gradient “CO2 drill like action”. This increased intracellular calcium influx augments the second messenger CO2 signal that just has made by its immediate intracellular entry. The CO2 induced heating reversibly alters the electrical capacitance of the plasma membrane, depolarizing the target cell to affect ion channel gating, and triggering thermosensitive ion channels forming membrane pores.
- The mechanism of autoimmune induction by both thermal and protonation effects unique to CO2, was shown to influence both TCR and BCR integrity and function of T and B cells respectively. It is caused via cellular cholesterol modulation and topological variations between TCRs and other membrane proteins which induce membrane bending and micro adhesion rings.
- Somatically mutated high-affinity autoantibodies are produced through the CO2 thermal cytosine deamination and protonation, to dissociate phosphate groups which attack cytosine amino group. On the other hand, the CO2 induction of mutated antibodies may be further catalyzed by the “MHC” susceptibility genes for autoimmune diseases.
Future Perspectives
- Continued investigation of the role of CO2 in the pathogenesis of various autoimmune diseases, should represent the most important intent in future studies. The precise determination of genes involved in immune mediated CO2 pathways along with prediction of possible mutations and their effects on immune response must be considered.
- The pivotal role of CO2 in eliciting immune response makes this identification more essential to increase understanding of mechanisms underlying the affinity of autoantibodies to self-antigens and hence improving the design of therapeutic monoclonal antibodies. Hopefully, the evidence presented herein linking CO2 to the pathogenesis of autoimmune diseases will encourage investigators of clinical trials to find new methods to overcome the undesired issues of antibodies against urease. The aim should aid in designing future studies of urease inhibitors to develop efficient new generations with the least possible side effects. It will uncover new avenue to eradicate H pylori and its dangerous complications. In addition, it will abort the development of autoimmune disorders because demolishing urease can stop sustained CO2 build-up, thus prevent autoimmune process, and make H pylori survival unlikely. Pending such studies, debate will continue regarding potential benefits and harms of hypercapnia, and whether therapeutic hypocapnia or normocapnia holds the best promise.
- Attention should be paid to a new line of therapies that include modalities to deplete CO2 rise and to compete with its intracellular diffusion by employing hyperbaric oxygen to neutralize CO2 deleterious effects. Overall, we present a versatile modality that may be broadly applicable in pharmaceutical industries, including diverse strategic mechanisms to search for immunologic modulators of both autoimmune disorders and cancer.
- To address the issue of various autoimmune mediated Ca2+channels and their precise targeted drug delivery, we must ensure the specificity of developed drugs for the Ca2+ channels to reduce cross-reactivity and damage to other tissues. We should be aware of the mechanisms by which drugs can more effectively modulate a specific Ca2+
- Our study raises multiple questions that should inspire further research dedicated to the processing of cellular channels, pathways and altered molecular receptor proteins, to target their consequent damaging effects.
Funding
The authors assure that they never received funding assistance from either individuals or organizations.
Acknowledgment
We thank Ahmed Ali, the expert in information system, and Shehab Ali, the expert in computer science for the discussions and help to this study.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare no conflict of interest.
References
- Atkins P, De Paula J. Physical chemistry.Macmillan; 2006 Mar 10.
- Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev. 2000 Jan 4; 80(2):681-715.
Google Scholar, Crossref, Indexed at
- Almanza-Hurtado A, Polanco Guerra C, Martínez-Ávila MC, et al. Hypercapnia from physiology to practice. Int J Clin Pract. 2022;2022(1):2635616.
Google Scholar, Crossref, Indexed at
- Shapiro MG, Homma K, Villarreal S, et al. Infrared light excites cells by changing their electrical capacitance. Nat Commun. 2012 Jan; 3(1):736.
Google Scholar, Crossref, Indexed at
- Garcia III AJ, Ramirez JM. Keeping carbon dioxide in check. Elife. 2017 May 17;6:e27563.
Google Scholar, Crossref, Indexed at
- Byung-Chul Oh. Phosphoinositides and intracellular calcium signaling: Novel insights into phosphoinositides and calcium coupling as negative regulators of cellular signaling. Exp Mol Med. 2023 Aug;55(8):1702-1712.
Google Scholar, Crossref, Indexed at
- Moccia F, Fiorio Pla A, Lim D, et al. Intracellular Ca2+ signalling: Unexpected new roles for the usual suspect. Front Physiol. 2023 Jul 27;14:1210085.
Google Scholar, Crossref, Indexed at
- Bootman MD, Bultynck G. Fundamentals of cellular calcium signaling: a primer. Cold Spring Harb Perspect Biol. 2020 Jan 1;12(1):a038802.
Google Scholar, Crossref, Indexed at
- Bkaily G, Jacques D. Calcium homeostasis, transporters, and blockers in health and diseases of the cardiovascular system. Int J Mol Sci. 2023 May 15;24(10):8803.
Google Scholar, Crossref, Indexed at
- Fahrner M, Grabmayr H, Romanin C. Mechanism of STIM activation. Curr Opin Physiol. 2020 Oct 1;17:74-9.
Google Scholar, Crossref, Indexed at
- Soboloff, J, Rothberg BS, Madesh M, et al. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012 Sep;13(9):549-565.
Google Scholar, Crossref, Indexed at
- Trebak, M, Kinet JP. Calcium signalling in T cells. Nat Rev Immunol. 2019 Mar;19(3):154-169.
Google Scholar, Crossref, Indexed at
- Kuo IY, Ehrlich BE. Location, location, and activation of a channel by calcium. Proc Natl Acad Sci. 2022 Oct 25;119(43):e2214826119.
Google Scholar, Crossref, Indexed at
- Davern, M, Donlon NE, O’Connell F, et al Acidosis significantly alters immune checkpoint expression profiles of T cells from oesophageal adenocarcinoma patients. Cancer Immunol Immunother. 2023 Jan;72(1):55-71.
Google Scholar, Crossref, Indexed at
- Navarro F, Casares N, Martín-Otal C, et al. Overcoming T cell dysfunction in acidic pH to enhance adoptive T cell transfer immunotherapy. Onco Immunology. 2022 Dec 31;11(1):2070337.
Google Scholar, Crossref, Indexed at
- Meng, L., Wu H, Wu J, et al. Mechanisms of immune checkpoint inhibitors: Insights into the regulation of circular RNAS involved in cancer hallmarks. Cell Death Dis. 2024 Jan 4;15(1):3.
Google Scholar, Crossref, Indexed at
- Sun, Q, Hong Z, Zhang C, et al. Immune checkpoint therapy for solid tumours: Clinical dilemmas and future trends. Sig Transduct Target Ther. 2023 Aug 28;8(1):320.
Google Scholar, Crossref, Indexed at
- Tai X, Van Laethem F, Sharpe AH, et al. Induction of autoimmune disease in CTLA-4−/− mice depends on a specific CD28 motif that is required for in vivo costimulation. Proc Natl Acad Sci. 2007 Aug 21;104(34):13756-13761.
Google Scholar, Crossref, Indexed at
- Izquierdo JH, Bonilla-Abadía F, Cañas CA, et al. Calcium, channels, intracellular signaling and autoimmunity. Reumatol Clin. (English Edition). 2014 Jan 1;10(1):43-47.
Google Scholar, Crossref, Indexed at
- Park YJ, Yoo SA, Kim M, et al. The role of calcium–calcineurin–NFAT signaling pathway in health and autoimmune diseases. Front Immunol. 2020 Mar 10;11:195.
Google Scholar, Crossref, Indexed at
- Watad A, Azrielant S, Bragazzi NL, et al. Seasonality and autoimmune diseases: The contribution of the four seasons to the mosaic of autoimmunity. J Autoimmun. 2017 Aug 1;82:13-30.
Google Scholar, Crossref, Indexed at
- Phelan DE, Mota C, Lai C, et al. Carbon dioxide-dependent signal transduction in mammalian systems. Interface Focus. 2021 Feb 12;11(2):20200033.
Google Scholar, Crossref, Indexed at
- Naseem A, Yuyang S, Viviane ND, et al. Differential activation of Ca 2+ influx channels modulate stem cell potency, their proliferation/viability and tissue regeneration. NPJ Regen Med. 2021;6(1).
Google Scholar, Crossref, Indexed at
- Mistrik M, Skrott Z, Muller P, et al. Microthermal-induced subcellular-targeted protein damage in cells on plasmonic nanosilver-modified surfaces evokes a two-phase HSP-p97/VCP response. Nat Commun. 2021 Jan 29;12(1):713.
Google Scholar, Crossref, Indexed at
- De Nadal E, Ammerer G, Posas F. Controlling gene expression in response to stress. Nat Rev Genet. 2011 Dec;12(12):833-845.
Google Scholar, Crossref, Indexed at
- Tsai SR, Hamblin MR. Biological effects and medical applications of infrared radiation. J Photochem Photobiol B. 2017 May 1;170:197-207.
Google Scholar, Crossref, Indexed at
- Thiel G, Schmidt T, Rössler OG. Ca2+ microdomains, calcineurin and the regulation of gene transcription. Cells. 2021 Apr 12;10(4):875.
Google Scholar, Crossref, Indexed at
- Yeh YC, Parekh AB. CRAC channels and Ca2+-dependent gene expression. Calcium Entry Channels in Non-Excitable Cells. 2017 Jul 14:93-106.
Google Scholar, Crossref, Indexed at
- Guo Q, Jin Y, Chen X, et al. NF-κB in biology and targeted therapy: New insights and translational implications. Sig Transduct Target Ther. 2024 Mar 4;9(1):1-37.
Google Scholar, Crossref, Indexed at
- Hossen MM, Ma Y, Yin Z, et al. Current understanding of CTLA-4: From mechanism to autoimmune diseases. Front Immunol. 2023 Jul 11;14:1198365.
Google Scholar, Crossref, Indexed at
- Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans.Immunity.2013 Jun 27;38(6):1092-1104.
Google Scholar, Crossref, Indexed at
- Casalino-Matsuda, SM, Wang N, Ruhoff PT, et al. Hypercapnia alters expression of immune response, nucleosome assembly and lipid metabolism genes in differentiated human bronchial epithelial cells. Sci Rep. 2018 Sep 10;8(1):13508.
Google Scholar, Crossref, Indexed at
- Winiewska-Szajewska M, Paprocki D, Marzec E, et al. Effect of histidine protonation state on ligand binding at the ATP-binding site of human protein kinase CK2. Sci Rep. 2024 Jan 17;14(1):1463.
Google Scholar, Crossref, Indexed at
- Marchesan E, Nardin A, Mauri S, et al. Activation of Ca2+ phosphatase Calcineurin regulates Parkin translocation to mitochondria and mitophagy in flies. Cell Death Differ. 2024 Jan 18:1-22.
Google Scholar, Crossref, Indexed at
- Klein SG, Alsolami SM, Arossa S, et al. In situ monitoring reveals cellular environmental instabilities in human pluripotent stem cell culture. Commun Biol. 2022 Feb 8;5(1):119.
Google Scholar, Crossref, Indexed at
- Armstrong B. Antigen–antibody reactions. ISBT Science Series. 2008 Jun;3(2):21-32.
Google Scholar
- Jespersen MC, Mahajan S, Peters B, et al. Antibody specific B-cell epitope predictions: Leveraging information from antibody-antigen protein complexes. Front Immunol. 2019 Feb 26;10:434287.
Google Scholar, Crossref, Indexed at
- Akbar R, Robert PA, Pavlović M, et al. A compact vocabulary of paratope-epitope interactions enables predictability of antibody-antigen binding. Cell Rep. 2021 Mar 16;34(11).
Google Scholar, Crossref, Indexed at
- Reverberi R, Reverberi L. Factors affecting the antigen-antibody reaction. Blood Transfus. 2007 Oct;5(4):227.
Google Scholar, Crossref, Indexed at
- Parija SC. Antigen–Antibody Reactions. Textbook of Microbiology and Immunology 2023 Mar 17: 189-209.
Google Scholar
- Devanaboyina SC, Lynch SM, Ober RJ, et al. The effect of pH dependence of antibody-antigen interactions on subcellular trafficking dynamics. In MAbs 2013 Nov 5(6): 851-859.
Google Scholar, Crossref, Indexed at
- Hironiwa N, Ishii S, Kadono S, et al. Calcium-dependent antigen binding as a novel modality for antibody recycling by endosomal antigen dissociation. InMAbs 2016 Jan 8(1): 65-73.
Google Scholar, Crossref, Indexed at
- Boswell CA, Tesar DB, Mukhyala K, et al. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem. 2010 Dec 15;21(12):2153-2163.
Google Scholar, Crossref, Indexed at
- Stank A, Kokh DB, Fuller JC, et al. Protein binding pocket dynamics. Acc Chem Res. 2016 May 17;49(5):809-815.
Google Scholar, Crossref, Indexed at
- Wang SW, Ko YA, Chen CY, et al. Mechanism of antigen presentation and specificity of antibody cross-reactivity elicited by an oligosaccharide-conjugate cancer vaccine. J Am Chem Soc. 2023 Apr 24;145(17):9840-9849.
Google Scholar, Crossref, Indexed at
- Vedadghavami A, Zhang C, Bajpayee AG. Overcoming negatively charged tissue barriers: Drug delivery using cationic peptides and proteins. Nano Today. 2020 Oct 1;34:100898.
Google Scholar, Crossref, Indexed at
- Leal J, Smyth HD, Ghosh D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm. 2017 Oct 30;532(1):555-572.
Google Scholar, Crossref, Indexed at
- Garcia-Gil M, Camici M, Allegrini S, et al. Metabolic aspects of adenosine functions in the brain. Front Pharmacol. 2021 May 14;12:672182.
Google Scholar, Crossref, Indexed at
- McGargill MA, Derbinski JM, Hogquist KA. Receptor editing in developing T cells. Nat Immunol. 2000 Oct;1(4):336-341.
Google Scholar, Crossref, Indexed at
- Wucherpfennig KW, Call MJ, Deng L, et al. Structural alterations in peptide–MHC recognition by self-reactive T cell receptors. Curr Opin Immunol. 2009 Dec 1;21(6):590-595.
Google Scholar, Crossref, Indexed at
- Strowitzki MJ, Nelson R, Garcia MP, et al. Carbon dioxide sensing by immune cells occurs through carbonic anhydrase 2–dependent changes in intracellular pH. J Immunol. 2022 May 15;208(10):2363-2375.
Google Scholar, Crossref, Indexed at
- Solouki S, Huang W, Elmore J, et al. TCR signal strength and antigen affinity regulate CD8+ memory T cells. J Immunol. 2020 Sep 1;205(5):1217-1227.
Google Scholar, Crossref, Indexed at
- Raza IG, Clarke AJ. B cell metabolism and autophagy in autoimmunity. Front Immunol. 2021 Jun 7;12:681105.
Google Scholar, Crossref, Indexed at
- Jaiswal A, Roy R, Tamrakar A, et al. Activation-induced cytidine deaminase an antibody diversification enzyme interacts with chromatin modifier UBN1 in B-cells. Sci Rep. 2023 Nov 10;13(1):19615.
Google Scholar, Crossref, Indexed at
- Shiraz AK, Panther EJ, Reilly CM. Altered germinal-center metabolism in B cells in autoimmunity. Metabolites. 2022 Jan 5;12(1):40.
Google Scholar, Crossref, Indexed at
- Ehrlich M, Norris KF, Wang RY, et al. DNA cytosine methylation and heat-induced deamination. Biosci Rep. 1986 Apr;6:387-393.
Google Scholar, Crossref, Indexed at
- Sethi S, Takashima Y, Nakamura S, et al. Acceleration of the Deamination of Cytosine through Photo-Crosslinking. Current Issues in Molecular Biology. 2023 May 29;45(6):4687-4700.
Google Scholar, Crossref, Indexed at
- Mikocziova I, Greiff V, Sollid LM. Immunoglobulin germline gene variation and its impact on human disease. Genes Immun. 2021 Aug;22(4):205-217.
Google Scholar, Crossref, Indexed at
- Zhao D, Chen J, Hu X, et al. Catalytic antibodies: Design, expression, and their applications in medicine. Appl Biochem Biotechnol. 2023 Feb;195(2):1514-1540.
Google Scholar, Crossref, Indexed at
- Bolshette N, Ezagouri S, Dandavate V, et al. Carbon dioxide regulates cholesterol levels through SREBP2. PLo S Biology. 2023 Nov 15;21(11):e3002367.
Google Scholar, Crossref, Indexed at
- Robinson GA, Waddington KE, Pineda-Torra I, et al. Transcriptional regulation of T-cell lipid metabolism: implications for plasma membrane lipid rafts and T-cell function. Front Immunol. 2017 Nov 24;8:314843.
Google Scholar, Crossref, Indexed at
- Horváth Á, Erostyák J, Szőke É. Effect of Lipid Raft Disruptors on Cell Membrane Fluidity Studied by Fluorescence Spectroscopy. Int J Mol Sci. 2022 Nov 8;23(22):13729.
Google Scholar, Crossref, Indexed at
- Gaud G, Achar S, Bourassa FX, et al. CD3ζ ITAMs enable ligand discrimination and antagonism by inhibiting TCR signaling in response to low-affinity peptides. Nat Immunol. 2023 Dec;24(12):2121-2134.
Google Scholar, Crossref, Indexed at
- Al-Aghbar MA, Jainarayanan AK, Dustin ML, et al. The interplay between membrane topology and mechanical forces in regulating T cell receptor activity. Commun Biol. 2022 Jan 11;5(1):40.
Google Scholar, Crossref, Indexed at
- Shi X, Bi Y, Yang W, et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature. 2013 Jan 3;493(7430):111-115.
Google Scholar, Crossref, Indexed at
- Chatterjee TK, Fisher RA. Mild heat and proteotoxic stress promote unique subcellular trafficking and nucleolar accumulation of RGS6 and other RGS proteins: Role of the RGS domain in stress-induced trafficking of RGS proteins. J Biol Chem. 2003 Aug 8;278(32):30272-30282.
Google Scholar, Crossref, Indexed at
- Yang P, Sun Y, Zhang M, et al. The inhibition of CD4+ T cell proinflammatory response by lactic acid is independent of monocarboxylate transporter 1. Scand J Immunol. 2021 Dec;94(6):e13103.
Google Scholar, Crossref, Indexed at
- Lu M, Peng K, Song L, et al. Association between Genetic Polymorphisms in Methylenetetrahydrofolate Reductase and Risk of Autoimmune Diseases: A Systematic Review and Meta‐Analysis. Dis Markers. 2022;2022(1):4568145.
Google Scholar, Crossref, Indexed at
- Ali AM. Helicobacter pylori and infantile colic. Arch Pediatr Adolesc Med. 2012 Jul 1;166(7):648-50.
Google Scholar, Crossref, Indexed at
- Schorr W, Swandulla D, Zeilhofer HU. Mechanisms of IL‐8‐induced Ca2+ signaling in human neutrophil granulocytes. Eur J Immunol. 1999 Mar;29(3):897-904.
Google Scholar, Crossref, Indexed at
- Bach JF. Revisiting the hygiene hypothesis in the context of autoimmunity. Front Immunol. 2021 Jan 28;11:615192.
Google Scholar, Crossref, Indexed at
- Komastu T, Ireland DD, Reiss CS. IL-12 and viral infections. Cytokine Growth Factor Rev. 1998 Dec 1;9(3-4):277-285.
Google Scholar, Crossref, Indexed at
- Callahan SM, Dolislager CG, Johnson JG. The host cellular immune response to infection by Campylobacter spp. and its role in disease. Infect Immun. 2021 Jul 15;89(8).
Google Scholar, Crossref, Indexed at
- Soderholm AT, Barnett TC, Sweet MJ, et al. Group A streptococcal pharyngitis: Immune responses involved in bacterial clearance and GAS-associated immunopathologies. J Leukoc Biol. 2018 Feb;103(2):193-213.
Google Scholar, Crossref, Indexed at







