Key words
Allergy, Anisakis species, IgE, flow cytometry
Introduction
Fish play an important role in human nutrition; they are valuable sources of proteins and contain large amounts of healthy fats (so called polyunsaturated fatty acids) and fat-soluble vitamins. However, they are also one of the most common causes of food allergy [1]. Common fish such as herring, tilapia, tuna, salmon and mackerel have all been linked with allergic reactions [1, 2].
Fish allergy is a so-called immunoglobulin E (IgE)-mediated food allergy. It is caused by a reaction to a muscle protein called parvalbumin, present in fish meat. People who are allergic to one type of fish, will also react to other types of fish, because the protein allergens of different fish are similar.This major allergen is extremely stable to heat, which means that boiling or frying of fish does not destroy the allergen [1, 2]. An adverse reaction linked to fish consumption can be also an IgE-mediated allergy to parasites infecting fish and not to fish meat. Since the parasitic infection of fish is common, this obviously has a diagnostic importance in reactions to seafood. However, a number of parasites, especially helminths, cause synthesis of IgE antibodies against a wide range of proteins released by the parasites during the stage of their life cycle spent in humans. Such parasitic proteins show many immunological similarities to common inhaled or ingested allergens [1, 2]. This parasite-specific IgE antibody is part of the defense system against foreign invaders, and hence protective. At the same time, it also participates in the pathological mechanisms behind a range of inflammatory reactions related to parasite infection [2, 3].
IgE is typically the least abundant isotype. Blood serum IgE level in a normal (non-atopic) individual is only 0.05% of the IgG concentration, it is less than 150 ng/ml, compared to 10 mg/ ml for the IgG [4].
Anisakis is a nematode parasitizing marine fish, which induces two major problems in humans; infection (anisakiasis), characterized by epigastric pain, nausea, vomiting and diarrhea; and allergic hypersensitivity reactions like urticaria and anaphylaxis. The reactions are mediated by IgE antibodies to Anisakis antigens, but were in the past often mistaken as fish allergy [5]. Various studies have shown that 80% of fish could be infested with this parasite [5, 6]. Humans acquire infection with the third stage larva of these parasites by eating raw or undercooked fish. The family Anisakidae includes different genera. There are in fact two types of Anisakis, Type I and Type II, and two types of Pseudoterranova, often referred to as cod worm, and Contracaecum. The commonest species is Anisakis Type I, namely Anisakis simplex. All these species were reported to infect humans in a similar mode as Anisakis simplex [7 – 10].
Although cooking at 70 0 C or freezing to - 20 0 C for 72 hours are believed to destroy the infectivity of this parasite, the allergenic capacity of its denaturized protein is not eliminated [11, 12]. The secretory antigens produced by the worms can induce antibody production against the worms even following ingestion of fully cooked fish containing Anisakis antigen [13]. Allergic conjunctivitis and asthma in persons handling fish meals also seem to occur due to the presence of anti-Anisakis antibodies [14]. It has been postulated that Anisakis allergy may be more prevalent than any specific food allergy in the adult population and comprises as much as 10% of idiopathic anaphylaxis [15].
Various immunological methods have been used for the detection of IgE, such as ELISA, immunoblotting, latex-agglutination test and flow cytometry [6, 11, 13, 15, 16]. The latter is a measurement of characteristics of single cells suspended in a flowing saline stream. A focused beam of laser light illuminates each moving cell and light is scattered in all directions, this is picked up by detectors and converted into a suitable form for computer analysis and interpretation. These profiles of cells are normally displayed as dot plots or histograms [17]. To the best of our knowledge, no reports were available about the efficacy of flow cytometry for the detection of the diversity in IgE response against Anisakis species parasitizing fish in lymphocytes of splenic suspensions.
The present work was designed to study the immunological response in experimental mice, through measurement of IgE antibodies in their lymphocytes, following the ingestion of Anisakis antigen using flow cytometry.
Methods
Parasites
Third stage larvae (L3) of Anisakis species were extracted manually from viscera and body cavity of herring purchased in local markets, and were counted individually [6, 9]. They were identified by being 3-4 cm in length and whitish in colour (Figure 1, 2). Under the microscope, each larva possessed a spicule at the anterior end (Figure 3).
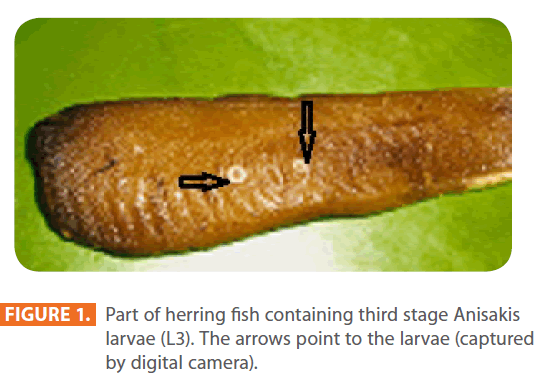
Figure 1: Part of herring fish containing third stage Anisakis larvae (L3). The arrows point to the larvae (captured by digital camera).
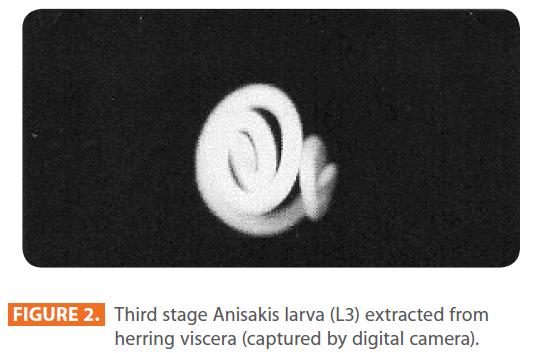
Figure 2: Third stage Anisakis larva (L3) extracted from herring viscera (captured by digital camera).
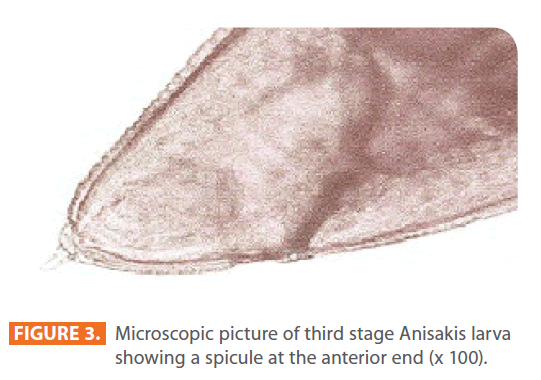
Figure 3: Microscopic picture of third stage Anisakis larva showing a spicule at the anterior end (x 100).
Preparation of Anisakis antigen
Three hundred larvae were grounded in a mortar with 10 ml phosphate buffer saline (PBS). The mixture was centrifuged for 15 minutes at 4500 x g and the pellet was discarded. The supernatant was separated, and its protein content was 2mg/ml as measured by the method of Bradford [18]. The supernatant was stored at - 20 o C without any addition as a crude extract [11, 15].
Animals
The present work was carried out on sixty male Swiss albino mice, three to five weeks of age, weighing 20 to 25 grams. They were kept in suitable cages with perforated covers, fed on standard pellet food and water in addition to libitum. They were free of parasites as shown by repeated stool examination by direct wet saline smear, iodine and Sheather’s sugar flotation method. Stool examination was performed before antigen inoculation, as well as weekly post inoculation till the end of the study. Animals were bred in the laboratory of the Parasitology Department. This animal study was approved by the Ethics Committee of Alexandria University. They were divided equally into two main groups:
Group I: Control group, included 30 non infected mice.
Group II: Experimental group, included 30 mice which were inoculated orally with Anisakis antigen by intra-gastric gavage [15, 19]. The dose was calculated to be 0.1 ml / mouse according to a pilot study. In the pilot study, three doses were tested initially; 0.05 ml, 0.1ml and 0.15ml. It was discovered that 0.5 ml was not enough to produce sensitization for the mice, thus it was discarded. On the other hand, both 0.1ml and 0.15 ml were sensitizing to the mice. Thus, we selected the minimal one which is the 0.1ml as an optimal concentration.
Both groups were subdivided into five subgroups (six mice each) according to the duration of sacrifice; zero, 1st, 3rd, 5th and 7th weeks post inoculation. All animals were sacrificed by an over dose of ether.
The spleens of mice of all subgroups were aseptically collected at the same duration of sacrifice. They were preserved at - 20o C to be processed for the detection of IgE antibodies in their lymphocytes by flow cytometry [16, 20].
Preparation of splenic suspensions
Suspensions were prepared by forcing the splenic tissues through a fine wire mesh sieve, using a piston of five ml disposable syringe. This was done to retain the connective tissue capsule of the spleen in the mesh sieve. This was followed by several extensive washes with PBS till a suspension was obtained. All obtained cell suspensions were maintained on ice to preserve their viability.
Lymphocytes were then separated from splenic suspensions by differential centrifugation on a density gradient as follows. In sterile 15 ml conical centrifuge tubes, three ml splenic suspensions were carefully layered over three ml sterile FicolHypaque, and centrifuged at 400 x g for 30 minutes. The lymphocytes mononuclear cell layer was cautiously collected by a sterile Pasteur pipette and washed thrice in PBS. Each wash was done by centrifugation at 400 x g for ten minutes. The supernatant fluid was aspirated and discarded, after each wash. The cell pellets of each subgroup of mice (6 mice) were collected and pooled together, and were resuspended in a dose of 5 x 105 cells / tube in three ml PBS and subjected to immunofluorescent staining. Fluorochrome intensity was measured by monoparameter flow cytometry [16, 20 – 24].
Detection of IgE antibodies was performed using Fluorescein isothiocyanate (FITC) anti – mouse IgE (23G3) PE (eBioscience, San Diego, California). The 23G3 monoclonal antibody reacts only with the heavy chain of mouse IgE, and not with any other class of mouse immunoglobulins.
Flow cytometry
Lymphocyte samples were analyzed on a FACS Calibur flow cytometer Becton Dickinson equipped with an argon-ion laser apparatus operating at 488 nm. The flow cytometer was calibrated by using CaliBRITE beads (Becton Dickinson, Mississauga, Ontario, Canada) and the samples were analyzed using monoparametric (monobasic) histograms. Surface marker analysis was conducted, after gating for forward and side scatter, using Cell Quest software program. It took about two minutes for the apparatus to process each sample and show the results on the computer screen [16, 20, 25].
Results
After staining the samples with specific fluorochromes (FITC), they were analyzed using monoparameter flow cytometry. All particles found in the samples appeared on a graph as dot plots (Figure 4). Beside this graph, a monobasic histogram appears on the screen of the computer. The x-axis of the histogram represents the intensity of the fluorescence detected by the positive cells according to the Cell Quest program, and the y-axis represents the number of cells read by the apparatus. Examples of negative and positive histograms are shown in figure 5 (a & b) respectively.
Figure 4: Flow cytometry graph showing dots representing a positive sample.
The arrow points to the line of the marker. In the control group (negative), the line of the marker was laying on the x-axis of the histogram (figure 5a). While in the experimental group (positive), the line of the marker was evident, appearing above the x-axis and passing through the graph (figure 5b). The more the line of the marker passes through the graph, the higher the percentage of IgE antibodies.
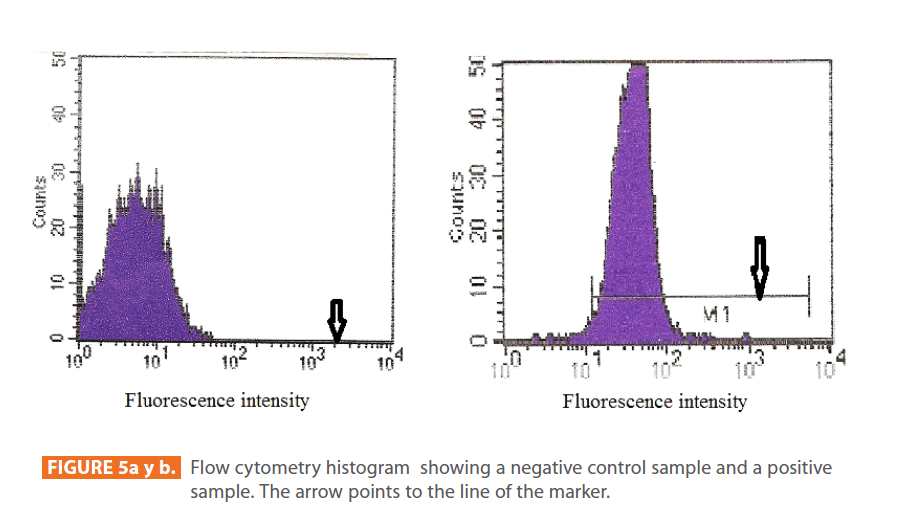
Figure 5a y b: Flow cytometry histogram showing a negative control sample and a positive sample. The arrow points to the line of the marker.
The percentage of IgE antibodies was enhanced in lymphocytes of animals exposed to Anisakis antigen from the first week (14%), peaking three weeks following initial exposure (29%), and starting to decrease by week five (26%). By the seventh week post-exposure, they declined to (3%). While in the control group, IgE remained more or less at the same level throughout the duration of the study, namely about 1% (Figure 6).
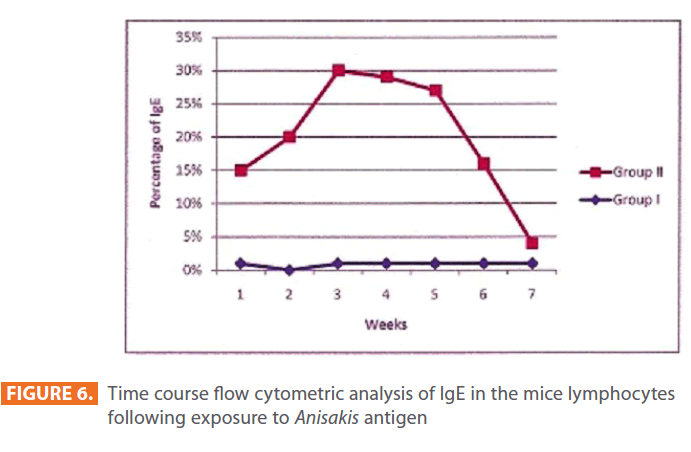
Figure 6: Time course flow cytometric analysis of IgE in the mice lymphocytes following exposure to Anisakis antigen
Discussion
Infection of humans with Anisakis species was first described in the 1960s in association with the consumption of raw or undercooked fish. During the 1990s it was realized that the ingestion of dead worms in fish can cause severe hypersensitivity reactions. These may be more prevalent than the infection itself, and this outcome could be associated with food preparations which were previously considered safe. Allergic symptoms are due to the presence of IgE antibodies that constitute an important component of the immune response to the parasite. The in vivo (skin-prick tests) and/or in vitro level of specific IgE are usually measured for diagnosis of sensitization to the parasite, and it is accepted that these determinations are highly sensitive methods [13, 14, 26, 27].
Measurement of IgE antibodies can aid in the diagnosis of parasitosis, as well as, potentially even more important, it can contribute essential information to the investigation of several allergic conditions, including fish allergy [15, 28]. Epidemiological and immunological studies indicate contribution of helminthes infection to the development of asthmatic conditions, depending on a direct relation which was present between the degree of helminthes infection and an increase in peak expiratory flow by the bronchodilator [29]. On the other hand, some authors suggest that the production of the non-specific IgE by helminthes infections can suppress the allergic response to environmental and parasite allergens via protection from mast cell or basophil degranulation by saturating IgE binding sites [30, 31]. However, in our study the Anisakis antigen was prepared from dead Anisakis larvae. Thus, there is no possibility that the produced IgE was related to parasitic infections. Furthermore, the detected IgE could not be due to a fish allergy caused by a reaction to the parvalbumin, present in fish meat, as the Anisakis larvae used in the present study were extracted manually from viscera and body cavity of herring, and no fish meat was given to mice.
In the present work, a murine model of allergy was generated by sensitization with proteins of Anisakis larval antigen. This model exhibited characteristics of sensitivity reaction very similar to those observed in humans in the form of enhancement in the IgE level. These findings were also observed by Iglesias et al.; 1995 [6] and Baeza et al.; 2005 [15], who reported that mouse specific IgE pattern had similarities with that detected in humans. They also reported that the histological and haematological alterations observed in infected mice, are similar to those occurring in humans.
The IgE level was enhanced in lymphocytes of splenic suspensions of animals exposed to Anisakis antigen from the first week of exposure, peaking after three weeks and decreased to a low level which was nearly similar to that of the control group at the seventh week post-exposure. This coincided with Akao and Yoshimura, 1989 [13] who reported that using ELISA assay, IgE antibodies were detectable as early as one week, following Anisakis simplex infections, and they disappeared as late as 35 days post-infection. They also reported that the antigens involved in IgE production were not affected by freezing or cooking temperatures, enhancing the same IgE pattern as that produced following infection. Furthermore, Manetz and Meade, 1999 [16] recorded an increase in the level of IgE by flow cytometry in the draining lymph nodes and spleen of animals eight days following sensitization with an IgE inducing allergens other than Anisakis antigen, and then, the IgE level returned to its normal level within the second month of sensitization.
Several methods have been used for detection of hypersensitivity reactions to Anisakis simplex proteins, such as ELISA, immunoblotting, latex-agglutination test [6, 11, 15, 31, 32]. However, few studies applied flow cytometry to diagnose Anisakis allergy, but using samples other than lymphocytes. Gónzalez- Muñoz et al; 2005 [33] detected allergen-induced basophil activation (BAT) by flow cytometry, using whole-blood samples and live basophils, detecting activation-associated membrane markers (CD63) of antigen binding to the IgE high-affinity receptor in response to Anisakis simplex crude extract. A significant activation of basophils (98%) was found for patients allergic to Anisakis simplex versus controls (3 %).
In the present study, the flow cytometric analysis was very rapid and proved to be a very suitable tool for detecting the lymphocytic IgE antibodies against Anisakis allergy. Similar findings about the reliability of the flow cytometry technique have been published by others. Toma et al.; 1996 [34] demonstrated that flow cytometric measurement of basophilbound IgE provided a useful method of analyzing the atopic cell population within the peripheral circulation, and it served as a critical parameter to evaluate the allergic inflammation in vivo. Moreover, Saite-Laudy et al.; 2000 [35] reported that flow cytometry showed a high sensitivity and a high specificity in diagnosis of venom allergy.
In conclusion, we have generated a murine model of sensitization to Anisakis proteins, which opens new possibilities to study the human allergic reactions to parasite antigens. The results of the present study would explain the high percentage of sensitization against Anisakis species, and demonstrate the usefulness of flow cytometry for immunological diagnosis of Anisakis allergy.
Conflict of Interest: Nil
Funding: This research was entirely funded by the Department of Parasitology, Faculty of Medicine, Alexandria University, Egypt.
215
References
- Lindqvist A, Ikezawa Z, Tanaka A, Yman L (1993) Seafood specific IgE in atopic dermatitis. Ann Allergy 70: 58.
- Force L, Torres JM, Carillo A, Busc J (1992) Evaluation of eight serological tests in the diagnosis of human echinococcosis and follow-up. Clin Infect Dis 15: 473-480.
- Magnaval JF, Fabre R, MauriËres P, Charlet J-P, de Larrard B (1992) Evaluation of an immunoenzymatic assay detecting specific anti- ToxocaraImmunoglobulin-E for diagnosis and post treatment follow-up of human toxocariasis. J ClinMicrobiol 30: 2269-2274.
- Winter WE, Hardt NS, Fuhrman NS (2000) Immunoglobulin E: importance in parasitic infections and hypersensitivity responses. Arch Pathol Lab Med 124(9):1382-1385.
- PlessisK ,Lopata A, Steinman H (2004) Adverse reactions to fish. Cur Allergy ClinImmunol 17(1): 4-8.
- Iglesias R, Leiro J, Ubeira FM, Santamarina MT, Sanmartin ML (1995) Anisakis simplex: Stage-specific antigens recognized by mice. J Helminthol 69: 319-324.
- Daschner A, Cuellar C, Sanchez-Pastor S, Pascual CY, Martin- Esteban M (2002) Gastro-allergic anisakiasis as a consequence of simultaneous primary and secondary immune response. Parasite Immunol 24: 243-251.
- Audicana MT, Ansotegui IJ, de Corres LF, Kennedy MW (2002) Anisakissimplex: dangerous-dead and alive? Trends Parasitol 18: 20-25.
- Sakanari JA, McKerrow JH (1989). Anisakiasis. ClinMicrobiol Rev 2(3):278-284.
- Myjak P, Szostakowska B, Wojciechowski J, Pietkiewicz H, Rokicki J (1994) Anisakid larvae in cod from the southern Baltic sea. Arch Fish Mar Res 42:149-161.
- Caballero ML, Moneo I (2004) Several allergens from Anisakis simplex are highly resistant to heat and pepsin treatments. Parasitol Res 15: 1-7.
- Audicana MT, Fernandez de Corres L, MuÒos D, Fernandez E, Navarro JA, et al. (1995) Recurrent anaphylaxis caused by Anisakis simplex parasitizing fish. J Allergy ClinImmunol 96:558-560.
- Akao N, Yoshimura H (1989). Latex agglutination test for immunodiagnosis of gastric anisakiasis, In: Ishikura H, Namiki M, editors. Gastric anisakiasis in Japan. Springer-Verlag, Tokyo. pp: 97-102.
- Daschner A, Alonso-Gomez A, Caballero T, Barranco P, Suarez de Parga JM, et al. (1998) Gastric anisakiasis: an underestimated cause of acute urticaria and angio-edema? Brit J Dermatol 139: 822-828.
- Baeza ML, Higaki LC, Martin E, Perez C, Infante S, et al. (2005) Anisakis simplex allergy: a murine model of anaphylaxis induced by parasitic proteins displays a mixed Th1/Th2 pattern. ClinExpImmunol 142: 433-440.
- Manetz TS, Meade BJ (1999) Development of Flow Cytometry Assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T Cell-Mediated hypersensitivity responses. ToxicolSci 48: 206-217.
- Ormerod MG (1990) Introduction to the principles of flow cytometry. Flow Cytometry, A practical Approach. Oxford University Express 1-28.
- Bradford M M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Perteguer MJ, Cuellar C (2001) Interleukin-4 production in balb/c mice immunized with Anisakis simplex. MemInstOswaldo Cruz 96(7): 979-982.
- Colovai AI, Giatzikis C, Ho EK, Farooqi M, Suciu-Foca N, et al. (2004) Flow cytometric analysis of normal and reactive spleen. Modern Pathol 17: 918-927.
- Hudson L, Hay FC (1989) The basic techniques. In: Practical immunology. 3rd ed. Oxford: Blackwell Scientific Publication.
- Coulson PS, Wilson RA (1993) Pulmonary T-helper lymphocytes are CD44 hi CD45 RB-effector / memory cells in mice vaccinated with attenuated cercariae of Schistosomamansoni. J Immunol 151: 3663-3671.
- Hofman FM, Kanesberg B, Smith D, Garrison D, Servier ED (1982) Stability of T- and B- cell numbers in human peripheral blood. Am J ClinPathol 77: 710.
- Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, et al. (2006) Intestinal helminthes protect in a murine model of asthma. J Immunol 177: 1628-1635.
- A 100DFK Aqua-glo G/C Direct Dual Flurochrome (FL/CY3), Comprehensive Kit, Waterborne Inc., New Orleans, LA, USA.
- Moneo I, Caballero ML, Rodriguez-Perez R, Rodriguez-Mahillo A, Gonzalez-Munoz M (2007) Sensitization to the fish parasite Anisakis simplex: clinical and laboratory aspects. Parasitol Res 101: 1051-1055.
- Audicana MT, Kennedy MW (2008) Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. ClinMicrobiol Rev 21(2): 360-379.
- Cooper PJ, Chico ME, Sandoval C (2000) Human infection with Ascarislumbricoidesis associated with a polarized cytokine response. J Infect Dis 182:1207–1213.
- Lynch N R, Hagel I, Perez M, Di Prisco M, Alvarez N, et al. (1992) Bronchoconstriction in helminthes infection. Int Arch Allergy Immunol 98: 77-79.
- Medeiros D, Silva AR, Rizzo JA, Motta ME, Oliveira FHB, et al. (2006) Total IgE level in respiratory allergy: study of patients at high risk for helminthes infection. J Pediatr 28(4): 374-378.
- Erb KJ (2007) Helminths, allergic disorders and IgE-mediated immune responses: where do we stand? Eur J Immunol 37(5): 1170-1173.











