Key words
Zinc, copper, LT50, LC50, mortality, rainbow trout
Introduction
The harmful effects of heavy metals on rainbow trout have been studied by various authors (Taylor et al., 2000; Hansen et al., 2002b; Bagdonas and Vosylienė 2006). Include alterations in metabolism and specific physiological functions (Glover and Hogstrand, 2003) leading to irreversible damages in behavior (Petrauskiene, 1999), growth, and reproduction (Kleerekoper, 1976; Nieboer and Richardson, 1980; Mance, 1987; Depledge et al., 1994; Phillips and Rainbow, 1994).
The effects of sublethal doses of zinc and copper on fish behavior were also studied and complicated responses found in their biological systems (Suresh et al., 1993).
Toxic substances dissolved in water increase often the sensitivity of aquatic organisms to temperature variations, changes in dissolved O2 and vice-versa. Also the growth performance can be impaired and reproduction capacity can be reduced. Metabolic effects of heavy metal exposure (i.e. oxygen consumption) can be explained by the accumulation of heavy metals on the gill surface impairing O2 diffusion capacity (Mutluay and Demirak, 1996; Svecevıèıus, 1999; Taylor et al., 2000 -2002; Bagdonas and Vosylienė 2006).
Heavy metals can be taken up by aquatic organisms via several routes (a) directly via the body surface or surface of the respiratory organs, (b) via feed, or (c) by a combination of them (Phillips and Rainbow, 1994). However which route is more important depends on environmental circumstances and has not always been properly documented (Depledge et al., 1994). Aquatic toxicology is also concerned with effects of the concentrations of the chemical substances occurring in natural waters, sediments and food. The aquatic environment is complex and exhibits a changeable structure. These changing conditions affect the chemical reactions of substances and pollutants (Cairns and Mount, 1990; Forbes and Forbes, 1994).
Most of the above mentioned heavy metals studies have determined the LT50 and LC50 values for various fish species but also assessed the accumulation in fish tissues illustrate in also that effects change with changing environmental conditions. Keeping these facts in our minds, the aim of the study was to find out the effects of zinc and copper ions for the rainbow trout in laboratory environmental conditions.
Material and Methods
Rainbow trout (Oncorhynchus mykiss) was purchased from a private fish farm and transported to the laboratory and placed in tanks (500 L), which the stocking density was 2000 fish/m3. The fish were fed on a commercial diet, containing 52% crude protein, 14% crude lipid and 19 Kj gross energy/g feed (Sibal A.Ş., Sinop, Turkey). The acclimation to test conditions lasted for 10 days. The same diet was used during the whole experiment. Tap water was aerated for 48 hours to remove chlorine. Half of the water in the stocking tanks was changed every other day with well-aerated water containing the same concentration of heavy metal.
Chemically pure salts of zinc chloride (ZnCl2) and copper sulphate (CuSO4 5H2O) dissolved in distilled water were used as toxicants. The final concentrations were recalculated according to the amount of zinc and copper ions.
The average weight and length of fish used in the zinc experiments range from 2.81 ± 0.24 to 3.21 ± 0.26 g and 6.37 ± 0.12 to 6.63 ± 0.11 cm respectively, while the weight and length for copper experiments range from 6.71 ± 0.78 to7.46 ± 0.63 g and 7.06 ± 0.32 to 8.41 ± 0.24 cm respectively. Temperature, dissolved O2, pH, total hardness and ammonia were measured daily, and the average values were 14.62 ± 0.41 ºC, 7.49 ± 0.15 mg/l O2, 7.48 ± 0.12, 249.56 mg/l as CaCO3 (Ca+++ Mg++ = 85.86 + 8.83 mg CaCO3 / l) and 0.013 ± 0.002 mg/l NH3-N, respectively.
Two seperate experiments were conducted to determine the lethal dosages of zinc and copper ions. The experiments were designed as 3 replicates in the tanks containing 100 L of water, each replicate had 8 exposure groups as well as one control and each test group was performed with ten fish.
Experiment I contained 8 zinc ion concentrations (5, 8, 10, 13, 15, 19, 23 and 27 mg/l) besides the control (not contain zinc) while Experiment II contained 8 copper ion concentrations (0.01, 0.025, 0.05, 0.075, 0.1, 0.5, 1 and 2 mg/l) besides the control (not contain copper). Concentration were determined by analysing the samples through atomic absorption spectrometer.
Observations were made after 15, 30 min, 1, 2, 4, 8 and 12 h on the first day, while follow-up observations were conducted after 24, 48, 72, 96 had 120 h. Death or abnormality in swimming behaviour of fish were noted. The tanks were checked daily; pH and dissolved O2 values were measured throughout the experimental period. Fish were not fed for 1 day before the start of experiments to the end of the 120-h experimental period. Thus, the volume of waste matter was minimized for not affect the fish conditions. Death was diagnosed either by lack of movement of the operculum or inactivity in swimming behaviour (Ünsal, 1998).
The terms LC50 and LT50 are in accordance with Lloyd (1992) and LC50 concentration values were analyzed by Probite Analysis (Finney, 1971). Data on mortalities recorded in the three replicates for each concentration were pooled. The mortality percentage of each concentration was verified using Abbortt’s formula (Anonymous, 1976). Weighted regression analysis based on probate (transformed percentage mortality) against log-dose was calculated for each metal independently, and considering these calculations for the lethal concentrations (LC50) and fiducially limits (FL) was determined.
Results and Discussion
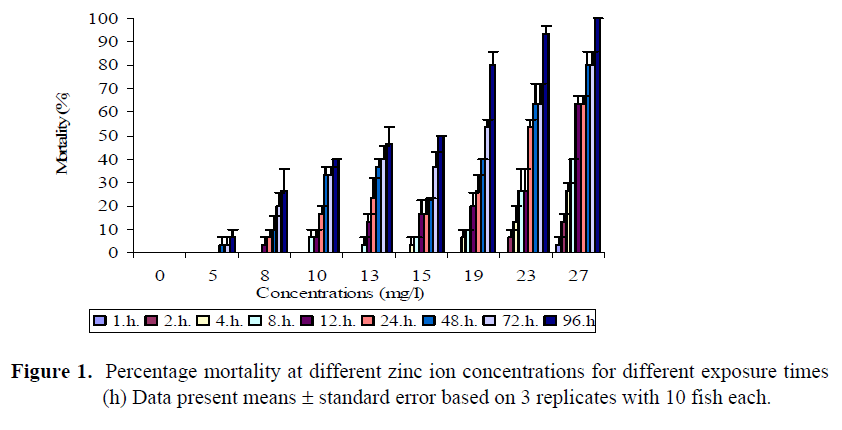
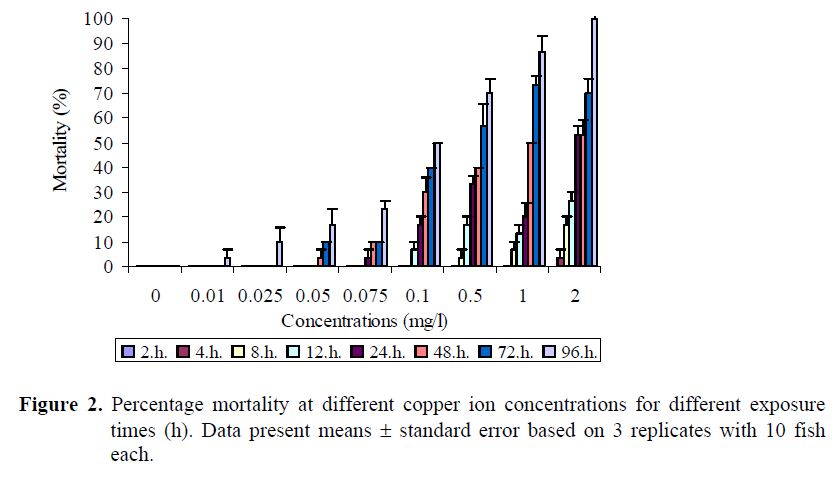
The mortality rates increased with increasing zinc and copper ions concentrations as shown in Figures 1 and 2. No mortality occured in the control groups. Also no fish died within the first 4 h in the concentrations up to 13 mg/l Zn whereas mortality was occurred in the concentrations from 15 to 27 mg/l. In the lowest zinc concentration, first mortality was seen after 48 hours. No mortality occured during the first two hour of exposure in any replicate of any of the copper ion concentrations employed. Figure 2 shows that mortality after 4 h exposure reached 3.3% in the concentration of 2 mg/l Cu. In addition, the death rates were 3.3 and 6.7% in the 0.5-1 mg/l Cu solutions respectively after 8 h exposure, and a death rate of 6.7% in the concentration of 0.1 mg/l Cu was recorded at the end of 12 h exposure. None of the fish in the control group died during the experimental period. In the highest concentration (2 mg/l Cu), the first fish died after 4 h exposure, and all of the fish died at the end of 96 h exposure.
Figure 1. Percentage mortality at different zinc ion concentrations for different exposure times (h) Data present means ± standard error based on 3 replicates with 10 fish each.
Figure 2. Percentage mortality at different copper ion concentrations for different exposure times (h). Data present means ± standard error based on 3 replicates with 10 fish each.
Mortalities in the highest three zinc levels were categorized in the same statistical group whereas mortalities in the lowest two zinc levels and the control group were categorized as another group and the rest formed the last group. When these three groups were compared, the mortality percentages were found to be significantly different from each other as shown in Table 1 (p<0.05). Mortalities in the lowest four copper levels and the control group were categorized in the same statistical group and the rest formed another group. When these groups were compared, the results were significantly different (Table 1, p<0.05).
Table 1. Mortality (%) in different zinc and copper ion concentrations at the end of 96h exposure experiment. Data present means ± standard error based on 3 replicates with 10 fish each
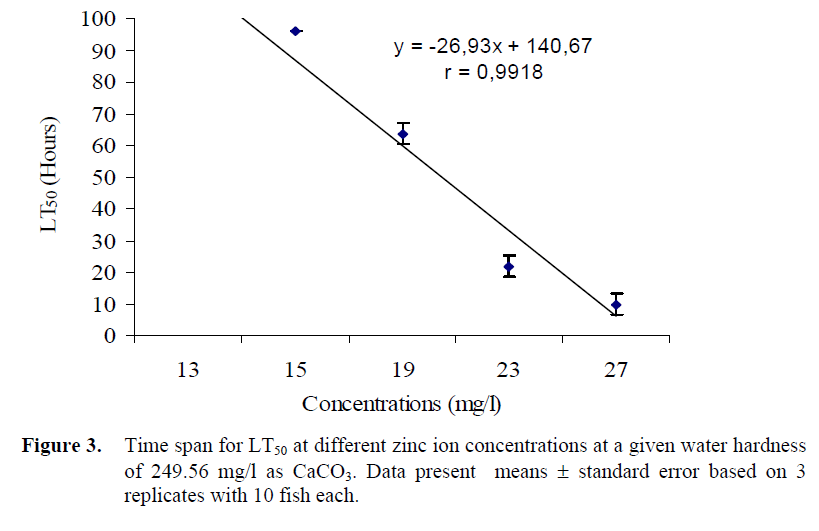
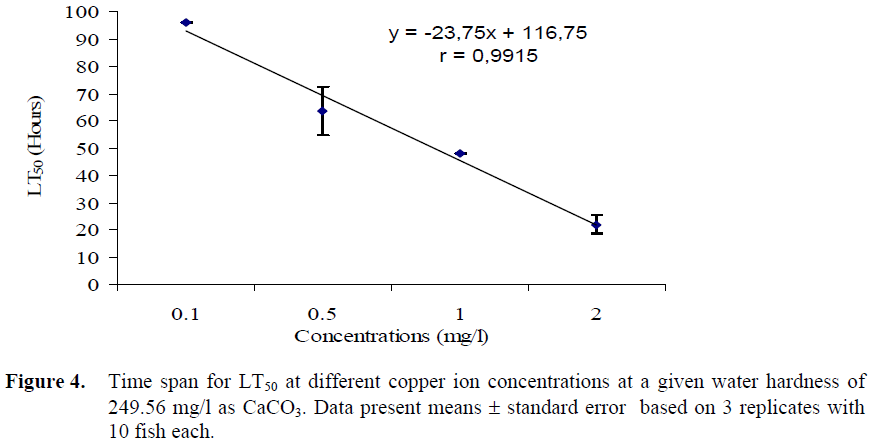
The LT50 for rainbow trout in different zinc and copper ion concentrations are shown in Figures 3 and 4 respectively. Since 50% mortality was not recorded in the 96 h exposure, the timespan of the experiments was extended up to 120 hours. The LT50 values in 15 and 27 mg/l Zn concentrations were 96 and 10 h, respectively. The LT50 values in 0.1 and 2 mg/l Cu concentrations were 96 and 22 h, respectively. There is negative correlation between the LT50 values and the zinc and copper ion concentrations; when the zinc and copper ion concentrations levels decreased, LT50 values increased.
Figure 3. Time span for LT50 at different zinc ion concentrations at a given water hardness of 249.56 mg/l as CaCO3. Data present means ± standard error based on 3 replicates with 10 fish each.
Figure 4. Time span for LT50 at different copper ion concentrations at a given water hardness of 249.56 mg/l as CaCO3. Data present means ± standard error based on 3 replicates with 10 fish each.
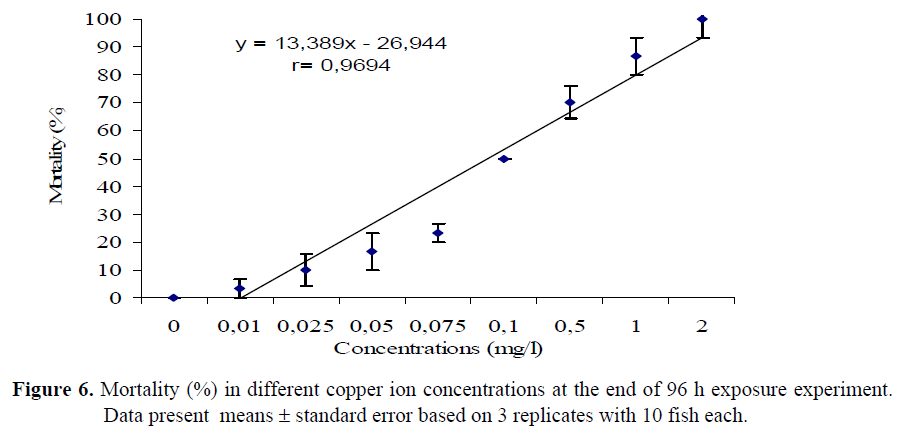
The mortality (%) in different zinc and copper ion concentrations within 96 h was evaluvated as well. The results illustrated that 50 and 100% of fish died in the concentrations of (LC50) 15 and 27 mg/l Zn solutions, respectively (Figure 5). For copper ion concentrations, 50 and 100 % mortality was found in (LC50) 0.1 and 2 mg/l Cu solutions, respectively (Figure 6).
Figure 5. Mortality (%) in different zinc ion concentrations at the end of 96 h exposure experiment. Data present means ± standard error based on 3 replicates with 10 fish each.
Figure 6. Mortality (%) in different copper ion concentrations at the end of 96 h exposure experiment. Data present means ± standard error based on 3 replicates with 10 fish each.
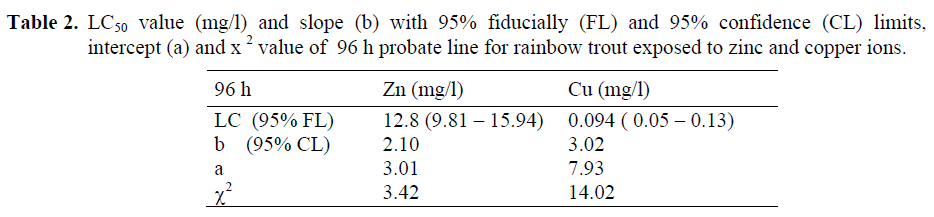
Lastly, the concentration values causing 50% mortality at the end of the 96-h period were analysed and the results were displayed in Table 2. LC50 was found only in the concentrations of 15 mg/l Zn and 0.1 mg/l Cu after 96 h exposure. These concentration values were analysed by Probite Analysis and LC50 value was calculated as 12.8 mg/l Zn and 0.094 mg/l Cu.
Table 2. LC50 value (mg/l) and slope (b) with 95% fiducially (FL) and 95% confidence (CL) limits, intercept (a) and x2 value of 96 h probate line for rainbow trout exposed to zinc and copper ions.
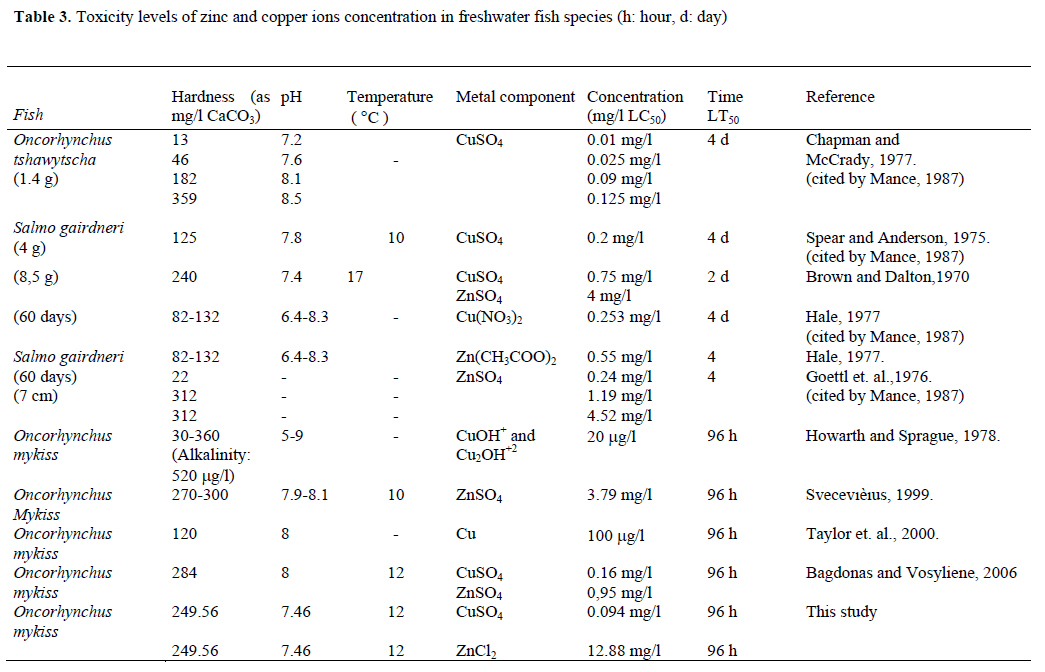
Several researchers reported different lethal dosages for zinc under different water conditions (Sprague and Ramsay, 1965; Goettl et al., 1976; Hale, 1977; Svecevıèıus, 1999; Bagdonas and Vosylienė 2006). The comparison of the results of the present study with the above mentioned studies was illustrated in Table 3. The differences in LC50 values might be caused by the different metal compounds used in the studies and environmental conditions in which the studies were applied. The toxicity limits of zinc in rainbow trout for water hardness of 10–500 mg/l as CaCO3 and a pH value of 6 were ≤ 0.03 and ≤ 0.50 mg/l, respectively, and the effects in hard water were found to be lower than that of soft water (Lloyd, 1992), and differences may also be due to the different rations of carbonate hardness to total hardness. Roy and Campbell (1995) reported varying LC50 values for young Atlantic salmon exposed to different Zn solution concentrations at different pH levels and the limits were reported to be 0.1–00.2 μM. Hansen et al. (2002b) also reported various effects of zinc in rainbow trout at different hardness, pH and temperature levels, and noted a low level toxicity at high hardness and low pH values.
Table 3. Toxicity levels of zinc and copper ions concentration in freshwater fish species (h: hour, d: day)
The results of this study indicated that mortality rate and time were influenced by the concentration levels of the heavy metals as well as the kind of metals used. Besides, it was found that there was a positive relationship between the mortality and concentration levels; when the con- centration level increased, the mortality rate increased as well. However, there was a negative relationship between the mortality time and concentration level; when the concentration level increased, the mortality time decreased.
In the previous studies, different LC50 and LT50 values were detected (Table 3). The experiments of zinc solutions were conducted in conditions of total hardness of 249.6 mg/l as CaCO3, temperature level of 12 °C and pH value of 7.5 Wong et al. (1977) and Lam et al. (1998) also conducted similar experiments using different fish species, namely common carp in the first and tilapia in the second study, and different heavy metal concentrations. There were some differences between our findings and the results of the above-mentioned researchers in the toxicity levels of zinc, possibly because of the different fish species used. This result clearly reflected that the toxicity levels of heavy metals varied depending on experimental fish species.
The time-spans for 50% mortality in different copper concentrations were analyzed. Schaeperclaus (1979) and Reichenbach-Klinke (1980) conducted experiments as well and found the lethal concentrations of copper to be 0.1 and 0.143 mg/l Cu, respectively. In another study, similar results were also observed (see Table 3; Chapman and McCrady, 1977). All of these results are very close to the results of our study (0.094 mg/l).
Several researchers reported different LC50 values for rainbow trout fed at different water conditions containing copper: an LC50 value of 0.01 mg/l as CuSO4 at a hardness of 13 mg/l as CaCO3 and pH value of 7.2 (Chapman and McCrady, 1977), high mortality rate and lower growth performance in water containing 144 μg/l Cu (Dixon and Hilton, 1985) and an LC50 value of 14 μg/l Cu (Marr et al., 1998). However, in the present study, the LC50 value of 0.094 mg/l Cu is lower than those mentioned above. This may be caused by the fish size used in the study, as reported by Howarth and Sprague (1978). Some contradictions in the LC50 values were also found between our results and those of Hansen et al. (2002a), supporting the varying effects of copper, depending on different water conditions, different fish sizes used and different copper salts in the experiments.
Brown and Dalton (1970) found a different LT50 level (2 d=48 h) from their experimental study using CuSO4 in similar water hardness and pH values (Table 3). This difference can be explained by the difference in LC50 values and fish species used. On the other hand, the 50% mortality in 96 h (LT50) exposure for Cu toxicity obtained by Taylor et al. (2000) and Bagdonas and Vosylienė (2006) supports our experimental results (Table 3). A LC50 value of 0.75 mg/l was analyzed for CuSO4 solution for 48 h at 17°C in Salmo gairdneri weighing 8.5 gr (Brown and Dalton, 1970), on the other hand, a LC50 value of 1 mg/l was determined for the same solution for 48 h at 12 °C in the same fish species weighing 6.71-7.46 g. This difference can be explained by different experimental water temperature at both studies.
Table 3 shows that LT50 values for Zn toxicity were analyzed as 4 days (96 h) in the present study, and also by Goettl et al. (1976). However, the LC50 values were different in our study and in Goettl’s, probably as a result of the differences in water hardness, Zn salt solutions and fish species used. A LC50 value of 4.52 mg/l was determined for ZnSO4 solution at the hardness of 312 mg/l as CaCO3 in Salmo gairdneri (Goettl et al. ,1976), on the other hand, in our study, a LC50 value of 12.9 mg/l was determined for the same solution at the hardness of 249.6 mg/l as CaCO3 in the similar fish species. The increase in the LC50 value in our study can be explained by the decrease in water hardness (Table 3).
Conclusion
The results of this study clearly illustrated that the toxic effects of zinc and copper to fish, i.e. the LT50 and LC50 values varied according to water conditions, such as temperature, pH, hardness, dissolved O2, the size and species of fish as well as the type of zinc or copper salt.
Acknowledgment
I assure you that whole activities involving experimental fish in the present study were conducted in accordance with national and institutional guidelines for the protection of human subjects and animal welfare.
The authors would like to thank Ondokuz Mayıs University for its financial support, which enable us to carry out this study.
References
- Anonymous, (1976) . S.M.E.W.W. (StandardMethods for the Examination of Water and Wastewater, Part 800). Bioassay methods foraquatic organisms, Amer. Wat. Works Ass.Wat. Pollut. Fed., 683-872. Washington DC., USA.
- Brown, V.M., Dalton, R. A., (1970) . The acute lethal toxicity to rainbow trout of mixtures of copper, phenol, zinc and nickel, Journal ofFish Biology, 2: 211-16.
- Bagdonas, E., Vosylienė, M. Z., (2006). A study of toxicity and genotoxicity of pper, zinc and their mixture to rainbow trout (Oncorhynchusmykiss). Bıologıja,1: 8–13.
- Cairns, J. Jr., Mount, D.I., (1990). Aquatic toxi-cology, Part 2 of a four-part series, Environ-mental Science Technology,24: 154-161.
- Chapman, G.A., McCrady, J.K., (1977). Copper toxicity: a question of form, In: Recent Ad-vances in Fish Toxicology: a Symposium,(R.A. Tubb, Ed.), 132-51. USEPA Report No. EPA 660 / 3 -77/ 085, Washington, DC., USA.
- Depledge, M.H., Weeks, J.M., Bjerregard, P., (1994). Heavy metals,In: P. Calow (Ed), Handbook of Ecotoxicology. Oxford Black-well, 2: 79–105, London.
- DIxon, D.G. and Hilton, J.W., (1985). Effect of available dietary carbohydrate and water temperature on the chronic toxicity of water-borne copper to Rainbow trout (Salmogaird-neri). Canadian Journal Fisheries and Aquatic Sciences, 42: 1007- 1013.
- Finney, D.J., (1971). Probit Analysis, 3. Edition, Cambridge University Press, 330, London.
- Forbes, V.E., Forbes, T.L., (1994). Ecotoxicologyin theory and practice, First edition. Chap-man and Hall, London.
- Goettl, J.P., Davies, P.H. and Sinley, J.R., (1976).
- Water pollution studies, In: Colorado Fishe-ries Research Review, 1972–1975. Fish. Res. Section, Color. Divis., Wildlife, 8: 68-75.
- Hale, J. G., (1977). Toxicity of metal mining wastes. Buletin Environmental ContamineToxicology, 17: 66-73.
- Hansen, J.A., Lipton, J., Welsh, P.G., Morris, J.,Cacela, D., Suedkamp, M.J., (2002a). Relationshipbetween exposure duration, tissueresidues, growth, and mortality in Rainbowtrout (Oncorhynchusmykiss) juveniles subchronicallyexposed to copper. Aquatic Toxicology 58(3-4): 175 - 188.
- Hansen, J.A., Welsh, P.G., Lipton, J., Cacela, D.,Dailey, A.D., (2002b). Relative sensitivity ofBull trout (Salvelinusconfluentus) and Rainbowtrout (Oncorhynchusmykiss) to acuteexposures of cadmium and zinc. EnvironmentalToxicology and Chemistry/ SETAC,21(1): 67–75.
- Howarth, R.S., Sprague, J.B., (1978). Copper lethality to rainbow trout in waters of various hardness and pH. Water Research, 12(7): 455-462.
- Kleerekoper, H., (1976). Effects of sublethal concentrations of pollutants on the behavior of fish. Journal Fisheries Research Board of Canada, 33: 2036 – 2039
- Lam, K.L., Ko, P.W., Wong, J.K.L., Chan, K.M., (1998). Metal toxicity and metallothionein gene expression studies in common carp and tilapia, Marine Environmental Research, 46 (1-5): 563–566.
- Lloyd, R., (1992). Pollution and Freshwater Fish, Fishing News Books, 161, London. Mance, G., (1987). Pollution Threat of Heavy Metals in Aquatic Environments, Elsevier, 372, London.
- Marr, J.C.A., Hanse, J.A., Meyer, J.S., Cacela, D., Podrabsky, T., Lipton, J., Bergman, H.L., (1998). Toxicity of cobalt and copper to rainbow trout: application of a mechanistic model for predicting survival. Aquatic Toxicology, 43(4): 225–238.
- Mutluay, H., Demirak, A., (1996). Water Chemistry, İ.Ü., Su Ürünleri Fakültesi, 624: 138, İstanbul, Turkish..
- Nieboer, E., Richardson, D.H.S., (1980). The replacement of the nondescript term ‘Heavy metals’ by a biologically and chemically significant classification of metal ions. Environmental Pollution, Series B, Chemical and Physical, 1: 3-26.
- Petrauskiene, L.,(1999). Effects of novel environment on rainbow trout exposed to copper. ActaZoologicaLituanica, 9(2): 95-102.
- Phillips, D.J.H., Rainbow, P.S., (1994). Biomonitoring of trace aquatic contaminants. Environmental Management Series, Chapman & Hall, London.
- Reichenbach-Klinke, H.N., (1980). Krankheiten und schaedigungen der fische, Gustav Fischer. Stuttgart. Roy, R., Campbell, P.G.C., (1995). Survival time modelling of exposure of juvenile Atlantic salmon (Salmosalar) to mixtures of aluminum and zinc in soft water at low pH. Aquatic toxicology, 33(2): 155–176.
- Schaeperclaus, W., (1979). Fisch-Krankheiten, Akademic, Berlin. Spear, P.A., Anderson, P.D., (1975). Fish size as a quantitative function of tolerance to heavy metals. Water Pollutiation Research of Canadian, 10: 170-179.
- Sprague, J.B., Ramsay, B.A., (1965). Lethal levels of mixed copper-zinc solutions for juvenile salmon. Journal of Fisheries Research Board of Canadian, 22(2): 425-432.
- Svecevıèıus, G, (1999). Acute toxicity of zinc to common freshwater fishes of lithuania. ActaZoologicaLituanicaHydrobiologia, 9(2): 1392-1657.
- Taylor, L.N., McGeer, J.C., Wood, C.M., McDonald, D.G., (2000). Physiological effects of chronic copper exposure to rainbow trout (Oncorhynchusmykiss) in hard and soft water: evaluation of chronic indicators. Enviromental Toxicology and Chemistry, 19: 2298- 2308.
- Taylor, L.N., Baker, D.W., Wood, C.M., McDonald, D.G., (2002). An in vitro approach for modellingbranchial copper binding in rainbow trout. Comparative Biochemistry and Physiology, 133(1-2): 111-124.
- Ünsal, M., (1998). Pollution Experiments (The evaluation of methods and results), T.C. Tarım ve Köy İşleri Bakanlığı Su Ürünleri Araştırma Enstitüsü Müdürlüğü, seri A, 11:168, Bodrum, Turkish.
- Bodrum, Turkish. Wong, M.H., Luk, K.C., Choi, K.Y., (1977). The effects of zinc and copper salts on Cyprunuscarpio and Ctenopharyngodonidellus, Acta Anatomy, 99: 450-454.
1537