Keywords
Tumor lysis syndrome (TLS); Liver metastasis; Oncologic emergency; Urothelial carcinoma of the renal pelvis cisplatin; Gemcitabine; Rasburicase
Introduction
Acute tumor lysis syndrome (TLS) is considered an oncologic emergency which may result in significant morbidity and mortality in cancer patients [1-3]. It results from rapid lysis of tumor cells and release of intracellular contents such as potassium, phosphorus and uric acid. Patients with ATLS commonly present with cardiac arrhythmias, seizures, muscle cramps, acute kidney injury (AKI), and vomiting [2]. TLS is most commonly seen in cases of hematologic malignancies such as Burkitt lymphoma and leukemia; however, cases of TLS have been reported in patients with rapidly growing, bulky, therapysensitive solid tumors [2-5]. To the best of our knowledge, there were only two cases describing TLS following chemotherapy for metastatic urothelial carcinoma [6,7].
Case Presentation
A 67-year-old female initially underwent a laparoscopic nephrectomy with bladder cuff excision in March of 2015. The surgical pathology showed low grade papillary urothelial carcinoma, with invasion through pelvic wall into peripelvic fibroadipose tissue (AJCC staging pT3). A routine follow-up computed tomography (CT) in August of 2016 was positive for two liver masses in segment 2 and 8 for which she underwent liver lesion biopsy at an outside hospital which was reported benign. Prior to her visit to our cancer center, she has been seeing a naturopathic physician for follow-up of her cancer. A subsequent Magnetic resonance imaging (MRI) three months later showed growth of both liver lesions. In Feb 2017, a CT chest, abdomen and pelvis with contrast at the time of consultation at our cancer center showed Interval development of multiple hepatic masses. The largest is partially necrotic and measures 9.8 × 13.7 cm (Figure 1).
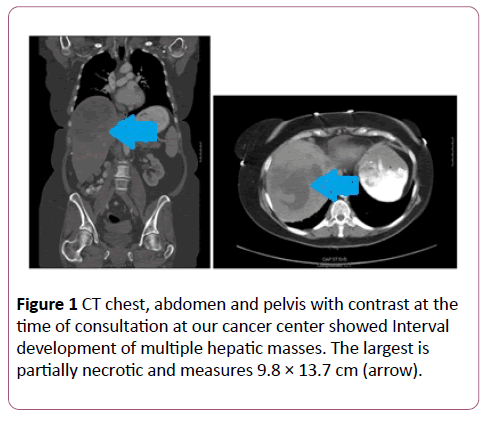
Figure 1: CT chest, abdomen and pelvis with contrast at the time of consultation at our cancer center showed Interval development of multiple hepatic masses. The largest is partially necrotic and measures 9.8 × 13.7 cm (arrow).
She subsequently underwent liver lesion biopsy. The tumor demonstrates strong GATA3, CK7 and CK 20 positivity. Based on the immunophenotypic profile, coupled with the histomorphology, the findings are consistent with metastatic urothelial carcinoma (Figure 2).
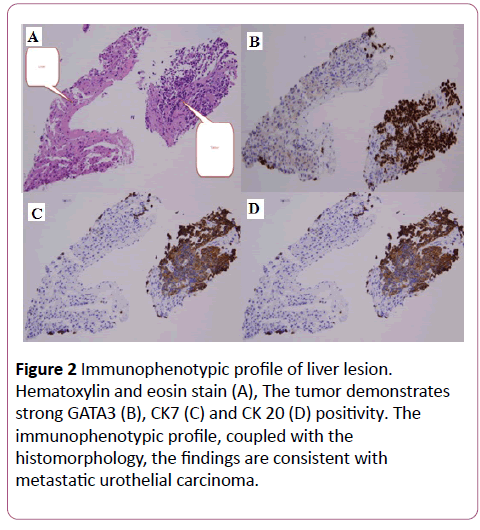
Figure 2: Immunophenotypic profile of liver lesion. Hematoxylin and eosin stain (A), The tumor demonstrates strong GATA3 (B), CK7 (C) and CK 20 (D) positivity. The immunophenotypic profile, coupled with the histomorphology, the findings are consistent with metastatic urothelial carcinoma.
She subsequently received Cycle 1 Day 1 chemotherapy at dose of cisplatin 70 mg/m2/ gemcitabine 800 mg/m2. On day 8 visit, the patient was found to have hyperuricemia with uric acid 12 mg/dL (range: 3-6 mg/dL), hypocalcemia with corrected calcium 7.7 mg/dL (range: 8.5-10.3 mg/dL), acute kidney injury with serum creatinine 4.04 mg/dL (range: 0.57-1.11 mg/dL, patient’s baseline is 0.85 mg/dL), phosphorus 4.2 mg/dL (range: 2.4-4.7 mg/dL), and lactate dehydrogenase 1,382 units/L (range: 125-243 units/L) (Table 1).
Laboratory Value
(Normal Range) |
Pre-chemotherapy |
7 days post - Cycle 1 Day 1 chemotherapy |
24 hours post-rasburicase 7.5 mg |
6 weeks post - Cycle 1 Day 1 chemotherapy |
| Potassium (3.6-5.3 mmol/L) |
4.1 |
4.0 |
4.9 |
4.4 |
| Phosphorus (2.4-4.7 mg/dL) |
N/A |
4.2 |
4.6 |
4.2 |
| Blood Urea Nitrogen (8-25 mg/dL) |
9 |
65 |
53 |
25 |
| Serum Creatinine (0.57-1.11 mg/dL) |
0.86 |
4.04 |
2.99 |
0.75 |
| Corrected Calcium (8.5-10.3 mg/dL) |
9.3 |
7.7 |
8.5 |
8.8 |
| Uric Acid (3-6 mg/dL) |
4 |
12 |
< 1 |
2 |
| T. Bili (0.2-1.2 mg/dL) |
1.8 |
0.8 |
0.6 |
0.5 |
| Lactate dehydrogenase (125-243 units/L) |
1,096 |
1,382 |
896 |
395 |
Table 1: Pertinent laboratory findings in 67-year-old female with suspected tumor lysis syndrome.
Patient was treated with normal saline hydration and received one dose of rasburicase 7.5 mg intravenous infusion. Most of her laboratory abnormalities improved shortly after treatment initiation and there was no need for inpatient admission. Currently she is responding to ongoing systemic chemotherapy with preservation of quality of life.
Discussion
We searched PubMed/Medline, for articles focused on TLS in patients with urothelial carcinoma published from March 1950 to March 2017. There were two only reported cases of advanced urothelial carcinoma who developed TLS documented in the literature, although only one occurred after gemcitabine [6], another case occurred after PD-1 Immunotherapy [7]. Our case is the third case of reported TLS in patients with urothelial carcinoma (Table 2). All three cases are urothelial carcinoma of renal pelvic origin/upper urinary tract. Tumors of the renal pelvis account for approximately 10% of all renal tumors and only 5% of all urothelial tumors of the urinary tract [8]. A randomized Phase III study comparing cisplatin and gemcitabine (GC) and methotrexate, vinblastine, adriamycin and cisplatin (MVAC) has demonstrated similar efficacy with respect to response, time-to-progression and overall survival, whereas GC is associated with less toxicity than MVAC [9]. Thus, GC is now considered a standard of care for patients with locally advanced and metastatic urothelial cancer.
| Author (year) Ref |
Primary cancer |
Chemotherapy |
Liver metastases |
Rasburicase |
Outcome |
| Lin (2007) |
renal pelvic urothelial carcinoma |
gemcitabine |
Yes |
No |
Death |
| Brunnhoelzl (2017) |
renal pelvic urothelial carcinoma |
Atezolizumab |
Yes |
No |
Death |
| This case (2017) |
renal pelvic urothelial carcinoma |
Cisplatin and Gemcitabine |
Yes |
Yes |
Alive |
Table 2: Review of case reports identifying tumor lysis syndrome in patient with solid tumors.
Both two previous cases were associated with poor clinical outcome leading to the death. Currently, our patient is alive and responding to ongoing systemic chemotherapy with preservation of quality of life. The early identification of TLS and prompt use of rasburicase likely contributed to the better outcome in current case. Rasburicase, a recombinant urate oxidase, may represent an effective alternative to allopurinol in rapidly reducing uric acid levels, improving patients' electrolyte status, and reversing renal insufficiency [4]. The drug initially was studied in pediatric patients with acute lymphoblastic leukemia and aggressive non-Hodgkin lymphoma; data are lacking in term of the benefit in patients with solid tumors.
Rasburicase is currently approved at a dosage of 0.15-0.2 mg/kg once/day for 5 days in pediatric patients with cancer to lower plasma uric acid concentrations and manage TLS [4]. Information on rasburicase dosing in adults is limited, with some data on using rasburicase as a single dose instead of multiple daily doses [10]. Our experience and others confirm that single dose of rasburicase is safe and highly effective in the treatment of chemotherapy-induced hyperuricemia in this setting.
Several of risk factors for TLS have been reported including dehydration, impaired pre-therapy kidney function, hyperuricemia, increased tumor cell proliferation rate and size, and chemosensitivity of the malignancy [1-4]. Our patient was not given prophylaxis before therapy, because the urothelial carcinoma was not considered a risk and no previous published report TLS secondary to current therapy. We speculate the possible contributing factors of developing TLS in this patient include high metastatic tumor volume which was sensitive to GC, which resulted in rapid tumor necrosis. Recent published findings showed patients with somatic alterations in DNA damage response and repair (DDR) genes are associated with improved sensitivity to platinum-based chemotherapy [11,12]. Further research on predictive biomarkers for TLS is warranted.
Conclusion
TLS is an oncologic emergency in the setting of high tumor cell proliferation, tumor burden, and chemosensitivity. Prompt recognition of TLS is essential in decreasing morbidity and mortality associated with it. In future, the integration of biomarkers in chemotherapy offers clinicians the ability to individualize treatment in order to better predict and manage drug toxicities such as TLS, improve quality of life, and maximize the efficacy of therapy.
18992
References
- Cairo MS, Coiffier B, Reiter A, Younes A (2010) TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumourlysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol149:578-586.
- Ansstas G, Chia Y, Ansstas M (2010) Tumor Lysis Syndrome in: Hematology and oncology subspecialty consult 3rd edition. Philadelphia: Lippincott Williams and Wilkins 434-436.
- Abraham J, Adesunloye B, Agarwala S (2014) Tumor Lysis Syndrome in: The Bethesda handbook of clinical oncology 4th edition. Philadelphia: Lippincott Williams and Wilkins 474-476.
- Alakel N, Middeke JM, Schetelig J, Bornhäuser M (2017) Prevention and treatment of tumor lysis syndrome, and the efficacy and role of rasburicase. Onco Targets Ther10:597-605.
- Gbaguidi X, Goodrich L, Roca F (2016) Bulky solid tumors in elderly adults: beware of spontaneous tumor lysis syndrome. J Am GeriatrSoc64:235-237.
- Lin CJ, Lim KH, Cheng YC (2007) Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol24:455-457.
- Brunnhoelzl D, Weed M, Trepeta R (2017) Tumor lysis syndrome following a single atezolizumab infusion for metastatic urothelial carcinoma involving both upper and lower tract. Arch Can Res5:1.
- Highman W (1986) Transitional carcinoma of the upper urinary tract: a histological and cytopathological study.J ClinPathol39:297-305.
- Von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, et al. (2005) Long-term survival results of a randomized trialcomparing gemcitabine plus cisplatin, with methotrexate, vinblastine,doxorubicin, plus cisplatin in patients with bladder cancer. J ClinOncol23:4602-4608.
- McDonnell AM, Lenz KL, Frei-Lahr DA, Hayslip J, Hall PD (2006) Single-dose rasburicase 6 mg in the management of tumor lysis syndrome in adults. Pharmacotherapy 26:806-812.
- Teo MY, Bambury RM, Zabor EC, Jordan E, Al-Ahmadie H, et al. (2017) DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin Cancer Res.
- Desai NB, Scott SN, Zabor EC, Cha EK, Hreiki J, et al. (2016) Genomic characterization of response to chemoradiation in urothelial bladder cancer. Cancer 122:3715-3723.








