Mini Review - (2022) Volume 10, Issue 8
Administration of Data Management in Clinical Research: Mini Review
Divya Dara*
Department of Clinical research, Geethanjali College of Pharmacy, Cheeryal, Keesara, Hyderabad, India
*Correspondence:
Divya Dara, Department of Clinical research, Geethanjali College of Pharmacy,
Cheeryal, Keesara, Hyderabad,
India,
Email:
Received: 04-Aug-2022, Manuscript No. IPACLR-22-13082;
Editor assigned: 08-Aug-2022, Pre QC No. IPACLR-22-13082(PQ);
Reviewed: 22-Aug-2022, QC No. IPACLR-22-13082;
Revised: 24-Aug-2022, Manuscript No. IPACLR-22-13082(R);
Published:
31-Aug-2022, DOI: 10.36648/2386-5180.22.10.425
Abstract
A crucial stage in clinical research is clinical data management (CDM), which produces high-quality, trustworthy, and statistically sound data from clinical trials. This results in a significantly shorter period of time between drug development and release. From the beginning to the end of a clinical trial, CDM team members are actively involved. They must have sufficient process knowledge to support upholding the CDM processes high levels of quality. At regular intervals throughout a trial, various CDM processes-including Case Report Form (CRF) designing, CRF annotation, database designing, data entry, data validation, inconsistency management, medical coding, data extraction, and database locking are evaluated for quality. To meet regulatory requirements and stay ahead of the market through quicker product commercialization, there is a greater need to strengthen CDM standards in the current environment. The CDM team can achieve these requirements by implementing regulatory-compliant data management technologies. Additionally, submitting data electronically is becoming required of businesses. Professionals in CDM should have the drive to keep up with the fast evolving technology, satisfy reasonable requirements for data quality, and fulfil reasonable expectations.
Keywords
Validation, e-CRF, Clinical data management systems, Clinical data
exchange standards consortium, and Clinical data management
Introduction
A clinical trial aims to answer the research question by producing
data that can be used to support or disprove a theory. The
outcome of the investigation is significantly influenced by the
quality of the generated data. The topic "what is Clinical Data
Management (CDM) and what is its significance?" is one that
research students frequently ask. A relevant and significant
component of a clinical study is clinical data management. In the
course of their study, all researchers engage in CDM activities,
whether consciously or unconsciously. While doing our research,
we engage in some of the CDM processes without specifying the
technical steps. The process of gathering, filtering, and managing
subject data in accordance with legal requirements is known as
CDM. The main goal of CDM procedures is to deliver high-quality
data by minimising errors and missing data while collecting as
much data as feasible for analysis [1]. In order to achieve this
goal, best practises are used to make sure that the data is
accurate, trustworthy, and handled properly. The introduction of software programmes that keep an audit trail and make it simple
to identify and fix differences has made this possible. Advanced
developments have made it possible for CDM to manage massive
trials and guarantee the data quality even in challenging trials.
Instruments for CDM
There are numerous software programmes available for managing
data, and these are referred to as Clinical Data Management
Systems (CDMS). A CDMS is now necessary in multicentric trials
to manage the massive volume of data. Several open source
tools are also accessible, although commercial CDMS make up
the majority of CDMS employed in pharmaceutical businesses.
The CDM tools ORACLE CLINICAL, CLINTRIAL, MACRO, RAVE,
and eClinical Suite are frequently utilised. There isn't much of a
functional difference between two software tools, and neither
system has a clear benefit over the other. The most well-known
open source tools include OpenClinica, openCDMS, TrialDB, and
PhOSCo. These CDM programmes can be downloaded without
charge and offer similar functionality to their paid equivalents.
CDM Process
Like a clinical trial, the CDM procedure starts with the goal in mind.
This indicates that the deliverable has been kept in mind throughout
the entire process. An error-free, valid, and statistically sound
database is what the CDM process is meant to give, much as a
clinical trial is made to provide an answer to the research question.
Discrepancy Control
Other name for this process is query resolution. Managing
differences entails reviewing them, looking into why they exist,
and either finding a solution supported by documentation or
announcing their irresolution. Distinction operation assists in
cleaning up the data and assembles sufficient evidence of the
data disagreement seen. Most CDMS contain a database for
discrepancies where all differences will be noted and maintained
with an audit trail [2]. Differences are either reported to the
investigator for clarification based on the kinds discovered, or
they are resolved internally via Self-Evident Corrections (SEC)
without forwarding DCF to the site. Spelling errors are the
most frequent SECs. DCFs will be dispatched to the location for
discrepancies that call for clarifications from the investigator.
DCFs can be created and printed with the aid of CDM tools.
The resolution or an explanation of the events that led to the
disparity in the data will be written by the investigators. When an
investigator provides a resolution, the database will be updated
with that information.
Occasionally, the CDM team checks all differences to make sure
they have been handled. The data discrepancies that have been
resolved are marked as "closed." It follows that subsequent data
validation efforts on the same data won't result in a discrepancy
for the same data point because those validation failures are no longer regarded as active. Closing differences, however, is
not always achievable. Sometimes the investigator won't be
able to explain why the disparity exists. Such conflicts will be
marked in the discrepancy database as "irresolvable" and will be
treated. The CDM procedure is most crucial step in discrepancy
management. The handling of discrepancies must be done with
the utmost care because it is a crucial part of data cleaning [3].
CDM’s Roles and Responsibilities
Different duties and responsibilities are given to the team
members in a CDM team. Graduation in a life science field
and familiarity with computer applications should be the very
minimum educational requirements for team members in CDM.
Medical graduates are ideal for the position of medical coder.
However, paramedical graduates are also hired in the sector as
medical coders. All CDM teams need to fill a few crucial jobs. The
following roles can be regarded as the bare minimum for a CDM
team:
• Medical coder
• Data Entry Associate
• Clinical Data Coordinator
• Quality Control Associate.
The clinical data coordinator creates all additional CDM-related
materials, checklists, and guidelines. The quality control associate
performs data audits and verifies the accuracy of data entry. A
different quality assurance individual may occasionally audit the
data entered [4,5]. The quality control associate also checks the
paperwork related to the protocols being followed. The team
responsible for data entry will keep track of when CRF pages are
received and enter the information into the database.
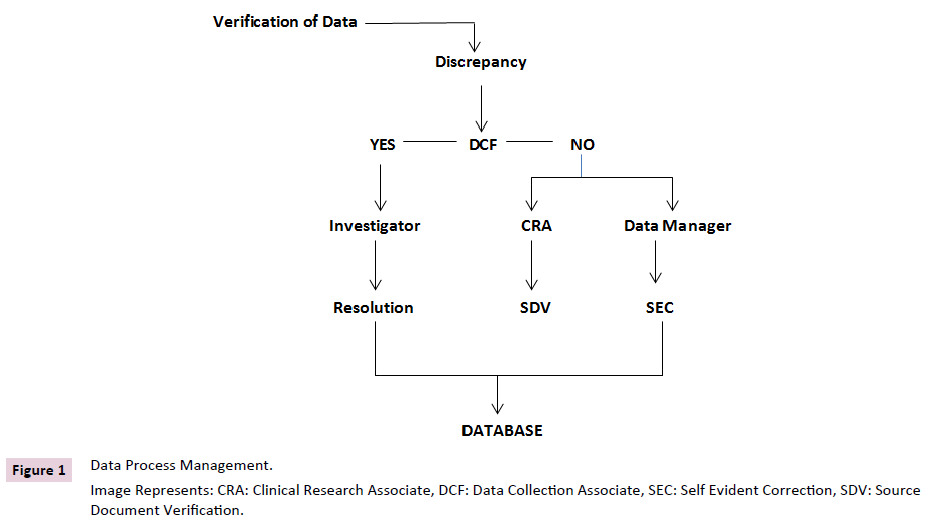
Figure 1. Electrophoretic tracing model interpreted as restriction of heterogeneity.
Conclusion
The need for medication development to be accelerated
by pharmaceutical companies and for regulatory agencies
to establish quality systems to guarantee the generation of
high-quality data for accurate drug evaluation has resulted
in the evolution of CDM. The CDM method and systems have
benefited from technology advancements, which have produced
encouraging results in terms of data generation speed and
quality. Professionals in CDM should simultaneously guarantee
that the standards for enhancing data quality are followed.
The establishment of guidelines to specify the processes to be
followed and the data standards, as well as the standardisation
of the data management process across businesses, would be
the biggest regulatory difficulty. The planning and execution of
data management systems in a dynamic operating environment
where the quick pace of technological advancement outpaces
the current infrastructure would present the biggest challenge
from the industry's standpoint. Despite these, CDM is developing
into a standard-based clinical research entity by balancing the demands placed on existing systems and their limitations with
the demands of commercial and technology advancements.
REFERENCES
- Gerritsen MG, Sartorius OE, Veen FM (1993) Data management in multi-center clinical trials and the role of a nation-wide computer network. A 5 year evaluation. Proc Annu Symp Comput Appl Med Care 659-662.
Indexed at, Google Scholar
- Lu Z, Su J (2010) Clinical data management: Current status, challenges, and future directions from industry perspectives. Open Access J Clin Trials 2: 93-105.
Google Scholar
- Fegan GW, Lang TA (2008) Could an Open-Source Clinical Trial Data-Management System Be What We Have All Been Looking For? PLoS Med 5(3): e6.
Indexed at, Google Scholar, Cross Ref
- Kuchinke W, Ohmann C, Yang Q, Salas N, Lauristen J, et al (2010) Heterogeneity prevails: The state of clinical trial data management in Europeâ?¯-â?¯results of a survey of ECRIN centres. Trials 11: 79.
Indexed at, Google Scholar, Cross Ref
- Cummings J, Masten J (1994) Customized dual data entry for computerized data analysis. Qual Assur 3(3): 300-303.
Indexed at, Google Scholar
Citation: Dara D (2022) Administration of Data Management in Clinical Research: Mini Review. Ann Clin Lab Res. Vol.10 No.8:425







