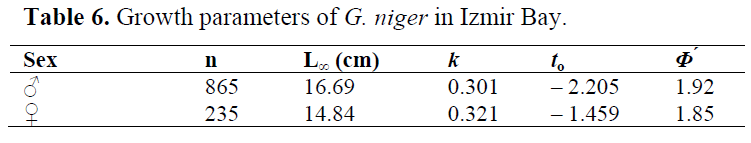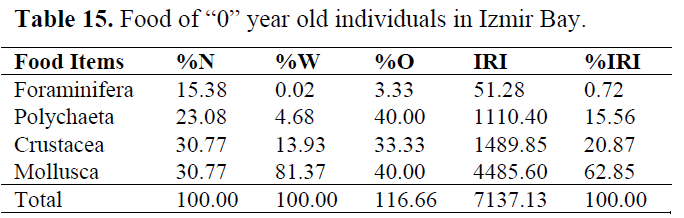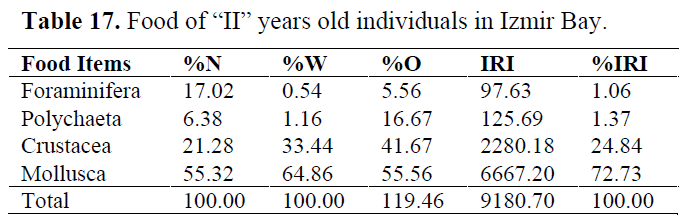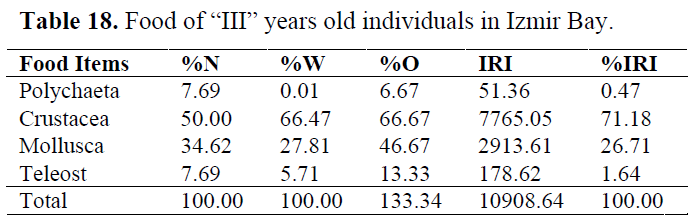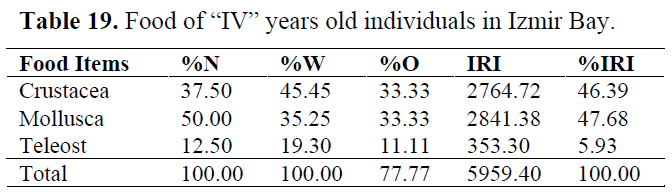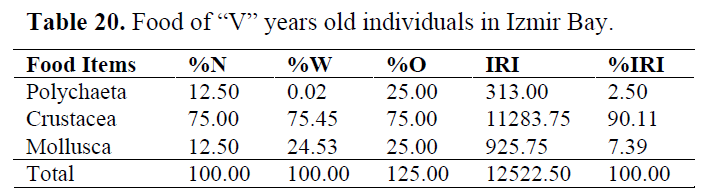Key words
Age and growth; reproduction; feeding; black goby; Gobius niger; Aegean Sea
Introduction
The black goby is widely distributed in Eastern Atlantic and Mediterranean Sea (include Black Sea), throughout North Africa from Cape Blanc, Mauritania north and eastwards to the Suez Canal; also along the eastern Atlantic coast northwards to Trondheim (Norway) and Baltic Sea (Miller, 1986). It can be found on sandy and muddy bottoms, in depth between 2 and 70 m (Hureau & Monod, 1973, Vesey & Langford, 1985). Also, it is able to live in brackish water down to %o 6 S (Vaas et al., 1975). This species is distributed on all coasts of Turkey (Bilecenoglu et al., 2002).
There are numerous studies about its abundance, distribution and habitat selection (Claridge et al., 1986; Wiederholm, 1987; Costello, 1992; Koutrakis et al., 2000; Gordo & Cabral, 2001; Letourneur et al., 2001; Malavasi et al., 2005), biology (Vaas et al., 1975; Fabi & Froglia, 1984; Vesey & Langford, 1985; Doornbos & Twisk, 1987; Arruda et al., 1993; Silva & Gordo, 1997; Bouchereau & Guelorget, 1998), growth (Fabi & Giannetti, 1985), feeding ecology and behavior (Casabianca & Kiener, 1969; McGrath, 1974; Fabi & Froglia, 1983; Magnhagen, 1988; Fernandez et al., 1995; Labropoulou & Markakis, 1998; Labropoulou & Papadopoulou-Smith, 1999), reproductive biology (Bonnin, 1971a, 1971b, 1975, 1977, 1981, 1984, 1989; Bonnin & Croizet, 1972, 1973; Holt & Byrne, 1988; Magnhagen, 1990, 1991; Joyeux et al., 1991b, 1992; Marconato et al., 1996; Pampoulie et al., 1999; Locatello et al., 2002; Mazzoldi & Rasotto, 2002; Pilastro et al., 2002; Rasotto & Mazzoldi, 2002; Immler et al., 2004; Mazzoldi et al., 2005; Scaggiante et al., 2005), genetic (Colombera & Rasotto, 1982; Vitturi & Catalano, 1989; Hoeglund & Thomas, 1992; Zander & Kesting, 1998; Sorice & Caputo, 1999; Mandrioli et al., 2001), parasites (Leiro et al., 1984; Loubes et al., 1984; Dezfuli et al., 1992; Zander, 2003; Kvach, 2005), and environmental effects and pollution (Bouchereau, 1997; Cunha & Antunes, 1999; Pampoulie et al., 2001; Antunes & Cunha, 2002; Arcos et al., 2002; Katalay & Parlak, 2002, 2004a, 2004b; Carnevali & Maradonna, 2003; Maradonna et al., 2004; Katalay et al., 2005; Migliarini et al., 2005). Also, length-weight relationships were given by Abdallah (2002), Cicek et al. (2006), and Verdiell- Cubedo et al. (2006).
Although this species is common in the Aegean Sea, surprisingly, there is no published study about its biology in Turkish waters. Since it has no commercial value in Turkey, probably, fisheries biologists did not give enough attention of its biology. However, this species has important ecologic values. Black goby is one of the species that used in monitoring pollution (as an indicator species) (Katalay & Parlak, 2002). Also, this species has an important role in the food chain in Izmir Bay (Bilecenoglu, 2003).
This paper provides the first information on the black goby, an important species of the Izmir Bay, in Aegean Sea, specifically on its age, growth, reproduction and feeding.
Material and Methods
Izmir Bay is situated at the western coast of the Anatolian peninsula, and is connected to the Aegean Sea. The bay is roughly ‘‘L’’ shaped. The leg of the ‘‘L’’ is about 20 km wide and 40 km long and the base of the ‘‘L’’ is about 5–7 km wide and 24 km long (Figure 1). The Izmir Bay has been divided into three areas according to their physical characteristics. These are Outer, Middle and Inner Bay (Sayin, 2003).
Figure 1. Map showing the location of the Izmir Bay.
All kind of fisheries activity is prohibited in inner part of Izmir Bay because it has highly polluted by both domestic and industrial wastes. Also, southeast part of the line combined Ard?ç Cape (38° 31' 58" N - 26° 37' 22" E) and Deveboynu (38° 39' 24" N - 26° 43' 42" E) is prohibited all kind of trawl by the Ministry of Agriculture and Rural Affairs (Anonymous, 2007). Therefore, purse seine, hook-and-line and gill net are important fisheries methods in the Izmir Bay (Bileceno?lu, 2003). Because presence of economically important demersal fish species, it is known that illegal fisheries can be occurred (Metin et al., 2000). Main fisheries activity in the Bay rely on pelagic fish stocks (sardine, anchovy, mackerel, etc.) (Cihangir et al., 1999). There is no statistical data about caught fish amount in Izmir Bay (Bilecenoglu, 2003). Therefore, Kara & Gurbet (1999) reported that there is totally 2256 boat in the Bay and nearly 20000 tones fish caught yearly.
Izmir Bay was sampled monthly between March 2003 and February 2004. Specimens obtained via trawl (n= 78), and fishing line (n= 1071). Trawl specimens were sampled by R/V EGESUF (27 m length and 463 HP). A conventional bottom trawl net of 22 mm cod-end mesh size was used and three hauls in same day were carried out and haul durations stabilized 30 minutes. The vessel speed was maintained at 2.5 knots. Depth of the fishing ground was 30 m. fishing line sampling carried out in first, middle and last weeks of every month. A fishing line named “sinek oltasi” was used (no. 18 needle, 0.20 mm main body and 25 g lead weight). Fish were caught in the Izmir Bay (where they abundant) in three locations between 09:00 and 12:00 a.m. (Table 1). All the specimens were injected to abdomen region with 4% alcohol and frozen.
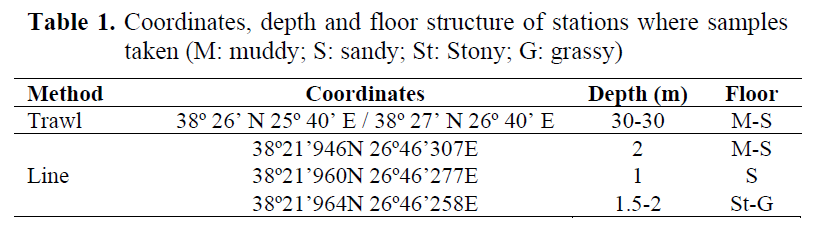
Table 1. Coordinates, depth and floor structure of stations where samples taken (M: muddy; S: sandy; St: Stony; G: grassy)
In the laboratory, the total length of all individuals was measured to the nearest millimeter below (later grouped in 1.0 cm length classes) and weighed to the nearest 0.01 g total weight. Sex was recorded and the gonads were weighed to the nearest 0.001 g (in the males, sperm-duct gland was not included in the gonadal weight). Sagittal otoliths were removed, cleaned, dried and stored in labeled plastic tubes.
For age determination, the otoliths of specimens were examined under a stereoscope microscope with a reflected light (x14 magnification). The otoliths were read by three readers and when there was no agreement among the three readings, the otolith was excluded. Age was expressed in years, the birthday of the fish being considered to be 1 January.
The mean lengths at age were analyzed separately per sex and were compared statistically using Student’s t test (p>0.05).
To examine fish growth, a von Bertalanffy growth equation (VBGE) was calculated using the iterated least square method (Sparre et al., 1989).
The relationship between weight and TL, W = aLb, was converted into logarithmic expression: ln W = ln a + b ln L. Parameters a and b were calculated by least-squares regression, as was the coefficient of determination (r2). Significant difference of b values from 3, which represent isometric growth, was tested with the t-test (Pauly, 1993).
To quantify the changes that occurred in the gonads during the annual sexual cycle and to determine the spawning season, the gonadosomatic index (GSI) (expressing gonadal weight as a percentage of total weight minus gonadal weight) (King, 1995) was calculated, between March 2003 and January 2004, monthly and per sex, their mean values being calculated from the individual ones. In order to determine first sexual maturation length and age, a logistic curve equation was used (King, 1995):
P = 1 / (1 + exp[ -r (L - Lm)])
here, P, proportion of mature individuals; r, slope of curve; L, total length, and Lm, mean length at maturity length or mean length of individuals where 50% of population is reproduced.
Sex ratio (males/females) was calculated by age.
Prey items in each stomach were identified to group level, measured, counted and weighed on an electronic balance (precision 0.0001 g). Diet composition was evaluated using three measures described by Hyslop (1980): the numerical index (%N); the gravimetric index (%W), and frequency of occurrence (%O). Based on Cortes' (1997) suggestion, the index of relative importance (IRI) was calculated and expressed as a percentage (%IRI). Subsequently, food items were grouped into categories of preference using the method proposed by Morato et al. (1998) and described by Sever et al. (2008). The categories were defined as follows:
IRI≥ 30*(0.15*Σ%O) main important prey (MIP)
30*(0.15*Σ%O)>IRI>10*(0.05*Σ%O) - secondary prey (SP)
IRI≤10*(0.05*Σ%O) occasional prey (OP)
Results and Discussion
Age and growth
The mean values of temperature recorded for the bay during the sampling period are shown in Figure 2. The lowest value of temperature (11.3°C) was found in February 2004 and the highest (27.8 °C) in August 2003.
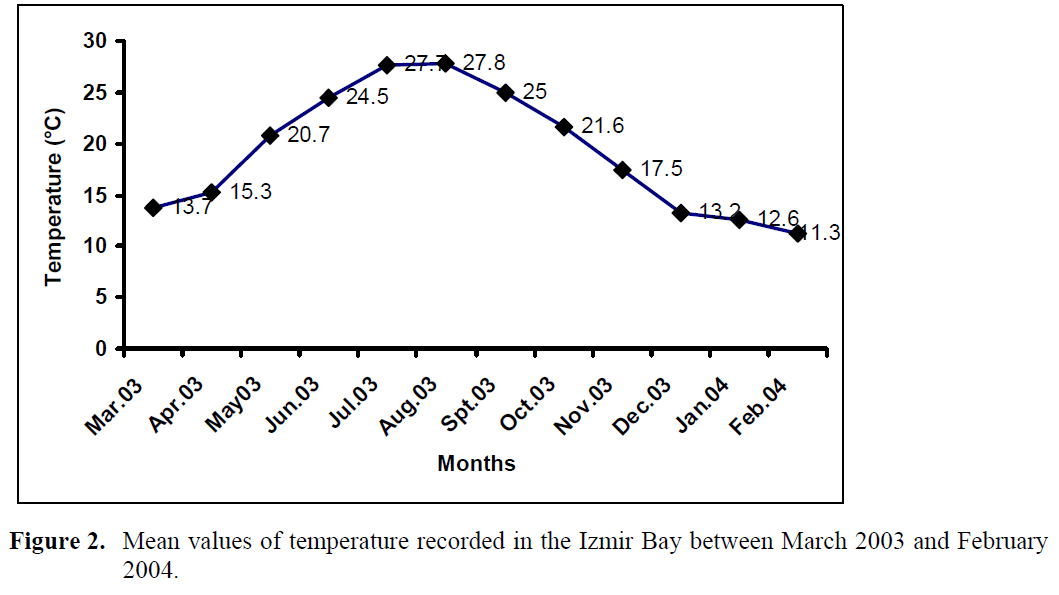
Figure 2. Mean values of temperature recorded in the Izmir Bay between March 2003 and February 2004.
Table 2 shows the descriptive statistics regarding length and weight data of all and sexed individuals.
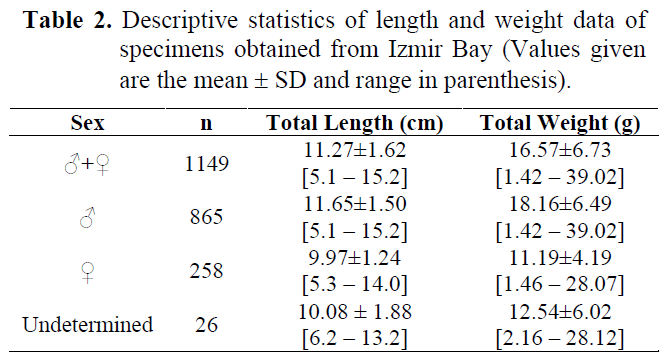
Table 2. Descriptive statistics of length and weight data of specimens obtained from Izmir Bay (Values given are the mean ± SD and range in parenthesis).
Among the 1149 black goby which were otolith’s taken, 49 individuals could not be aged since both there were undetermined specimens (n= 26) and there was no agreement between readers (n= 23). Thus, 865 (78.6%) males and 235 (21.4%) females were used for direct reading on otoliths (Table 3 and 4).
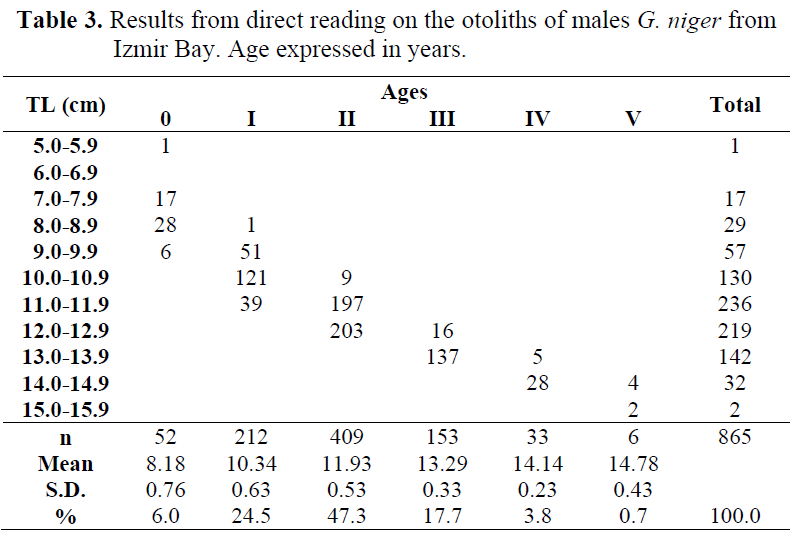
Table 3. Results from direct reading on the otoliths of males G. niger from Izmir Bay. Age expressed in years.
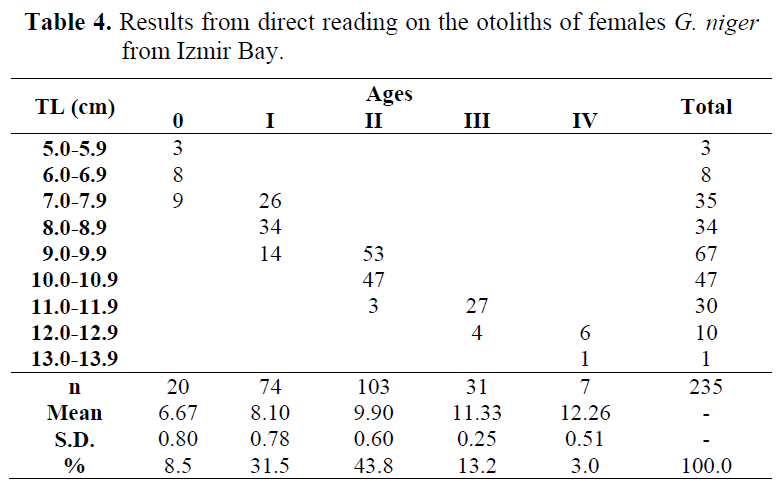
Table 4. Results from direct reading on the otoliths of females G. niger from Izmir Bay.
The length range by age group for both males and females was different. Application of the Student’s t-test to the mean lengths and standard deviations per age groups showed that there was significant difference between sexes (Table 5). The test was not applied to both males and females of age V because there was no female in this age. So, results from direct reading on the otoliths were not pooled, and calculations were made by sex separately.
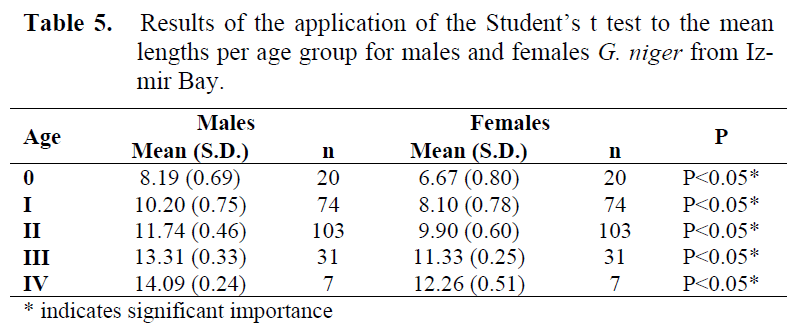
Table 5. Results of the application of the Student’s t test to the mean lengths per age group for males and females G. niger from Izmir Bay.
To describe the growth of the black goby population found in Izmir Bay, a VBGE, based on the males and females data, was established (Table 6).
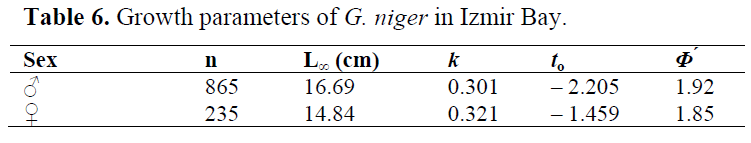
Table 6. Growth parameters of G. niger in Izmir Bay.
The length-weight relationships were calculated for each sex (Table 7).
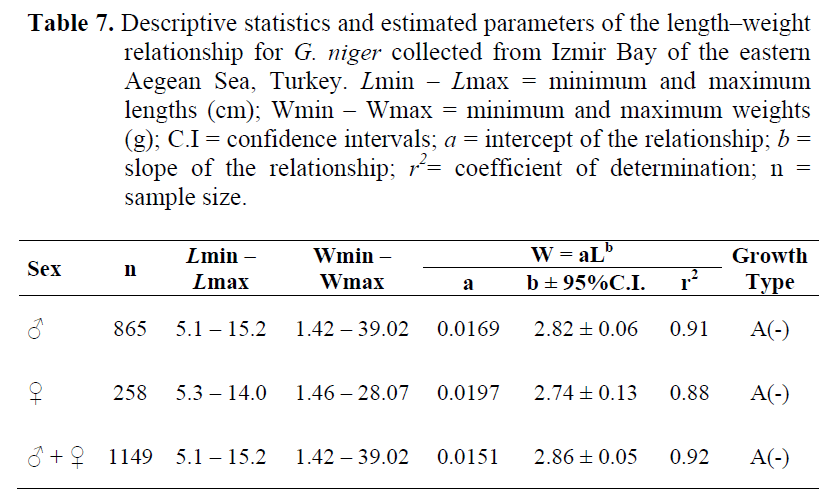
Table 7. Descriptive statistics and estimated parameters of the length–weight relationship for G. niger collected from Izmir Bay of the eastern Aegean Sea, Turkey. Lmin – Lmax = minimum and maximum lengths (cm); Wmin – Wmax = minimum and maximum weights (g); C.I = confidence intervals; a = intercept of the relationship; b = slope of the relationship; r2= coefficient of determination; n = sample size.
Reproduction
Monthly changes in gonadosomatic index (GSI) for females are shown in Figure 3. GSI values remain similar between November and February, and then show a marked increase in March. Therefore, this month seems to be the beginning of the spawning season which lasts until November.
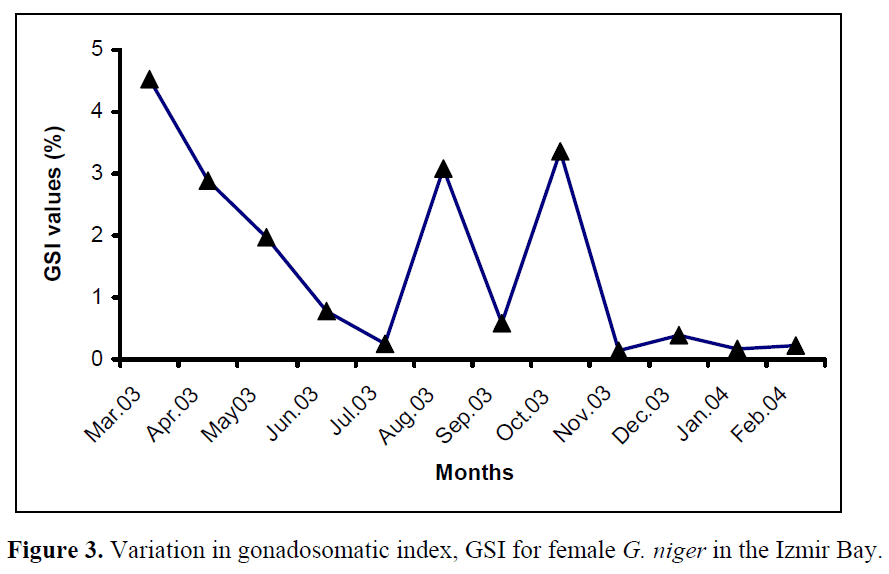
Figure 3. Variation in gonadosomatic index, GSI for female G. niger in the Izmir Bay.
Comparing the GSI values attained by females it can be noticed that, during the reproduction period, females reach GSI values of about 18.2%.
The sex ratio was found as 3.3:1 males were dominant in all ages (Table 8), as in all months and seasons.
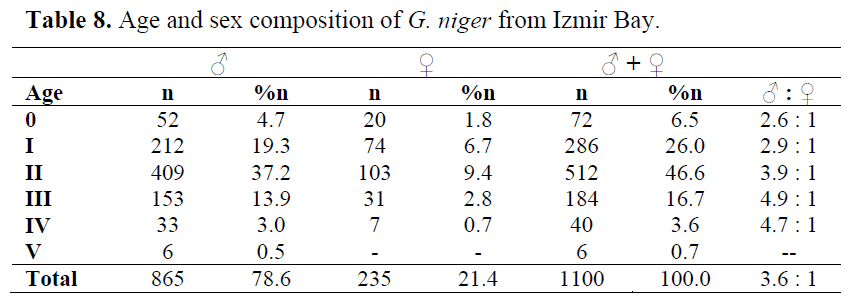
Table 8. Age and sex composition of G. niger from Izmir Bay.
Females reached sexual maturity at 7.80 cm TL and before I year old (0.86 year) (Figure 4 and 5). The smallest female had well developed gonad was 7.50 cm TL.
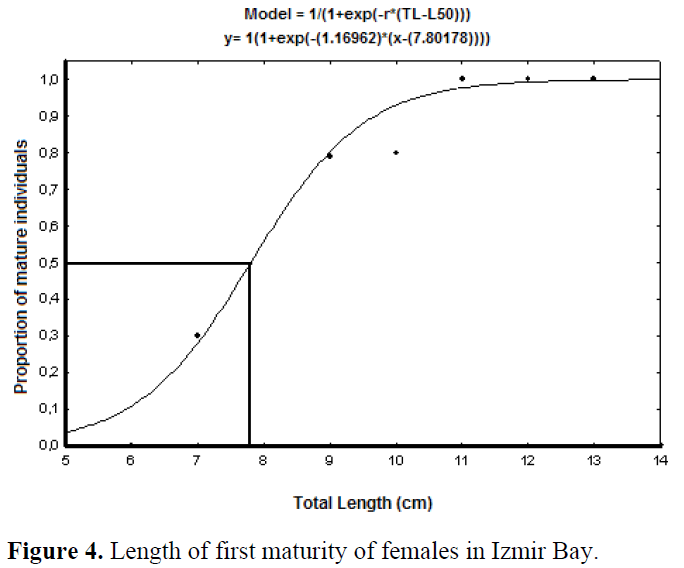
Figure 4. Length of first maturity of females in Izmir Bay.
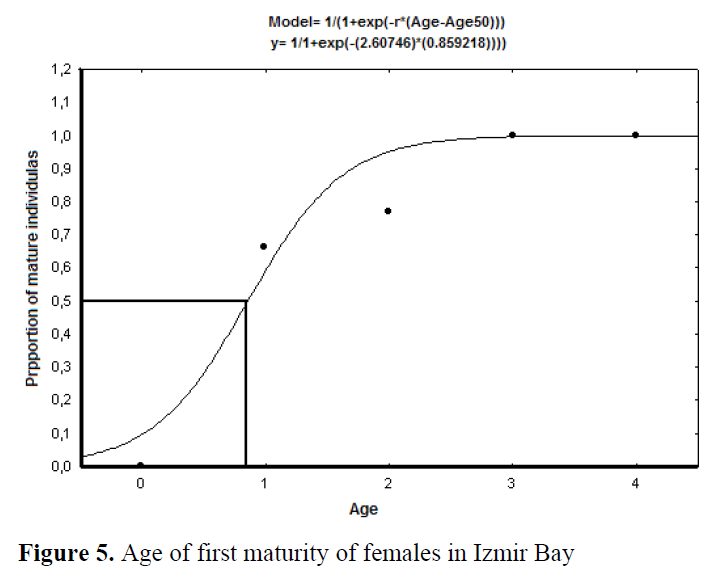
Figure 5. Age of first maturity of females in Izmir Bay
Food
Of the 508 black goby stomachs examined, 489 had food (96.3%) and 19 were empty (3.7%). Mollusca, Crustacea and Polychaeta were found to be most important prey group (MIP; IRI≥561) in the diet. Foraminifera constituted the secondary prey group (SP; 561>IRI>63), whereas Teleost fishes were an occasional prey group (OP; IRI≤63). Mollusca, Crustacea and Polychaeta constituted of 98.73% of the diet. Foraminifera and Teleostei comprised 1.14 and 0.13% of the diet, respectively (Table 9).
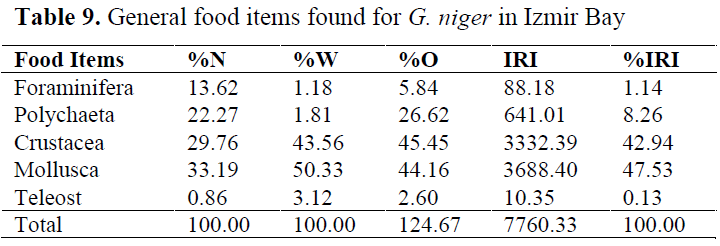
Table 9. General food items found for G. niger in Izmir Bay
In order to determine whether any difference between seasons, stomach contents were examined for each season (Table 10-13). Generally, Crustacea and Mollusca were found as important prey items in all seasons. Teleosts never consumed in spring, while they eaten occasionally in other seasons (Table 14).
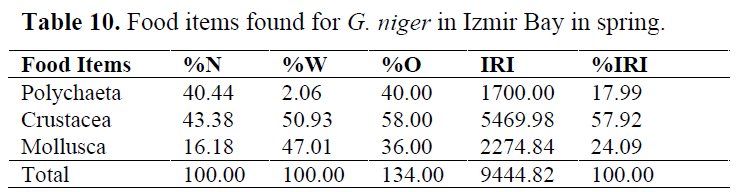
Table 10. Food items found for G. niger in Izmir Bay in spring.
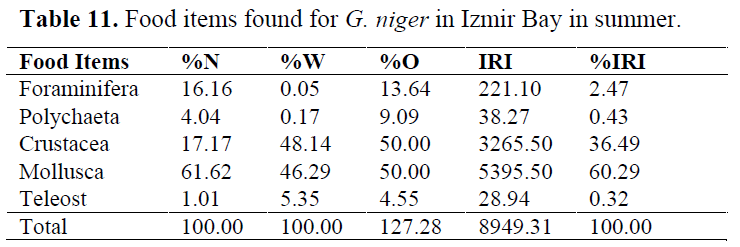
Table 11. Food items found for G. niger in Izmir Bay in summer.
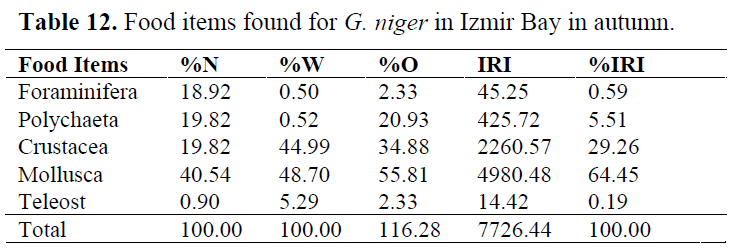
Table 12. Food items found for G. niger in Izmir Bay in autumn.
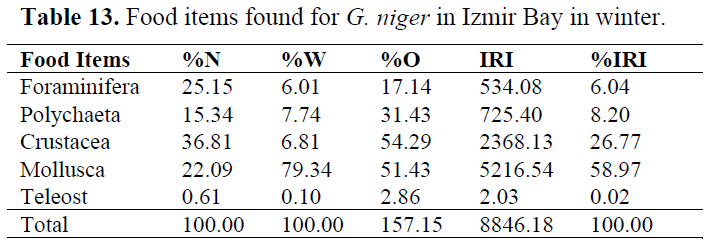
Table 13. Food items found for G. niger in Izmir Bay in winter.
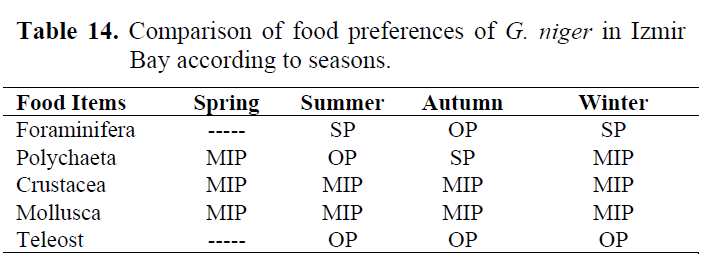
Table 14. Comparison of food preferences of G. niger in Izmir Bay according to seasons.
In order to determine whether any difference between ages, stomach contents are examined for each age group (Table 15-20). Generally, Crustacea and Mollusca were found as important prey items in all ages. While Teleosts were consumed as occasianlly by 1 year old specimens, they became secondary in 3 and important in 4 years old (Table 21).
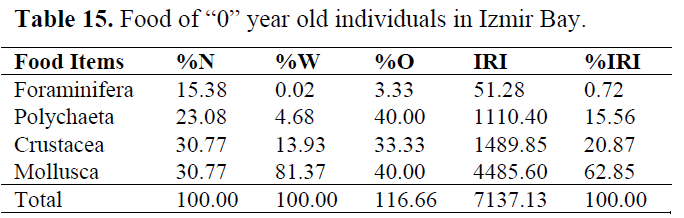
Table 15. Food of “0” year old individuals in Izmir Bay.
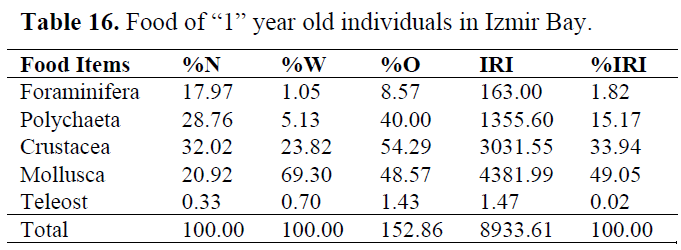
Table 16. Food of “1” year old individuals in Izmir Bay.
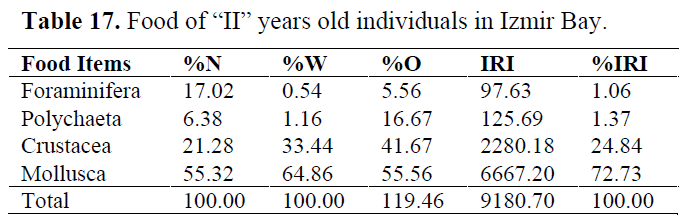
Table 17. Food of “II” years old individuals in Izmir Bay.
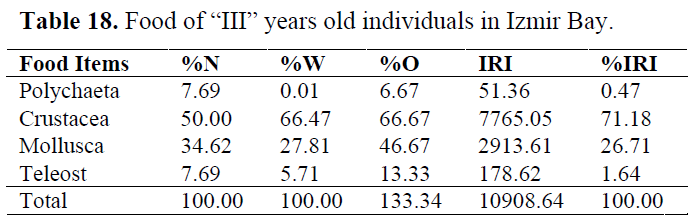
Table 18. Food of “III” years old individuals in Izmir Bay.
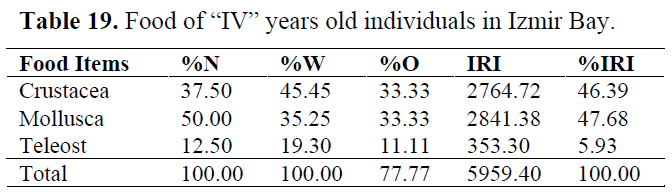
Table 19. Food of “IV” years old individuals in Izmir Bay.
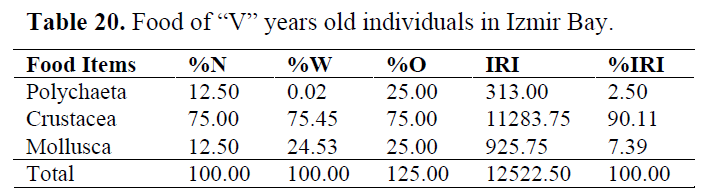
Table 20. Food of “V” years old individuals in Izmir Bay.
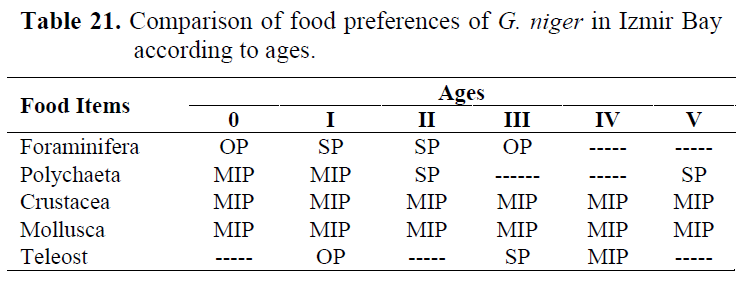
Table 21. Comparison of food preferences of G. niger in Izmir Bay according to ages.
In order to determine whether any difference between sexes, stomach contents were examined for each sex. Mollusca and Crustacea were found as important prey items in both sexes. Teleosts consumed occasionally (Table 22 and 23).
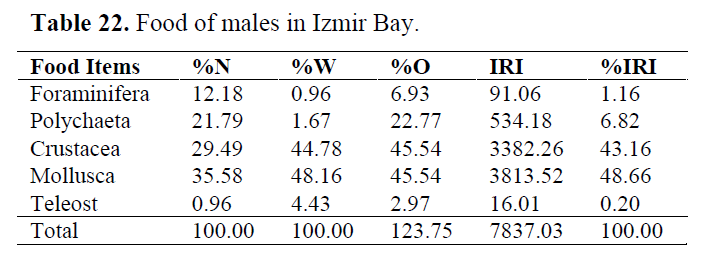
Table 22. Food of males in Izmir Bay.
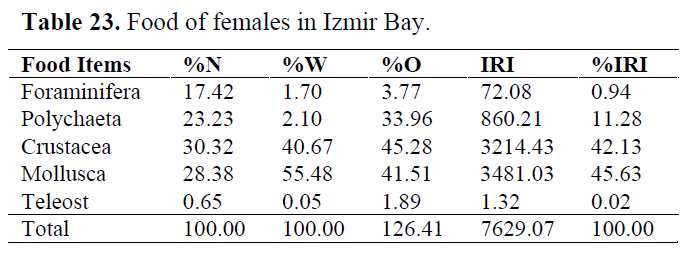
Table 23. Food of females in Izmir Bay.
Age and growth
There are various studies providing information about maximum lengths of the species both in Mediterranean and Atlantic. These studies give us a chance to make a comparasion (Table 24). As it is seen, maximum length in Izmir Bay is a little higher than those obtained in other parts of Mediterranean (except central Adriatic) and quite higher than those obtained from Atlantic. It seems that Mediterranean populations can be attained much bigger lengths from Atlantic ones. Whereas, expected outcome is that individuals living in Atlantic must be bigger. Sampling methods used may affect to this situation. It can be difficult to obtain bigger individuals in Atlantic by trawls since black gobies possess nest in the sand or under some substratum like stone, rocks or shells. Therefore, we obtained a big part of samples via fishing line in the shallow zone. This allowed us be able to see individuals when fishing and bigger male individuals attacker bait. Additionally, if we consider that most of the Mediterranean studies are made in lagoon areas, temperature and food availability can affect in this outcome.
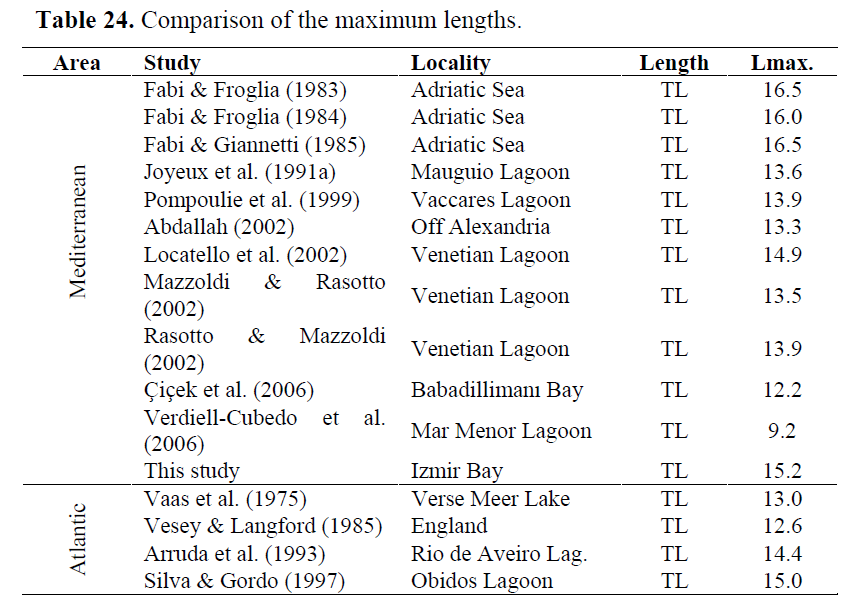
Table 24. Comparison of the maximum lengths.
Age determination from direct observations on the otoliths resulted in the establishment of six age groups (0, I, II, III, IV and V) for the population. Among the 1100 specimen aged, 865 (78.6%) were males and 235 (21.4%) were females. Age group II and I included 46.6% and 26.0% of the population, respectively. Age group V was consisted of only males (Table 25).
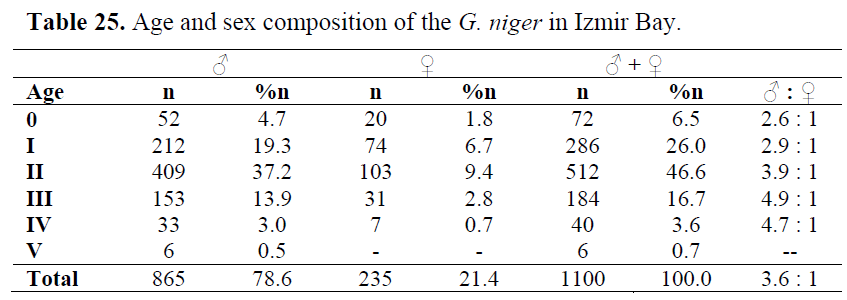
Table 25. Age and sex composition of the G. niger in Izmir Bay.
The maximum age reached by specimens of black goby (5 years) from the Izmir Bay is within the longevity limits observed over the biogeographical distribution area (Table 26). Indeed, if black goby longevity (5 year) is similar on the Adriatic (Fabi & Giannetti, 1985) and Norwegian coasts (Nash, 1984), the life span seems to be shorter (2-3 years) in the Atlantic lagoons and estuaries. From these results, it can be concluded that the populations living in the Aegean and Adriatic Sea are the ones that reach the maximum age (5) and close to the maximum length ever recorded (16.5cm).
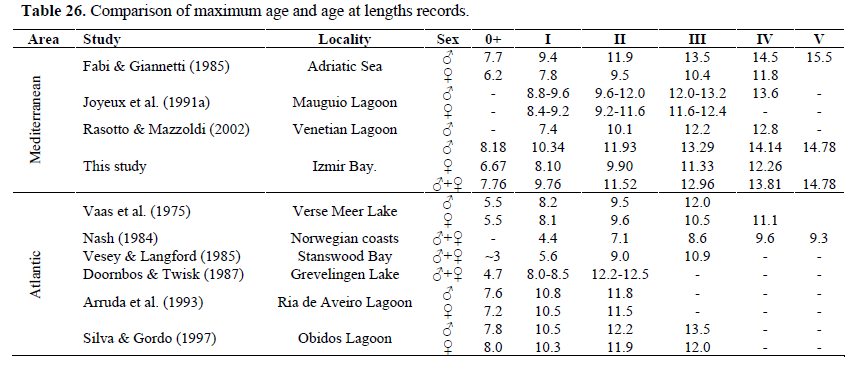
Table 26. Comparison of maximum age and age at lengths records.
In terms of mean length per age group significant differences were found between the two sexes (Table 5). Males attain a bigger length than females. These differences between male and female growth rates and life span have already been noticed by Vaas et al. (1975), Fabi & Froglia (1984), Fabi & Giannetti (1985), Joyeoux et al. (1991a), Arruda et al. (1993), Silva & Gordo (1997) and Bouchereau & Guelorget (1998) (Table 26). Just about any factor that might possibly influence growth has been shown to have an effect, including temperature, food availability, nutrient availability, light regime, oxygen, salinity, pollutants, current speed, predator density, intraspecific social interactions and genetics (Helfman et al., 1997). These factors, often working in combination, create large variations in size of fishes of the same and different ages (Helfman et al., 1997).
In this study, growth rate of the population has been found relatively low. Since the earlier spawning fish has slower somatic (body) growth (Helfman et al., 1997), this finding was expected. However, the growth coefficient is highly variable among different studies (k= 0.19-0.91) (Table 27). The asymptotic length (L∞) estimated in this study was within the observed total length of males and females. When compared with the the previous studies made in Mediterranean (Table 27), calculated growth performance index (Φ) value for the Izmir Bay was higher than that found by Fabi & Giannetti (1985). Analyzing the same table for Atlantic, it can be seen that our Φ value was lower than those calculated by Vesey & Langford (1985) and Silva & Gordo (1997). Although there were observed differences, no significant differences were found among our Φ value and ones obtained for Mediterranean and Atlantic from previous studies (p> 0.05).
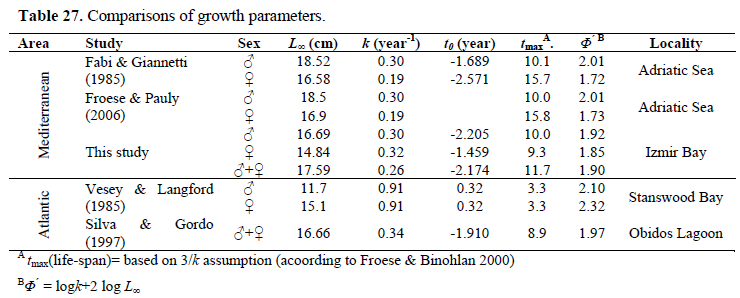
Table 27. Comparisons of growth parameters.
Bouchereau & Guelorget (1998) interpreted the differences in the growth rates: “G. niger growth is different in the Atlantic and the Mediterranean. Growth rate in the Mediterranean populations is, during the first year, clearly higher than of Atlantic populations. Afterwards, it decrases strongly in the Mediterranean but regulate itself in Atlantic. After the third year, differences in size are less evident, but final performances are better in the Mediterranean (136 mm) than in the Atlantic (130 mm).
A comparison of published length–weight relationships for the species is given in Table 28. The values of the slope (b) ranged from 2.86 to 3.39 and our results remained within the ranges given. Our b value (2.86) has constituted of lowest limit. Concerning growth type, the length-weight relationships revealed negative allometry. The length-weight relationship in fishes can be affected by a number of factors, including season, habitat, gonad maturity, sex, diet and stomach fullness, health and preservation techniques, and differences in the length ranges of the specimen caught (Tesch, 1971; Wootton, 1998), which were not accounted for in the present study. Thus, differences in lengthweight relationships between this and other studies could potentially be attributed to the combination of one or more of the factors given above.
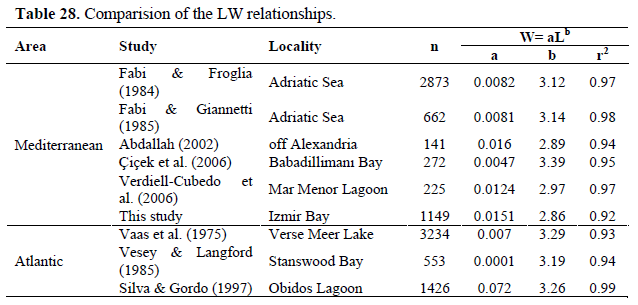
Table 28. Comparision of the LW relationships.
Reproduction
The male:female ratios obtained both in Mediterranean and Atlantic have shown in Table 29. In all seasons, including reproduction period, males were found as dominant. In previous studies, a decrease in the availability of the males has been reported, especially in spring and summer months (reproduction period). According to Miller (1984), this fact is very common among gobioid fishes since during the breeding season, there is a marked reduction in the proportion of males due to the egg protection behaviour, because they guard eggs under shells or stones and so less easily cauhgt. This egg protection behaviour of males also has been observed in free divings, especially made in reproduction period. So, the dominancy of the males in Aegean Sea does not mean that they have no egg protection. The most important factor in this differency is probably sampling method. Altought, studies claimed a decrease in the availability of the males especially in the reproduction period used beach siene nets (Arruda et al., 1993; Silva & Gordo, 1997; Bouchereau & Guelorget 1998; Pampoulie et al., 1999) and trawl (Vaas et al., 1975; Fabi & Froglia, 1984; Fabi & Giannetti, 1985; Vesey & Langford, 1985), in this study most of specimens was caught by hand line.
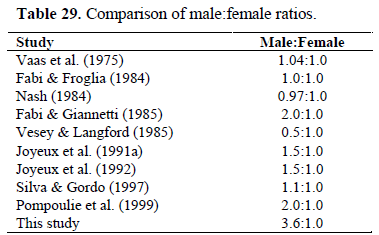
Table 29. Comparison of male:female ratios.
Monthly changes in gonadosomatic index for females are shown in Figure 3. A comparison between the spawning season of the black goby population living in Izmir Bay with those from other areas (Table 30) shows that there is a certain homogeneity in the spawning period, although the Northern populations have a shorter breeding season than the Southern ones. The longer spawning period of the latter can not be dissociated from the climatic conditions. In fact, the warmer temperature and longer day length have a large influence on gonadal development, favouring a longer spawning period (Joyeux et al., 1992; Silva & Gordo, 1997).
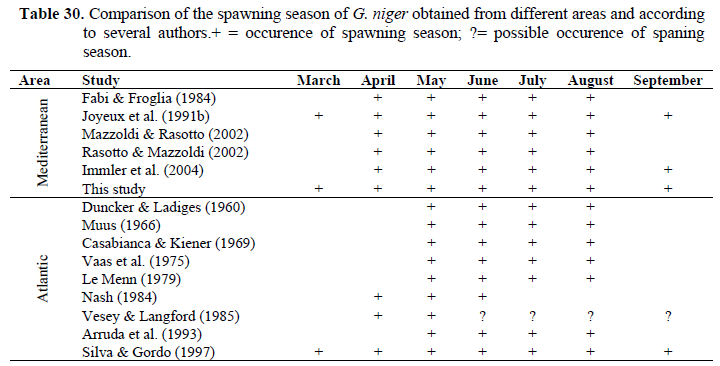
Table 30. Comparison of the spawning season of G. niger obtained from different areas and according to several authors.+ = occurence of spawning season; ?= possible occurence of spaning season.
Most individuals of the Izmir Bay populations probably reproduce in their first year. Females reached sexual maturity at 7.80 cm TL and before I year old (0.86 year) (Figure 4 and 5). The smallest female had well developed gonad was 7.50 cm TL. Obtained first maturity ages and lengths from previous studies were compared in Table 31.
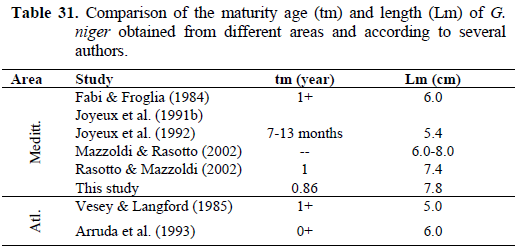
Table 31. Comparison of the maturity age (tm) and length (Lm) of G. niger obtained from different areas and according to several authors.
Vaas et al. (1975) reported that reproduction of the black goby starts when the water temperature exceeds 12 °C (in May in Veerse Meer). Measured water temperatures in Izmir Bay in March appeared to be support the relationship between reproduction and water temperature.
Food
In the Izmir Bay, the diet of black goby is based on small benthic invertebrates, generally similar to that of other populations (Table 32). Therefore, Foraminifera comprised 8.26% of the diet. We believed that those foraminiferans are ingested accidentally, together with the animal constituents of the diet.
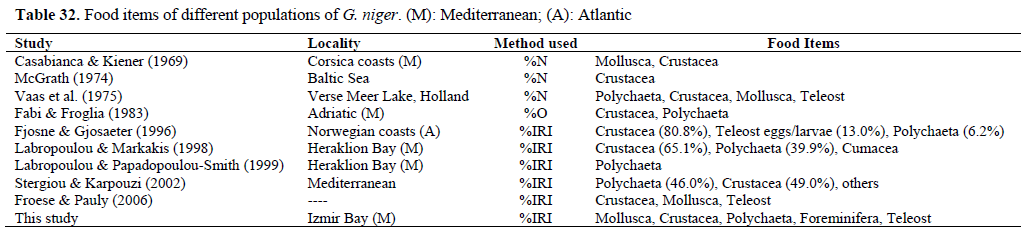
Table 32. Food items of different populations of G. niger. (M): Mediterranean; (A): Atlantic
1450
References
- nAnonymous, (2007), Republic of Turkey Ministry of Agriculture and Rural Affairs General Directorateof Protection And Control, “Notification 1/1 Regulating Commercial Fishing”, Ankara, Published in the Official Gazette date: 19.10.2007- 26675 Notification Number: 2007-43
- nAntunes, M., Lopes Da Cunha, P., (2002),Skeletal anomalies in Gobiusniger(Gobiidae) from Sado estuary, Portugal.Cybium, 26: 179-184
- nArcos, J. M., Ruiz, X., Bearhop, S., Furness, R.W., (2002), Mercury levels in seabirds and their fish prey at the Ebro Delta (NW Mediterranean): the role of trawler discards as a source of contamination. MarineEcology Progress Series, 232: 281-290
- nArruda, L. Azevedo, M., J. N., Neto, A. I., (1993), Abundance, age-structure and growth and reproduction of gobies (Pisces; Gobiidae) in the Ria de Averio Lagoon (Portugal). Estuarine, Coastal and Shelf Science, 37:509-523
- nBilecenoglu, M., Taskavak, E., Mater, S., Kaya, M., (2002), Checklist of the marine fishes of Turkey. Zootaxa113. Magnolia Pres, Auckland, 194 p
- nBilecenoglu, M., (2003), ModellingTrophicm Relationships Among Fishes Inhabiting Izmir Bay (in Turkish). PhD Thesis, Supervisor Kaya, M., Ege University Graduate School of Natural and Applied Sciences. Izmir, 156 pages
- nBonnin, J. P., (1971a), Organotypic cultures of thyroids of a marine teleostean fish: GobiusnigerL. Effects of TSH and prolactin. ComptesRendus des Séances de la Société deBiologieet de sesFiliales, 165: 1284-1291
- n-----(1971b), Study of the thyreotropic function in GobiusnigerL. by means of the association in vitro association of pituitary glands and thyroids. ComptesRendus des Séances de laSociété de Biologieet de sesFiliales, 165: 2321-2327
- n----(1975), Association in organ culture of the hypophysis and glandular tissue of the testis of GobiusnigerL. ComptesRendus desSéances de la Société de Biologie et de sesFiliales, 169: 920-923
- n----(1977), Radioimmunoassay of hormonal activity of GobiusnigerL. testicular interstitial tissue in organ culture: spontaneous activity and stimulation by pituitary gland. ComptesRendusHebdomadaires des Séances de l'Académiedes Sciences. Série D: Sciences naturelles, 284: 1827-1830
- n----(1981), Effect of ovine prolactin on plasma testosterone and the male genital tract of GobiusnigerL. ComptesRendus des Séancesde l'Académie des Sciences. Série III:Sciences de la vie, 292: 319-322
- n----(1984), Response of the seminal vesicles of GobiusnigerL. to castration, followed or without treatment with free or conjugated testosterone. ComptesRendus des Séances del'Académie des Sciences. Série III: Sciencesde la vie, 298: 119-122
- n----(1989), Prolactin and specific binding of testosterone in cultured cells of the seminal vesicle of GobiusnigerL. ComptesRendusdes Séances de l'Académie des Sciences.Série III: Sciences de la vie, 309: 435-440
- nBonnin, J. P., Croizet, M., (1972), Evaluation of hormonal activity in culture of thyroid explants of GobiusnigerL. ComptesRendusdes Séances de la Société de Biologie et desesFiliales, 166: 347-350
- n----(1973), In vitro action of dibutyryl-3'-5'-AMPc on thyroid explants of GobiusnigerL. ComptesRendus des Séances de la Société deBiologie et de sesFiliales, 167: 1106-1109.
- nBouchereau, J. L., (1997), Biodiversity of tactics used by three Gobiidae (Pisces; Teleostei): Pomatoschisthusminutus(Pallas, 1770), P.microps(Kroyer, 1838), Gobiusniger Linnaeus, 1758, to survive in a Mediterranean lagoon environment. Oceanological Studies, 2/3: 153-170
- nBouchereau, J-L., Guelorget, O., (1998), Comparision of three Gobiidae (Teleostei) life history strategies over their geographical range. OceanologiaActa, 21: 503-517
- nCarnevali, O., Maradonna, F., (2003), Exposure to xenobiotic compounds: looking for new biomarkers. General and ComparativeEndocrinology, 131: 203–209
- nCasabianca, M. L., Kiener, A., (1969), Gobides des etangscorses: systematique,.ecologie, regime alimentaire et position dans les chainestrophiques. Vie et Milieu, 20: 611- 634
- nCihangir, B., Önen, M., Kocataş, A., Ergen, Z., Mater, S., Koray, T., Katağan, T., Özel, İ., Demirkurt, E., Tıraşın, E.M., Ünlüoğlu, A., Çınar, M.E., Çolak, F., Çoker, T., Öztürk, B., Doğan, A., (1999), Some biological properties of Izmir Bay. Ecosystem '99:Workshop On The Role Of The Physical,Chemical And Biological Processes InMarine Ecosystems, 19-48, Izmir
- nClaridge, P.N., Hardisty, M.W., Potter, I.C., Williams, C.V., (1986), Abundance, Life History and ligulolsis in the gobies (Teleostei) of the inner seven estuary. Journal of the Marine Biological Associationof the United Kingdom, 65: 951-968
- nCarnevali, O., Maradonna, F., (2003), Exposure to xenobiotic compounds: looking for new biomarkers. General and ComparativeEndocrinology, 131: 203–209
- nCasabianca, M. L., Kiener, A., (1969), Gobides des etangscorses: systematique,.ecologie, regime alimentaire et position dans les chainestrophiques. Vie et Milieu, 20: 611- 634
- nMater, S., Koray, T., Katağan, T., Özel, İ., Demirkurt, E., Tıraşın, E.M., Ünlüoğlu, A., Çınar, M.E., Çolak, F., Çoker, T., Öztürk, B., Doğan, A., (1999), Some biological properties of Izmir Bay. Ecosystem '99:Workshop On The Role Of The Physical,Chemical And Biological Processes InMarine Ecosystems, 19-48, Izmir
- nClaridge, P.N., Hardisty, M.W., Potter, I.C., Williams, C.V., (1986), Abundance, Life History and ligulolsis in the gobies (Teleostei) of the inner seven estuary. Journal of the Marine Biological Associationof the United Kingdom, 65: 951-968
- nDoornbos, G., Twisk, F., (1987), Density, growth, and annual food consumption of gobiid fish in the saline lake Grevelingen, Netherlands. Netherlands Journal of Sea Research, 21: 45- 74
- nDuncker, G.W., Ladiges, W., (1960), Die Fische der Nordmark. Hamburg. Fabi, G., Froglia, C., (1983), Food and feeding of GobiusnigerL. in the central Adriatic Sea (Osteichthyes: Gobiidae). Rapports
- nCommission Internationale pour l’ExplorationScientifique de la MerMéditerree, 28: 99-102
- nFabi, G., Froglia, C., (1984). A note on the biology and fishery of the black goby (Gobiusniger) in the Adriatic Sea. Fish.Rep./FAO Rapp., 290: 167-170
- nFabi, G., Giannetti, G., (1985), Groth parameters of the balck goby (GobiusnigerL.) in the Adriatic Sea, based on otolith readings. Rapports Commission Internationale pourl’ExplorationScientifique de la MerMéditerree, 29: 87-90
- nFernandez, L., Freire, J., Gonzalez, G.E., (1995), Diel feeding activity of demersal fishes in the Ria de Arousa (Galicia, NW Spain): an area of intense mussel raft culture. Cahiers deBiologie Marine, 36: 141-151
- nFjosne, K., Gjosaeter, J., (1996), Dietary composition and the potential of food competition between )-group cod (GadusmorhuaL.) and some toher fish species in the littoral zone. ICES Journal of MarineScience, 53: 757-770
- nFroese, R., Binohlan, C., (2000), Empirical relationships to estimate asymptotic length, length at first maturity, and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequencydata. Journal of Fish Biology, 56: 758-773
- nFroese, R., Pauly, D., (2006), Fishbase World Wide Web electronic publications. www.fishbase.org, 20 Sept. 2006
- nGordo, L.S., Cabral, H.N., (2001), The fish assemblage structure of a hydrologically altered coastal lagoon: the Obidos lagoon (Portugal). Hydrobiologia, 459: 125-133
- nHelfman, G. S., Collette, B.B., Facey, D.E., (1997), The diversity of fishes. Blackwell Science, United States of America. Hoeglund J., Thomas, K., (1992), The black goby Gobiusnigeras a potential paratenic host for the parasitic nematode Anguillicolacrassus in a thermal effluent of the Baltic. Diseasesof Aquatic Organisms, 13:175-80
- nHolt, E.W.L., Byrne, L.W., (1988), Notes on the reproduction of Teleostean fishes in the South-western district. Mittheil. Zool. Neap., 8: 333-340
- nHureau, J-C., Monod, T., (1973), Check-list of the Fishes of the North-Eastern Atlantic and Mediterranean (CLOFNAM I), Unesco, Paris
- nHyslop, E. J., (1980), Stomach content analysis –a review of methods and their application. Journal of Fish Biology, 17: 411-429
- nImmler, S., Mazzoldi, C., Rasotto, M.B., (2004). From sneaker to parental male: change of reproductive traits in the black goby, Gobiusniger(Teleostei, Gobiidae). Journal ofExperimental Zoology, 301: 177-185
- nJoyeux, J.C., Bouchereau, J.L., Tomasini, J.A., (1991a), Croissanceet structure demographie de la population de GobiusnigerLinne, 1758. (poissionteleosteen) dansunolagunenordmediterraneene. Cahiers de BiologieMarine, 32: 415-437
- nJoyeux, J.C., Bouchereau, J.L., Tomasini, J.A.,(1991b), La reproduction de Gobiusniger
- n(Pisces, Gobiidae) danslagune de Mauguio- France: rapports gonosomatiques, fecondites, ponte, oeufsetlarves. Vie etMillieu, 41: 97- 106
- nJoyeux, J.C., Tomasini, J.A., Bouchereau, J.L., (1992), Modalites de la reproduction de Gobiusniger(Teleostei, Gobiidae) dansunelagunemediterraneenne. Vie et Milieu, 42: 1- 13
- nKara, Ö.F., Gurbet, R., (1999), Investigation on Industrial Fishery of the Aegean Sea (in Turkish). Republic of Turkey Ministry of Agriculture and Rural Affairs 5: 1-135
- nKatalay, S., Parlak, H., (2002), The effects of water pollution on blood paremeters of black gobby (GobiusnigerLinn, 1758) (in Turkish). E.U. Journal of Fisheries &Aquatic Sciences, 19: 115-121
- nKatalay, S., Parlak, H., (2004a), The effects of cadmium on erythrocyte structure of black goby (GobiusnigerL., 1758) (in Turkish). E.U. Journal of Fisheries & AquaticSciences, 21: 99-102
- nKatalay, S., Parlak, H., (2004b), The Effects of Pollution on Haematological Parameters of Black Goby (GobiusnigerL., 1758) in Foça and Aliağa Bays. E.U. Journal of Fisheries &Aquatic Sciences, 21: 113-117
- nKatalay, S., Parlak, H., ÇakalArslan, Ö., (2005), The accumulation of some heavy metals in the liver tissues of black gobby (Gobiusniger L., 1758) living in the Aegean Sea (in Turkish). E.U. Journal of Fisheries &Aquatic Sciences, 22: 385-388
- nKing, M.., (1995), Fisheries Biology Assesment and Management. Fishing News Books, Oxford
- nKoutrakis, E.T., Kokkinakis, A.K., Eleftheriadis, E.A., Argyropoulou, M.D., (2000), Seasonal changes in distribution and abundance of the fish fauna in the two estuarine systems of Strymonikos gulf (Macedonia, Greece). Belgian Journal of Zooogy, 130: 41-48
- nKvach, Y., (2005), A comparative analysis of helminth faunas and infection parameters of ten species of gobiid fishes (Actinopterygii: Gobiidae) from the North-western Black Sea. ActaIchthyologicaetPiscatoria, 35: 103- 110
- nLabropoulou, M., Markakis, G., (1998), Morphological-dietary relationships within two assemblages of marine demersal fishes. Environmental Biology of Fishes, 51: 309-319
- nLabropoulou, M., Papadopoulou-Smith, K.N., (1999). Foraging behaviour patterns of four sympatric demersal fishes. Estuarine Coastaland Shelf Science, 49: 99-108
- nLe Menn, F., (1979), Some aspects of vitellogenesis in a teleostean fish: GobiusnigerL. Comparative Biochemistry andPhysiology, 62: 495-500
- nLetourneur, Y., Darnaude, A., Salen-Picard, C., Harmelin-Vivien, M., (2001), Spatial and temporal variations of fish assemblages in a shallow Mediterranean soft-bottom area (Gulf of Fos, France). OceanologicaActa, 24: 273-285
- nLocatello, L., Mazzoldi, C., Rasotto, M.B., (2002), Ejaculate of sneaker males is pheromonallyinsconspicuous in the black goby, Gobiusniger(Teleostei, Gobiidae. Journal of Experimental Zoology, 293: 601- 605
- nLoubes, C., Maurand, J., Gosc, C., De Burun, I., Borral, J., (1984), Etude ultrastructurale de
- nLoma dimorphan. Sp., microsporidie parasite de poissonsGobiidaelanguedociens. Protistologica4: 579-589
- nMagnhagen, C., (1988), Changes in foraging as a response to predation risk in two gobiid fish species, Pomatoschistusminutusand Gobiusniger. Marine Ecology Progress Series, 49: 21-26.Magnhagen, C., (1990), Reproduction under
- npredation risk in the sand goby, Pomatoschistusminutus, and the black goby, Gobiusniger: the effect of age and longevity. Behavioral Ecology and Sociobiology, 26: 331–335
- nMagnhagen C., (1991), Predation risk as cost of reproduction. Trends in Ecology andEvolution, 6:183–186
- nMalavasi, S., Franco, A., Fiorin, R., Franzoi, P., Torricelli, P., Mainardi, D., (2005), The shallow water gobiid assemblage of the Venice Lagoon: abundance, seasonal variation and habitat partitioning. Journal ofFish Biology, 67: 146-165
- nMandrioli, M., Manicardi, G.C., Machella, N., Caputo, V., (2001), Molecular and cytogenetic analysis of the goby Gobiusniger(Teleostei, Gobiidae). Genetica, 110: 73–78
- nMaradonna, F., Polzonetti, V., Bandiera, S.W., Migliarini, B., Carnevali, O., (2004), Modulation of the hepatic CYP1A1 system in the marine fish Gobiusniger, exposed to xenobiotic compounds. EnvironmentalScience and Technology, 38: 6277-6282
- nMarconato, A., Rasotto, M.B., Mazzoldi, C., (1996), On the mechanism of sperm release in three gobiid fishes (Teleostei: Gobiidae). Environmental Biology of Fishes, 46: 321- 327
- nMazzoldi, C., Rasotto, M.B., (2002), Alternative male mating tactics in Gobiusniger. Journalof Fish Biology, 61: 157–172
- nMazzoldi, C., Petersen, C.W., Rasotto, M.B., (2005), The influence of mating system on seminal vesicle variability among gobies (Teleostei, Gobiidae). Journal of ZoologicalSystematics, 43: 307-314.
- nMcGrath, D., (1974), Preliminary studies on the feeding of GobiusnigerL. and Gobiusflavescens(Fabricius) (Pisces, Gobiidae) in the Northern Baltic proper. Contr. Asko Lab., 4: 25 pp
- nMetin, C., Tosunoğlu, Z., Tokaç, A., Lök, A., Aydın, C., Kaykaç, H., (2000), Seasonal
- nvariations of demersal fish composition in Gülbahçe Bay (İzmir Bay). Turkish Journal of Zoology, 24: 437-446
- nMigliarini, B., Campisi, A.M., Maradonna, F., Truzzi, C., Annibaldi, A., Scarponi, G., Carnevali, O., (2005), Effects of cadmium exposure on testis apoptosis in the marine teleost Gobiusniger. General and
- nMiller, P.J., (1984), Thetokology of gobioid fishes. in Potts, G.W., Wootton, R.J (eds),Fish reproduction: strategies and tactics, 119-153, London
- nMiller, P. J., (1986), Gobiidae, in Whitehead et al., eds, Fishes of the Northern-Eastern Atlantic and the Mediterranean, UNESCO,1019-1085, Paris.
- nMorato, T. M., Sola, E., Gros, M.P., Menezes, G., Pinho, M.R., (1998), Trophic relationships and feeding habits of demersal fishes from the Azores: importance to multispecies assessment. In. International Council for theExploration of the Sea ICES CM 1998/O: 7, 34 pp
- nMuus, B.J., (1966), Zeevissengids. Elsevier, Amsterdam. Nash, R.D.M., (1984), Aspects of biology of the
- nblack goby, GobiusnigerL., in: Oslofjorden, Norway. Sarsia, 69: 55-61
- nPampoulie, C., Priour, F., Bouchereau, J.L., Rosecchi, E., Crivelli, A.J., (1999), Reproductive traits of Gobiusniger (Teleostei: Pisces) following a salinity stress: is it really a sedentary lagoon species. Journal of the Marine Biological Associationof the United Kingdom, 79: 961-962
- nPampoulie, C., Chauvelon, P., Rosecchi, E., Bouchereau, J.L., Crivelli, A.J., (2001), Environmental factors influencing the gobiid assemblage of a Mediterranean Lagoon: Empirical evidence from a long-term study. Hydrobiologia, 445: 175–181
- nPauly D., (1993), Use of Fish Base in the context of the BDRM. Annexe IX5. Page 141 in Atelier sur la Mise en Ouvre de la Base de DonnéeRégionale Maritime (BDRM), Dakar, Sénégal, 15-19 février 1993. Conference Ministeriellesur la Coopération entre les Etatsafricainsriverains de l'Océanatlantique,
- nDakar/Brussels. Pilastro, A., Scaggiante, G., Rasotto, M.B., (2002), Individual adjustment of sperm
- nexpenditure accords with sperm competition theory. Proceedings of the National-Academyof Sciences of the United States of America,99: 9913-9915
- nRasotto, M.B., Mazzoldi, C., (2002), Male traits associated with alternative reproductive tactics in Gobiusniger. Journal of FishBiology, 61: 173–184
- nSayın, E., (2003), Physical features of the Izmir Bay. Continental Shelf Research, 23: 957- 970
- nScaggiante, M., Rasotto, M.B., Romualdi, C., Pilastro, A., (2005), Territorial male gobies respond aggressively to sneakers but do not adjust their sperm expenditure. BehavioralEcology, 16: 1001-1007
- nSever, T.M., Filiz, H., Bayhan, B., Taskavak, E., Bilge, G., (2008), Food habits of the hollowsnout grenadier, Caelorinchuscaelorhincus(Risso, 1810), in the Aegean Sea, Turkey. Belgian Journal of Zoology, 138: 81-84
- nSilva, M. N., Gordo, L.S., (1997), Age, growth and reproduction of the black goby, Gobiusniger, from Obidos Lagoon, Portugal. Cahiers De Biologie Marine, 38: 175-180.
- nSorice, M., Caputo, V., (1999), Genetic variation in seven goby species (Perciformes: Gobiidae) assessed by electrophoresis and taxonomic inference. Marine Biology, 134: 327-333
- nSparre, P., Ursin, E., Venema, S.C., (1989), Introduction to tropical fish stock assesment. Part 1-Manual. FAO Fish. Tech. Pap. No: 306/1: 1-163. Stergiou, K. I., Karpouzi, V.S., (2002), Feeding habits and trophic levels of Mediterranean fish. Reviews in Fish Biology and Fisheries, 11: 217-254
- nTesch, F.W., (1977), The eel. Biology and management of anguillid eels (Translated by P.H. Greenwood). Chapman and Hall, London.
- nVaas, K.F., Vlasbom, A.G., Koeijer, P.de., (1975), Studies on the black goby (Gobies niger, Gobiidae, Pisces) in the Verse Meer, SW Netherlands. Netherlands Journal of Sea Research, 9: 56-68.
- nVerdiell-Cubedo, D., Oliva-Paterna, P.J., Torralva, M., (2006), Length-weight relationships for 22 fish species of the mar menor lagoon (western Mediterranean Sea). Journal of Applied Ichthyology, 22: 293-294.
- nVesey, G., Langford, T.E., (1985). The biology of the black goby, Gobiusniger L. in an English south-coast bay. Journal of Fish Biology, 27: 417-429
- nVitturi, R., Catalano, E., (1989), Multiple chromosome polymorphism in the gobiid fish Gobiusnigerjozo L. 1758 (Pisces, Gobiidae). Cytologia, 54: 231-235
- nWiederholm, A.M., (1987), Habitat selection and interactions between three marine fish species (Gobiidae). Oikos, 48: 28-32.
- nWootton, R.J., (1998). Ecology of Teleost fishes. Kluwer Academic Publishers, Dordrecht, Netherlands.
- nZander, C. D., Kesting, V., (1998), Colonization and seasonality of goby (Gobiidae, Teleostei) parasites from the southwestern Baltic Sea. Parasitology Research, 84: 459-466
- nZander, C. D., (2003), Four-year monitoring parasite communities in gobiid fishes.of the south-western Baltic. Parasitology Research, 90: 502–511