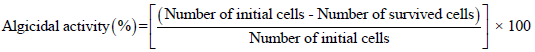Keywords
Algicide; Dinophyceae; HABs; Prodigiosin; Serratia
Introduction
Harmful algal blooms (HABs) have been accompanied by huge economic losses through massive fish deaths and a threat with shellfish poisoning in marine and freshwater ecosystems (Hallegraeff, 1993; Landsberg, 2002; Sellner et al., 2003). Moreover, approximately 2,000 cases of human poisoning resulting from algal toxins are reported each year (Zingone and Enevoldsen, 2000). A number of methods of bloom control have been investigated. Many chemical (e.g. copper sulphate, ozone) and physical (e.g. clay/algal flocculation, ultrasonic irradiation) methods have been proposed to mitigate or control HABs (Anderson, 1997; Kim, 2006). However, most of these methods are inapplicable because of high costs and secondary pollution. Therefore, research on economic and feasible approaches for algae removal has important theoretical and practical significance (Pei et al., 2005). Recently, some studies have demonstrated that the bacteria could lyse alga cells by producing extracellular substances, such as protease (Lee et al., 2000b), hydroxylamine (Berger et al., 1979), antibiotics (Dakhama et al., 1993), and aminophenol (Yoshikawa et al., 2000; Yamanoto et al., 1998).
Bacteria that can control algal blooms are called algicidal bacteria. Many bacteria with strong algicidal activity against HAB species have been isolated and investigated (Kodani et al., 2002; Su et al., 2007; Ma et al., 2011). Algicidal bacteria have a broad range of potential industrial and environmental applications from the point view of diversity, biodegradability, low toxicity, and biocompatibility (Fiechter, 1992). The greatest advantage of biosurfactants when compared with synthetic surfactants is that they are easily biodegraded, making them environmentally acceptable in contrast to some synthetic algicides (Mulligan, 2005). Additionally, several studies have stated that algicidal bacteria may only be effective on a specific genus or species of algae (Fukami et al., 1992; Doucette et al., 1999), while others target a broader range of algal classes (Imai et al., 1995; Lovejoy et al., 1998; Kang et al., 2008; Roth et al., 2008a). Several strains of algicidal bacteria have been isolated, but few algicides have been purified. Great difficulties exist in the isolation and purification of algicidal compounds because of the apparent variation in characteristics across different species of algicidal bacteria (Skerratt et al., 2002).
In this study, the algicidal bacterium PDGS120915 was isolated from lightly contaminated stream water. Furthermore, characterization of the lytic effect was examined against several HAB species. These results were investigated to determine the potential source of algicidal bacteria or controlling HABs.
Materials and Methods
Algal Cultures
Alexandrium catenella, Gymnodinium impudicum, and Cochlodinium polykrikoides were supplied by the Korea Marine Microalgae Culture Center (KMCC). All algal cultures were grown in f/2 medium (Guillard and Ryther, 1962) at 20°C, pH 8, and 120 μE/m2/sec in a 12-h light and 12-h dark cycle.
Bacterial Strain
The bacteria sample was collected from lightly polluted stream water in Busan, Korea. To screen the algicidal bacteria, the isolated strain was inoculated into logarithmic-phase algal cultures. The algicidal substance-producing bacterium designated PDGS120915 was obtained on Luria Bertani (LB) medium at 25°C.
Identification of Algicidal Bacteria
An isolated strain was grown at 25°C for 1 day on LB medium. Standard physiological and biochemical characteristics were examined using API kits (API 20E and APIZYM; BioMerieux, France). For the sequence analysis, bacterial genomic DNA was extracted and purified using a DNA extraction kit according to the manufacturer’s instructions (Promega Co., Madison, USA). 16S rDNA was amplified by PCR using the universal primers 8F (5’- AGAGTTTGATCCATGGC-3’) and 1492R (5’-GTTACCTTGTTACGACTT-3’). The obtained nucleotide sequences were analyzed by the BLASTN database (https://www.ncbi.nlm.nih.gov/BLAST) at the National Center for Biotechnology Information (NCBI). A neighbor-joining phylogenetic tree was constructed using MEGA 5.0.
Purification of algicidal compound
The bacterial cells were harvested, and pigment extraction was performed using acidified ethanol (5% HCl and 95% ethanol). Subsequently, the extracted pigments were chromatographed on an XTerra MS C18 reverse-phase column (125Å, 2.5 μm, 2.1 mm × 20 mm, Waters). HPLC was carried out with the Bio-Rad HPLC system (Bio-Rad, USA) at a 0.3 ml/min flow rate.
Characterization of Algicidal Effect
Activities of different treatments: Bacterial cells, bacterial cultures, cell-free filtrates, and purified compounds were inoculated into each algal culture. Afterward, the inoculates were cultivated in adjusted algae conditions.
Effects of growth phase of bacteria: To investigate the algicidal activities related to bacterial growth phase, the bacterial growth and algicidal activity of each time were examined.
Effects of concentration: The purified compound was inoculated into each algal culture at concentrations of 1, 3, 5, 7, and 10 ppb, and f/2 medium was used as a control. All experiments were performed in triplicate. The cultivation used the same conditions as those used for the algal culture.
Mode of activity: Isolated bacterium PDGS120915 was cultured in LB medium at 25°C for 24-h. Cells were harvested by centrifugation at 12,000 × g for 20 min, and supernatants were passed through 0.22-μm filters to obtain cell-free filtrates. Bacterial cultures, cell-free filtrates, and purified compounds were inoculated into each algal cell culture, respectively. Then, they were cultivated at 20°C, pH 8, and 120 μE/m2/sec in a 12-h light and 12-h dark cycle.
Analysis of algicidal activity: To measure the algicidal activities of the isolated bacterial strain, the algal cells were quantified using a hemocytometer under a light microscope. Lugol’s iodine reagent fixed algal cell counting. The following formula was used to calculate the algicidal effect:
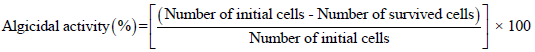
Results
Physiological identification
The bacterium PDGS120915 was determined to be a gram-negative rod shape and reddish colony obtained on an LB agar plate. The results of its biochemical and enzymatic characteristics are shown in Table 1. The strain utilized citrate, fructose, galactose, glucose, maltose, mannitol, mannose, sorbitol, sucrose, and trehalose, as well as the hydrolysis of gelatin and casein. In addition, the enzyme activities were positive on lysine decarboxylase, ornithine decarboxylase, catalase, esterase, lipase, acid phosphatase, naphthol-AS-BI-phosphohydrolase, and N-acetyl-β-glucosaminidase (Ji et al., 2015).
| Characteristic |
120915 |
Characteristic |
120915 |
| Spore |
- |
Arginine dihydrolase |
- |
| Motility |
+ |
Lysine decarboxylase |
+ |
| Anaerobic growth |
+ |
Ornithine decarboxylase |
+ |
| Utilization of |
|
Cytochrome oxidase |
- |
| Arabinose |
- |
Catalase |
+ |
| Cellobiose |
- |
Alkaline phosphatase |
- |
| Citrate |
+ |
Esterase (C4) |
+ |
| Fructose |
+ |
Esterase Lipase (C8) |
+ |
| Galactose |
+ |
Lipase (C14) |
- |
| Glucose |
+ |
Leucinearylamidase |
- |
| Lactose |
- |
Valinearylamidase |
- |
| Maltose |
+ |
Cystinearylamidase |
- |
| Mannitol |
+ |
Trypsin |
- |
| Mannose |
+ |
α-chymotrypsin |
- |
| Melibiose |
- |
Acid phosphatase |
+ |
| Raffinose |
- |
Naphthol-AS-BI-phosphohydrolase |
+ |
| Sorbitol |
+ |
α-galactosidase |
- |
| Sucrose |
+ |
β-galactosidase |
- |
| Trehalose |
+ |
β-glucuronidase |
- |
| Xylose |
- |
α-glucosidase |
- |
| Hydrolysis of |
|
β-glucosidase |
- |
| Gelatin |
+ |
N-acetyl-β-glucosaminidase |
+ |
| Urea |
- |
α-monnosidase |
- |
| Casein |
+ |
α-fucosidase |
- |
| Starch |
- |
|
|
| β-hemolysis |
+ |
|
|
| Production of |
|
|
|
| Acetoin |
- |
|
|
| H2S |
- |
|
|
| Indole |
+ |
|
|
| Mixed acid |
- |
|
|
| Gas |
- |
|
|
Table 1: Biochemical and enzymatical characteristics of Serratia sp. PDGS120915
Identification of algicidal substance-producing microorganism
The sequence obtained was available in GenBank under the accession number KC007128. The 16S rDNA sequence of strain PDGS120915 was aligned through comparison with available sequences from the GenBank database. The sequences of PDGS120915 shared the highest homology with Serratia marcescens subsp. sakuensis KREDT. Using the 16S rDNA sequences, phylogenetic analysis was performed. The phylogenetic tree based on bacterial 16S rDNA sequences showed a close relationship between PDGS120915 and the genus Serratia (Ji et al., 2015) (Figure 1).
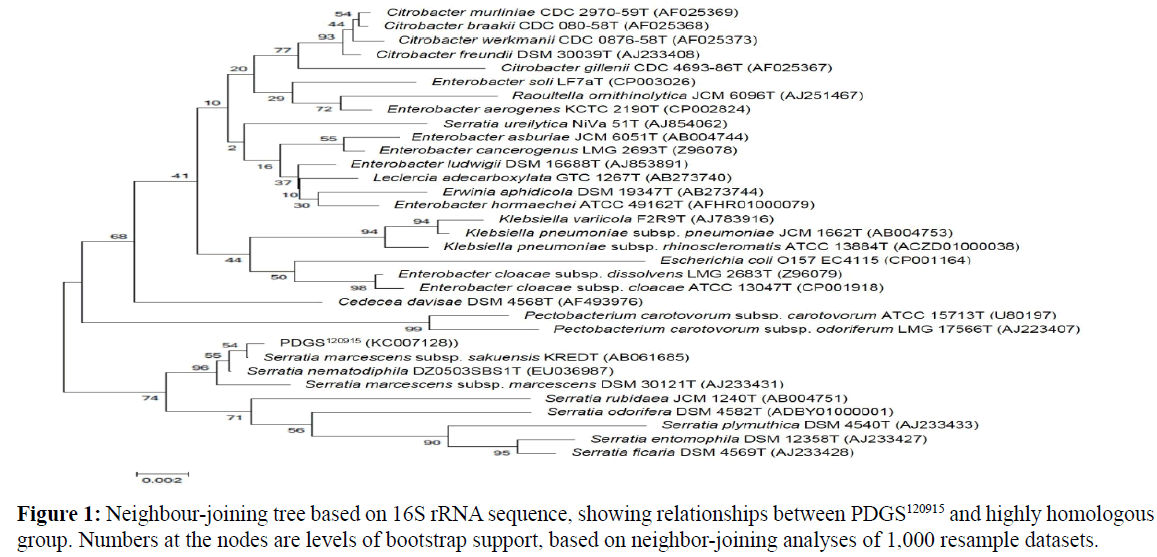
Figure 1: Neighbour-joining tree based on 16S rRNA sequence, showing relationships between PDGS120915 and highly homologous group. Numbers at the nodes are levels of bootstrap support, based on neighbor-joining analyses of 1,000 resample datasets.
Confirmation of algicidal compound
Prodigiosin, extracted from S. marcescens (Castro et al., 1959), had a maximum absorption spectrum of 537–538 nm. In this study, the maximum absorption spectrum of prodigiosin extracted from Serratia sp. PDGS120915 was observed to have similar characteristics (Ji et al., 2015).
Algicidal activity of Serratia sp. PDGS120915 against harmful algae
The algicidal activities were investigated and divided into two parts (i.e. direct and indirect). One of the parts was treated with bacterial cells and another part with filtrates. We found that the bacterial cells had almost no algicidal activity. However, the cell-free filtrates and purified prodigiosin showed more that 80% algicidal activity, similar to that of the original bacterial cultures (Figure 2).
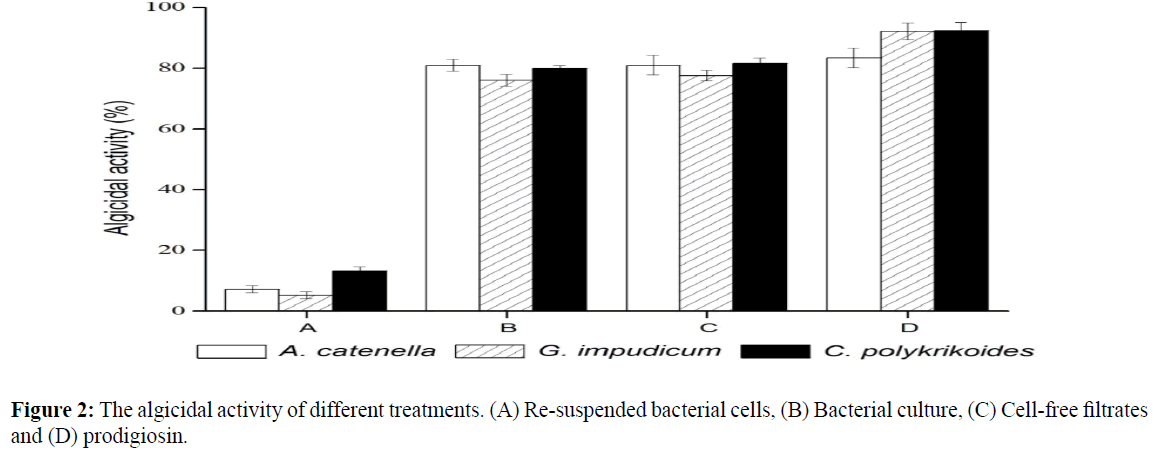
Figure 2: The algicidal activity of different treatments. (A) Re-suspended bacterial cells, (B) Bacterial culture, (C) Cell-free filtrates and (D) prodigiosin.
Interaction between algicidal activity and bacterial growth
The correlation between bacterial growth and algicidal activity was examined over 24-h at 2-h intervals. The results demonstrated that the algicidal activity of the PDGS120915 was bacterial growthdependent; thus, the strongest algicidal activity occurred in the stationary phase(Figure 3).
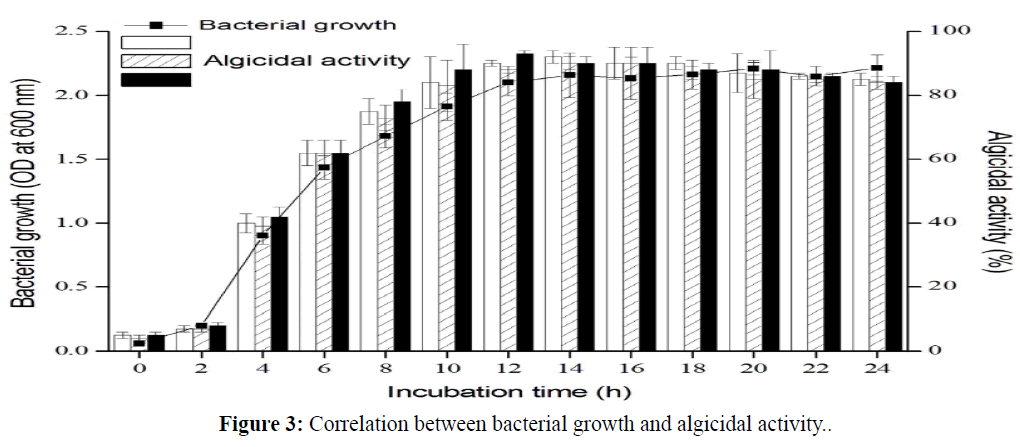
Figure 3: Correlation between bacterial growth and algicidal activity.
Algicidal activity according to prodigiosin concentration
The algicidal activities at different concentrations of prodigiosin were examined. Although at 1 ppb concentration, algicidal activity was not strong, when the concentration was more than 5 ppb, the algicidal activities were over 90%. Every time the concentration increased, stronger algicidal activity was observed (Figure 4). Even though increase ratio was under 1% by stages between 5 and 10 ppb, this seems to be a concentration dependent reaction.
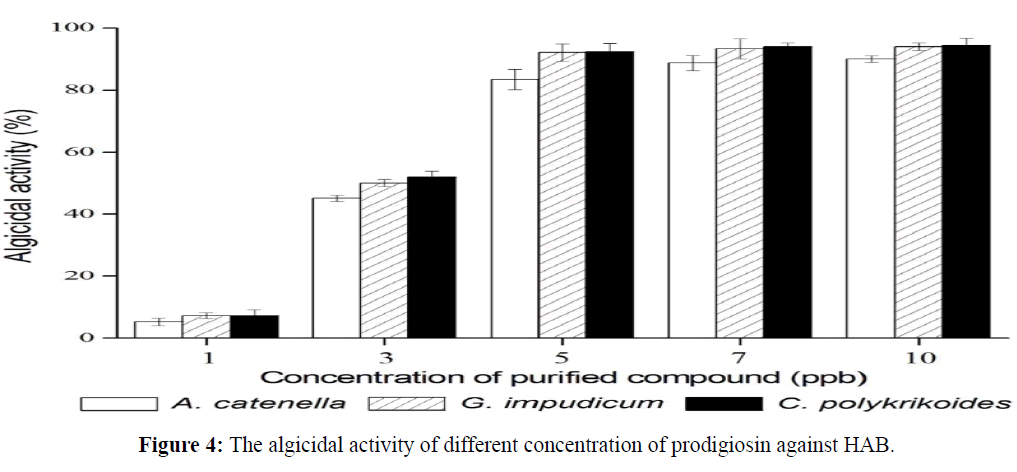
Figure 4: The algicidal activity of different concentration of prodigiosin against HAB.
The algicidal range of Serratia sp. PDGS120915
The algicidal range of Serratia sp. PDGS120915 was examined against other HAB classes. The 5 ppb purified prodigiosin was added to each algal culture reached at the mid-exponential phase. The cultivation was adequate for the growth of each algal cell. Algicidal activity was estimated using the aforementioned equation. The algicidal activities against raphidophyceae, coscinodiscophyceae, and bacillariophyceae were as follows (Figure 5): Chattonella marina (38.9%), Heterosigma akashiwo (30.8%), Skeletonema costatum (24.7%), and Pseudonitzschia pungens (22.1%).
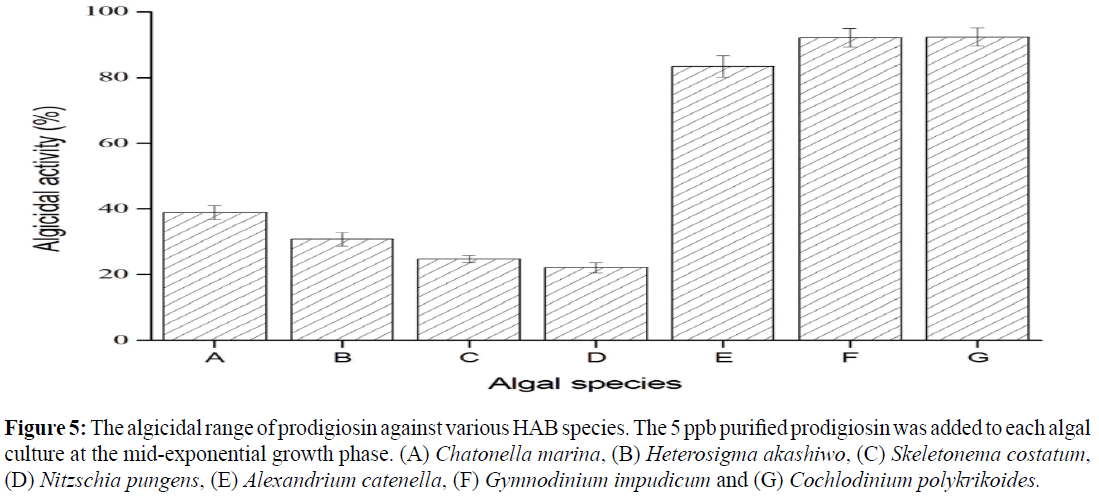
Figure 5: The algicidal range of prodigiosin against various HAB species. The 5 ppb purified prodigiosin was added to each algal culture at the mid-exponential growth phase. (A) Chatonella marina, (B) Heterosigma akashiwo, (C) Skeletonema costatum, (D) Nitzschia pungens, (E) Alexandrium catenella, (F) Gymnodinium impudicum and (G) Cochlodinium polykrikoides.
Discussion
During the past several decades, HABs have tended to increase worldwide and have negative influences, such as killing fish, shellfish, and other marine life in the ocean ecosystem (Friedman and Levin, 2005). Sprinkling clay to mitigate HABs is the only method used in practice, but it has limitations in its application to the marine ecosystem. Therefore, many researchers have examined biological control methods for HABs, such as the algicidal effect (Fukami et al., 1992; Iwata et al., 2004) and the interaction between marine bacteria and phytoplankton (Furuki and Kobayashi, 1992; Fukami et al., 1997). We found that a potent algicidal strain, PDGS120915, isolated from the lightly contaminated stream water in Busan, Korea. Based on 16S rDNA sequence analysis, this strain was identified as the genus Serratia. Yang et al. (2013) also isolated a strain belonging to that genus, which can effectively lyse the algal cells of the Microcystis aeruginosa, which is a species of freshwater cyanobacteria. However, no reports have documented a bacterial strain belonging to Serratia that can lyse the toxic algal cells of A. catenella, G. impudicum, and C. polykrikoides, which are widely included as HABs and distributed in the coast of Korea.
In general, the algicidal mode of bacteria could be summarized as either direct or indirect attacks (Mayali and Azam, 2004; Pokrzywinski et al., 2012). Direct attacks mean that algicidal bacteria make contact with the algal cells and lyse them, while indirect attacks are algicidal activity dependent on active compounds produced by the microorganism (Mayali and Azam, 2004). Only a few compounds from algicidal bacteria have been characterized. These compounds comprise peptides or enzymes (Chen et al., 2011; Imamura et al., 2000; Lee et al., 2000; Paul and Pohnert, 2011; Wang et al., 2012), biosurfactants (Wang et al., 2005), pigments (Nakashima et al., 2006; Sakata et al., 2011), and antibiotic-like substances (Dakhama et al., 1993). In the present study, PDGS120915 exhibits indirect attacks against A. catenella, G. impudicum, and C. polykrikoides. These results determined different treatments for the algal cells. It was indicated that our strain released an algicidal compound into the culture broth, and we identified this compound as prodigiosin. The algal cell rupture from cellular swelling was observed after the treatment of prodigiosin. It is presumed that the intervention of the ion channel of prodigiosin leads to increasing water inflow within the intracellular space, causing the rupture of cells due to cellular swelling. This result is similar to that of Kim et al.’s research (Kim et al., 2008).
Several reports describe the algicidal activity of prodigiosin, and a preliminary test for prodigiosin’s algicidal activity has been carried out. Jeong et al. (2005) reported that when prodigiosin from Hahella chejuensis was used to treat C. polykrikoides culture, the algal cells were rapidly burst. Takuji et al. (2006) reported that PG-L-1, a prodigiosin-like pigment, had potent algicidal activity against C. marina and H. akashiwo. In our studies, we purified prodigiosin from Serratia sp. PDGS120915 and examined the algicidal activity against the dominant algal bloomforming species A. catenella, G. impudicum, and C. polykrikoides. Our results showed that prodigiosin from Serratia sp. PDGS120915 could be a useful bio-compound for mitigating or controlling HABs. However, further investigation of how to use it and the killing process that takes place in natural conditions is required. Additionally, in order to evaluate the benefits of algicidal agent spraying, we are considering the necessary addition of a mesocosm study.
The cost of algicidal agent spraying is divided into direct and indirect costs. Direct costs are all expenses involved in spraying. (i.e. production charges, transportation charges, equipment and vessel charges, labor costs, etc.). Indirect costs refer to the negative effects on marine ecosystems and organisms. We have obtained 0.81 kg/ton of prodigiosin from a wild-type strain. An expendable production cost (LB media) except equipment for separation and extraction is 2,320,000 won (unit price/ton). As a result of calculation in the presumable working concentration range at 10– 100 ppb, the processing cost per ton (m3) of sea water is 28.6–286 won (in vitro test). If decreasing production costs and increasing production yield are achieved through the investigated optimal production conditions, we expect a reduction of the processing cost per ton (m3). This information will be an important factor for the potential application of algal control.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of KOREA (NRF) funded by the Ministry of Education (2014R1A1A4A01009382).
9295
References
- Sellner, K., Doucette, G., Kirkpatrick, G. (2003). Harmful algal blooms: causes, impacts and detection. J. Ind. Microbiol. Biotechnol.30, 383-406
- nLandsberg, J.H.(2002). The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 10, 113-390
- nHallegraeff, G.M. (1993). A review of harmful algal blooms and their apparent global increase.Phycologia32, 79-99
- nZingone, A., Enevoldsen, H.O. (2000). The diversity of harmful algal bloom: a challenge for science and management. Ocean Coast.Manag.43, 725-748
- nKim, H. (2006). Mitigation and controls of HABs. In: Ecology of Harmful Algae. Springer, 327-338
- nAnderson, D.M., (1997). Turning back the harmful red tide. Nature.388, 513-514
- nPei, H.Y., Hu, W.R., Qu, Y.B. et al., (2005).Degradation characteristics of two Bacillus strains on the Microcystisaeruginosa. J. Environ. Sci. 17, 205-207
- nLee, S.O., Kato, J., Takiguchi, N., Kuroda, A., Ikeda, T. et al., (2000). Involvement of an extracellular protease in algicidal activity of the marine bacterium Pseudoalteromonas sp. strain A28. Appl. Environ. Microbiol.66, 4334-4339
- nBerger, P.S., Rho, J., Gunner, H.B. (1979). Bacterial suppression of chlorella by hydroxylamine production. Water Res. 13, 267-273
- nDakhama, A., De la Notie, J., Lavoie, M.C. (1993). Isolation and identification of antialgal substances produced by Pseudomonas aeruginosa. J. Appl. Phycol. 5, 297-306
- nYoshikawa, K., Adachi, K., Nishijima, M., Takadera, T., Tamaki, S. et al., (2000).ß-Cyanoalanine production by marine bacteria on cyanide-free medium and its specific inhibitory activity toward cyanobacteria. Appl. Environ. Microbiol.66, 718-722
- nYamamoto, Y., Kouchiwa, T., Hodoki, Y., Hotta, K., Uchida,H. et al., (1998). Distribution and identification of actinomycetes lysing cyanobacteria in eutrophic lake. J. Appl. Phycol. 10, 391-397
- nSu, J.Q., Yang, X.R., Zheng, T.L., Tian, Y., Jiao, N.Z. et al., (2007).Isolation and characterization of a marine algicidalbacterium against the toxic dinoflagellateAlexandriumtamarense. Harmful Algae 6, 799-810
- nMa, H., Krock, B., Tillmann, U., Muck, A., Wielsch, N. et al.,(2011). Isolation of activity and partial characterization of large non-proteinaceous lytic allelochemicals produced by the marine dinoflagellateAlexandriumtamarense. Harmful Algae 11, 65-72
- nKodani, S., Imoto, A., Mitsutani, A., Murakami, M. (2002). Isolation and identification of the altialgal compound, harmane (1-methyl- ß-carboline), produced by the algicidal bacterium, Pseudomonas sp. K44-1. J. Appl. Phycol. 14, 109-114
- nFiechter, A. (1992). Biosurfactants: moving towards industrial application. Trends Biotechnol.10, 208-217
- nMulligan, C.N.(2005).Environmental applications for biosurfactants. Environ. Pollut.133, 183-198
- nSkerratt, J.H., Bowman, J.P., Hallegraeff, G., James, S., Nichols, P.D. (2022).Algicidal bacteria associated with blooms of a toxic dinoflagellate in a temperate Australian estuary. Mar. Ecol. Prog. Ser. 244, 1-15
- nRoth, P.B., Twiner, M.J., Mikulski, C.M., Barnhorst, A.B., Doucette, G.J.(2008). A comparative analysis of two algicidal bacteria active against the red tide dinoflagellateKareniabrevis. Harmful Algae 7, 682-691
- nDoucette, G.J., McGovern, E.R., Babinchak, J.A.(1999). Algicidal bacteria active against Gymnodiniumbreve (Dinophyceae). I. Bacterial isolation and characterization of killing activity. J. Phycol. 35, 1447-1454
- nFukami, K., Yuzawa, A., Nishijima, T., Hata, Y.(1992).Isolation and properties of a bacterium inhibiting the growth of Gymnodiniumnagasakiense. Nippon Suisan Gakkaishi58, 1073-1077
- nImai, L., Ishida, Y., Sakaguchi, K., Hata, Y.(1995). Algicidal marine bacteria isolated from northern Hiroshima Bay, Japan. Fish. Sci. 61, 624-632
- nKang, Y.K., Cho, S.Y, Kang, Y.H., Katano, T., Jin, E.S., et al., (2008).Isolation, identification and characterization of algicidal bacteria against Stephanodiscushantzschii and Peridiniumbipes for the control of freshwater winter algal blooms. J. Appl. Phycol. 20, 375-386
- nGuillard, R.R.L., Ryther, J.H., (1962). Studies of marine planktonic diatoms.I. Cyclotella nana Hustedt, and Detonulaconfervacea (Cleve).Gran.Can. J. Microbiol.8, 229-235
- nFriedman, M.A., Levin, B.E.(1978). Neurobehavioral effects of harmful algal bloom (HAB) toxins: a critical review. J. Int. Neuropsychol. Soc. 11, 331-338
- nIwata, Y., Sugahara, I., Kimura, T., Kowa, H., Matsumoto, A. et al., (2004). Properties of an algicidal bacterium (Flavobacterium sp.) against Kareniamikimotoi isolated from Ise Bay, Japan. Nippon Suisan Gakkaishi.70, 537-541
- nFuruki, M., Kobayashi, M. (1991).Interaction between Chattonella and bacteria and prevention of this red tide. Mar. Pollut. Bull. 23, 189-193
- nFukami, K., Nishijima, T., Ishida, T. (1997).Stimulative and inhibitory effects of bacteria on the growth of microalgae.Hydrobiologia.358, 185-191
- nYang, F., Wei, H.Y., Li, X.Q., Li, Y.H., Li, X.B. et al.,(2013).Isolation and characterization of an algicidal bacterium indigenous to Lake Taihu with a red pigment able to lyse Microcystisaeruginosa.Biomed. Environ. Sci. 26, 148-154
- nMayali, X., Azam, F. (2004).Algicidal bacterial in the sea and their impact on algal blooms. J. Eukaryot. Microbiol.51, 139-144
- nPokrzywinski, K.L., Place, A.R., Warner, M.E., Coyne, K.J. (2012). Investigation of the algicidal exudate produced by Shewanella sp. IRI-160 and its effect on dinoflagellates. Harmful Algae.19, 23-29
- nChen, W.M., Sheu, F.S., Sheu, S.Y. (2011). Novel L-amino acid oxidase with algicidal activity against toxic cyanobacteriumMicrobystisaeruginosa synthesized by a bacterium Aquimarina sp. Enzyme Microb. Tech. 49, 372-379
- nImamura, N., Motoike, I., Noda, M., Adachi, K., Konno, A. et al., (2000).Argimicin A, a novel anti-cyanobacterial compound produced by an algae-lysing bacterium. J. Antibiot. 53, 1317-1319
- nLee, S.O., Kato, J., Takiguchi, N., Kuroda, A., Ikeda, T. et al., (2000). Involvement of an extracellular protease in algicidal activity of the marine bacterium Pseudoalteromonas sp. strain A28. Appl. Environ. Microbiol.66, 4334-4339
- nPaul, C., Pohnert, G. (2011). Interactions of the algicidal bacterium Kordiaalgicida with diatoms: regulated protease excretion for specific algal lysis. Plos One 6
- nWang, B., Yang, X., Lu, J., Zhou, Y., Su, J. et al.,(2012). A marine bacterium producing protein with algicidal activity against Alexandriumtamarnese. Harmful Algae 13, 83-88
- nWang, X., Gong, L., Liang, S., Han, X., Zhu, C. et al., (2005).Algicidal activity of rhamnolipidbiosurfactants produced by Pseudomonas aeruginosa. Harmful Algae 4, 433-443
- nNakashima, T., Miyazaki, Y., Matsuyama, Y., Muraoka, W., Yamaguchi, K.(2006).Producing mechanism of an algicidal compound against red tide phytoplankton in a marine bacterium gamma-proteobacterium. Appl. Microbiol. Biot.73, 684-690
- nSakata, T., Yoshikawa, T., Nishitarumizu, S. (2011).Algicidal activity and identification of an algicidal substance produced by marine Pseudomonas sp. C55a-2. Fisheries Sci. 77, 397-402
- nDakhama, A., Noie, J.D.L., Lavoie, M.C. (1993). Isolation and identification of antialgal substances produced by Pseudomonas aeruginosa. J. Appl. Phycol. 5, 297-306
- nKim, D., Kim, J.F., Yim, J.H., Kwon, S.K., Lee, C.H. (2008). Red to red-the marine bacterium Hahellachejuensis and its product prodigiosin for mitigation of harmful algal blooms. J. Microbiol. Biotechnol.18, 1621-1629
- nJeong, H., Yim, J.H., Lee, C., Choi, S.H., Park, T.K. et al., 2005.Genomic blueprint of Hahellachejuensis, a marine microbe producing an algicidal agent. Nucleic Acids Res. 33, 7066-7073
- nNakashima, T., Kim, D., Miyazaki, Y., Yamaguchi, K., Takeshita, S., (2006).Mode of action of an antialgal agent produced by a marine gamma Proteobacterium against Chattonella marina.Aquat.Microb. Ecol. 45, 255-262
- nJi, K., Jeong, T.H., Kim, Y.T. (2015). Anti-MRSA Properties of Prodigiosin from Serratia sp. PDGS 120915. J. Life. Sci. 25, 29-36.