Research - (2022) Volume 13, Issue 7
Alzheimer’s disease - A neurodegenerative fight
Akshat Patel*,
Preksha Saparia,
Heer Shah,
Kirtan Solanki,
Aashal Patel and
Maulin Sahayata
Gujarat Cancer Society Medical College, Hospital, & Research Centre (GCSMCH & RC), Asarwa, Ahmedabad, Gujarat, India
*Correspondence:
Akshat Patel, Gujarat Cancer Society Medical College, Hospital, & Research Centre (GCSMCH & RC), Asarwa, Ahmedabad, Gujarat,
India,
Email:
Received: 13-Jul-2022, Manuscript No. ipjnn-22-12856;
Editor assigned: 15-Jul-2022, Pre QC No. P-12856;
Reviewed: 20-Jul-2022, QC No. Q-12856;
Revised: 24-Jul-2022, Manuscript No. R-12856;
Published:
31-Jul-2022
Abstract
Alzheimer's is not about the past - the successes, the accolades, the
accomplishments. Alzheimer's is about the present and the struggle,
the scrappy bowl, the fight to live with a disease”.
Alzheimer’s disease may cause a person to become confused,
get lost in familiar places, misplace things, or have trouble with
language. Groups of nerve cells have special jobs. Some are involved
in thinking, learning and memory. Others help us see, hear, smell and
tell our muscles when to move. Brain cells operate like tiny factories.
They receive supplies, generate energy, construct equipment and
get rid of waste. Cells also process and store information and
communicate with other cells.
Keeping everything running requires coordination as well as large
amounts of fuel and oxygen. Scientists believe Alzheimer's disease
prevents parts of a cell's factory from running well. But just like a
real factory, backups and breakdowns in one system cause problems
in other areas. As damage spreads, cells lose their ability to do their
jobs and, eventually, die.
The brains of individuals with Alzheimer's have an abundance of
plaques and tangles. Plaques are deposits of a protein fragment
called beta-amyloid that build up in the spaces between nerve
cells. Tangles are twisted fibers of another protein called tau that
builds up inside cells. Though autopsy studies show that most
people develop some plaques and tangles as they age, those with
Alzheimer's tend to develop far more and in a predictable pattern,
beginning in the areas important for memory before spreading to
other regions.
Scientists do not know exactly what role plaques and tangles play in
Alzheimer's disease. Most experts believe that they disable or block
communication among nerve cells and disrupt processes the cells
need to survive. The destruction and death of nerve cells causes
memory failure, personality changes, problems in carrying out daily
activities and other symptoms of Alzheimer's disease.
Keywords
Alzheimer’s; Plagues; Tangles; Beta-amyloid; Tau;
Autopsy
Introduction
Alzheimer's disease (AD) is a neurodegenerative disease
that usually starts slowly and progressively worsens [1]. It
is the cause of 60–70% of cases of dementia [2-4]. The
most common early symptom is difficulty in remembering
recent events [1]. As the disease advances, symptoms can
include problems with language, disorientation (including
easily getting lost), mood swings, loss of motivation, selfneglect,
and behavioural issues [2]. As a person's condition
declines, they often withdraw from family and society [5].
Gradually, bodily functions are lost, ultimately leading to
death [6]. Although the speed of progression can vary, the
typical life expectancy following diagnosis is three to nine
years [3,7].
Alzheimer's disease is characterized by progressive loss of
memory and cognitive function in middle aged individuals
(Fig. 1.). Thus the condition is frequently associated with:
• Memory failure for recent events
• Lack of spontaneous activity and initiative with loss
of intellectual functions
• Extra pyramidal and a kinetic hypertonic symptom.
• Loss of spatial orientation.
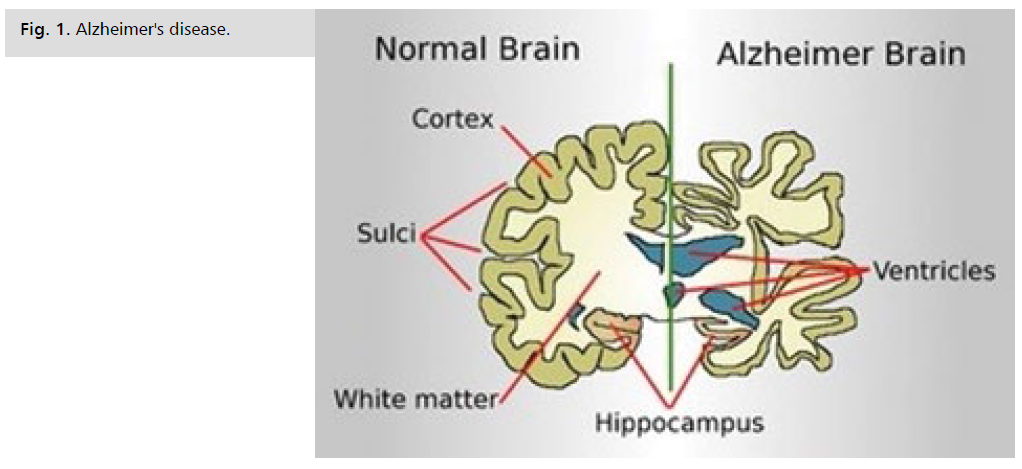
Fig 1: Alzheimer's disease.
After 2 or 3 years, dementia (memory loss) becomes
well established and focal symptoms occur, such as aphasia
(speech disorder), apraxia (inability to perform voluntary
movements) and agnosia (inability to recognize objects
inspite of intact sensory modality). Similar features in the
old age (over 65 years) are called senile dementia [8].
The sequence of events in the affected neurons

Epidemiology
Alzheimer’s disease is the most common type of dementia
diagnosed today, accounting for 60-70% of the estimated
50 million people globally who suffer with dementia [9].
It is a degenerative brain disease which is understood to
begin 20 years or more before symptoms become apparent
in those affected. Neurons in the brain become damaged or
destroyed due to the accumulation of the protein fragment
beta-amyloid (Aβ) outside the neurons, called beta-amyloid
plaques, as well as accumulations of an abnormal form of
the protein tau inside the neurons. As a result of this buildup,
the body activates immune system cells called microglia
that try to clear the toxic proteins, resulting in chronic
inflammation when the microglia can no longer keep up
with the amount of toxins produced.
The highest prevalence and incidence rates of AD are
reported in North America and Western Europe, followed
by Latin America, China, and the Western Pacific. It
is officially the sixth leading cause of death in the U.S.,
where 5.8 million Americans have been diagnosed with
the disease. This figure is projected to rise to 14 million by
2050. Approximately 200,000 of those living with AD are
younger than 65 years of age, but the vast majority (81%)
are over age 75 [10,11].
Symptoms
Alzheimer’s disease is a progressive condition, meaning
that the symptoms get worse over time. Memory loss is a
key feature, and this tends to be one of the first symptoms
to develop.
The symptoms appear gradually, over months or years.
If they develop over hours or days, a person may require
medical attention, as this could indicate a stroke.
Symptoms of Alzheimer’s disease include:
• Memory loss: A person may have difficulty taking
in new information and remembering information.
This can lead to:
• Repeating questions or conversations
• Losing objects
• Forgetting about events or appointments
• Wandering or getting lost
• Cognitive deficits: A person may experience
difficulty with reasoning, complex tasks, and
judgment. This can lead to:
• A reduced understanding of safety and risks
• Difficulty with money or paying bills
• Difficulty in making decisions
• Difficulty in completing tasks that have several
stages, such as getting dressed
• Problems with recognition: A person may become
less able to recognize faces or objects or less able
to use basic tools. These issues are not Due to
problems with eyesight’s
• Problems with spatial awareness: A person may have
difficulty with their balance, trip over, or spill things
more often, or they may have
• Difficulty orienting clothing to their body when
getting dressed
• Problems with speaking, reading, or writing: A
person may develop difficulties with thinking of
common words, or they may make more speech,
spelling, or writing errors
• Personality or behavior changes: A person may
experience changes in personality and behavior that
include:
a. becoming upset, angry, or worried more often
than before
b. a loss of interest in or motivation for activities
they usually enjoy
c. a loss of empathy
d. compulsive, obsessive, or socially inappropriate
behavior [12,13]
Stages of Alzheimer's Disease
You can help support your loved one with Alzheimer's by learning more about how the condition unfolds (Fig. 2.).
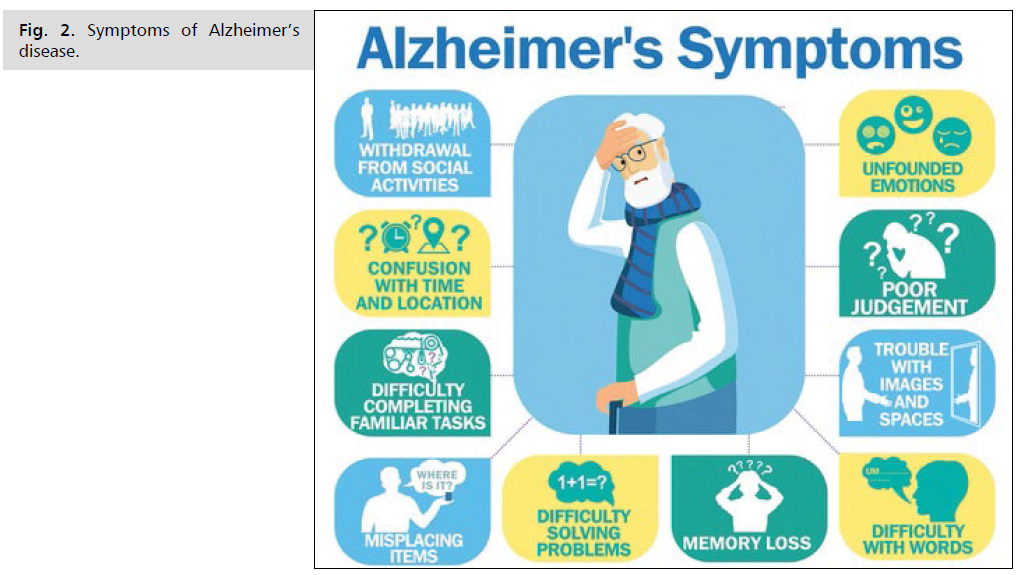
Fig 2: Symptoms of Alzheimer’s disease.
The stages don't always fall into neat boxes, and the symptoms might vary but they can be a guide and help you plan for your friend or relative's care. Doctors call these different stages the progression of the disease. There is no cure for Alzheimer's disease, so it can help to know what to expect so you can plan to meet your loved one’s needs in each stage. There are no hard-and-fast lines between mild and moderate stages, but over time, you can expect changes like the ones below.
There are 3 stages of Alzheimer’s:
Stage 1. Early stage Alzheimer’s (mild)
Stage 2. Middle stage Alzheimer’s (moderate)
Stage 3. Late stage Alzheimer’s (severe)
Be aware that it may be difficult to place a person with Alzheimer's in a specific stage as stages may overlap (Fig. 3. and 4.).
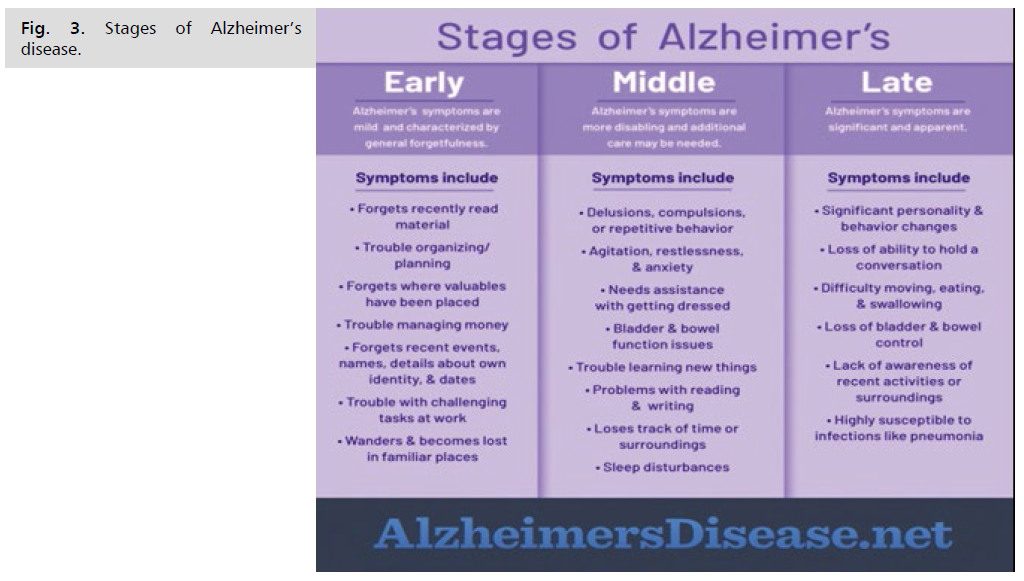
Fig 3:Stages of Alzheimer’s disease.
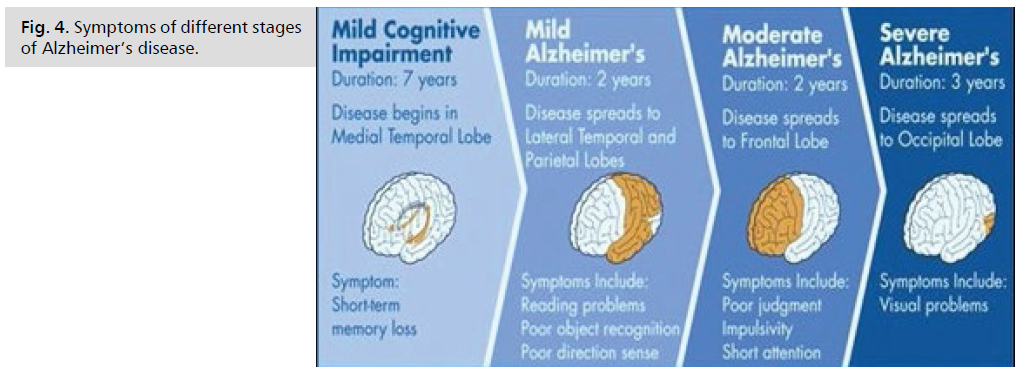
Fig 4: Legend
Stage 1: Early-stage Alzheimer's (mild)
In the early stage of Alzheimer's, a person may function independently. He or she may still drive, work and be part of social activities. Despite this, the person may feel as if he or she is having memory lapses, such as forgetting familiar words or the location of everyday objects.
Symptoms may not be widely apparent at this stage, but family and close friends may take notice and a doctor would be able to identify symptoms using certain diagnostic tools.
Common difficulties include:
• Coming up with the right word or name.
• Remembering names when introduced to new people.
• Having difficulty performing tasks in social or work settings.
• Forgetting material that was just read.
• Losing or misplacing a valuable object.
• Experiencing increased trouble with planning or organizing.
Stage 2: Middle-stage Alzheimer's (moderate)
Middle-stage Alzheimer's is typically the longest stage and can last for many years. As the disease progresses, the person with Alzheimer's will require a greater level of care.
During the middle stage of Alzheimer’s, the dementia symptoms are more pronounced. The person may confuse words, get frustrated or angry, and act in unexpected ways, such as refusing to bathe. Damage to nerve cells in the brain can also make it difficult for the person to express thoughts and perform routine tasks without assistance.
Symptoms, which vary from person to person, may include:
• Being forgetful of events or personal history.
• Feeling moody or withdrawn, especially in socially or mentally challenging situations.
• Being unable to recall information about themselves like their address or telephone number, and the high school or college they attended.
• Experiencing confusion about where they are or what day it is.
• Requiring help choosing proper clothing for the season or the occasion.
• Having trouble controlling their bladder and bowels.
• Experiencing changes in sleep patterns, such as sleeping during the day and becoming restless at night.
• Showing an increased tendency to wander and become lost.
• Demonstrating personality and behavioral changes, including suspiciousness and delusions or compulsive, repetitive behavior like hand-wringing or tissue shredding.
In the middle stage, the person living with Alzheimer’s can still participate in daily activities with assistance. It’s important to find out what the person can still do or find ways to simplify tasks. As the need for more intensive care increases, caregivers may want to consider respite care or an adult day center so they can have a temporary break from care giving. While the person living with Alzheimer’s continues to receive care in a safe environment.
Stage 3: Late-stage Alzheimer's (severe)
In the final stage of the disease, dementia symptoms are severe. Individuals lose the ability to respond to their environment, to carry on a conversation and, eventually, to control movement. They may still say words or phrases, but communicating pain becomes difficult. As memory and cognitive skills continue to worsen, significant personality changes may take place and individuals need extensive care.
At this stage, individuals may:
• Require around-the-clock assistance with daily personal care.
• Lose awareness of recent experiences as well as of their surroundings.
• Experience changes in physical abilities, including walking, sitting and, eventually, swallowing
• Have difficulty communicating.
• Become vulnerable to infections, especially pneumonia.
• The person living with Alzheimer’s may not be able to initiate engagement as much during the late stage, but he or she can still benefit from interaction in ways that are appropriate, like listening to relaxing music or receiving reassurance through gentle touch. During this stage, caregivers may want to use support services, such as hospice care, which focus on providing comfort and dignity at the end of life. Hospice can be of great benefit to people in the final stages of Alzheimer’s and other dementias and their families [14].
Pathophysiology of Alzheimer's Disease
The two pathologic hallmarks of Alzheimer disease are:
• Extracellular beta-amyloid deposits (in senile plaques)
• Intracellular neurofibrillary tangles (paired helical filaments)
The beta-amyloid deposition and neurofibrillary tangles lead to loss of synapses and neurons, which results in gross atrophy of the affected areas of the brain, typically starting at the mesial temporal lobe. The mechanism by which beta-amyloid peptide and neurofibrillary tangles cause such damage is incompletely understood. There are several theories. The amyloid hypothesis posits that progressive accumulation of beta-amyloid in the brain triggers a complex cascade of events ending in neuronal cell death, loss of neuronal synapses, and progressive neurotransmitter deficits; all of these effects contribute to the clinical symptoms of dementia. A sustained immune response and inflammation have been observed in the brain of patients with Alzheimer disease. Some experts have proposed that inflammation is the third core pathologic feature of Alzheimer disease (Fig. 5.).
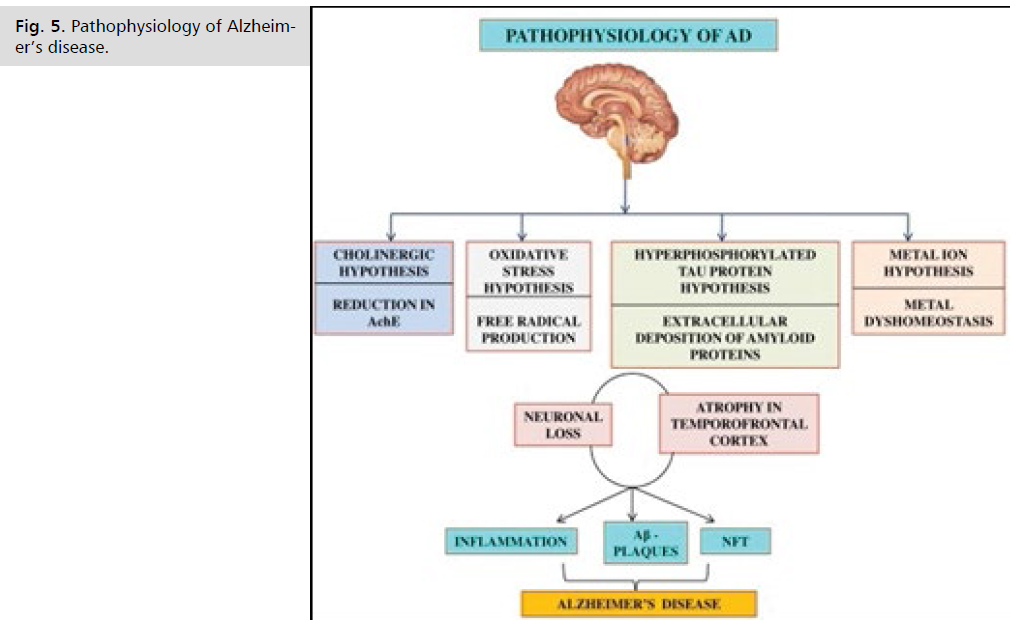
Fig 5: Pathophysiology of Alzheimer’s disease.
Prion mechanisms have been identified in Alzheimer disease. In prion diseases, a normal cell-surface brain protein called prion protein becomes misfolded into a pathogenic form termed a prion. The prion then causes other prion proteins to misfold similarly, resulting in a marked increase in the abnormal proteins, which leads to brain damage. In Alzheimer disease, it is thought that the beta-amyloid in cerebral amyloid deposits and tau in neurofibrillary tangles have prion-like, self-replicating properties [15].
Neuroanatomy of Alzheimer's Disease
It is proposed that Alzheimer's disease is initiated by entry of some environmental factor into the brain via the olfactory pathway and that this factor spreads through the brain from neuron to neuron along identified axonal connections. The proposed agent may be a virus or viruslike particle [16]. The objective of this study was to identify the regions of decreased grey matter (GM) volume which were associated with specific neuropsychiatric behaviours in patients with mild Alzheimer's disease. Voxel-based morphometry was used to correlate GM derived from T1- weighted MRI images of 31 patients with mild Alzheimer's disease and specific neuropsychiatric symptoms and behaviours measured by the Neuropsychiatric Inventory. Delusions were associated with decreased GM density in the left frontal lobe, in the right front parietal cortex and in the left claustrum. Apathy was associated with GM density loss in the anterior cingulate and frontal cortex bilaterally, the head of the left caudate nucleus and in bilateral putamen. Agitation was associated with decreased GM values in the left insula and in anterior cingulate cortex bilaterally [17].
By controlling for confounds of varying task difficulty and subsequent performance, remarkably similar brain activations were identified during successful paired associate learning in patients with Alzheimer’s disease and in healthy comparison subjects. The study methods provide a useful model for further applications of functional imaging involving cognitive activation paradigms in the study of neuropsychiatric disorders [18].
Is Alzheimer’s genetics
Family history is not necessary for an individual to develop Alzheimer’s. However, research shows that those who have a parent or sibling with Alzheimer's are more likely to develop the disease than those who do not have a first-degree relative with Alzheimer’s. Those who have more than one first-degree relative with Alzheimer’s are at an even higher risk. When diseases like Alzheimer's and other dementias tend to run in families, either genetics (hereditary factors), environmental factors or both may play a role [14].
Genetics and Alzheimer’s
There are two categories of genes that influence whether a person develops a disease: (1) risk genes and (2) deterministic genes. Researchers have identified hereditary Alzheimer's genes in both categories (Fig. 6.).
Fig 6: Genetics of Alzheimer’s disease.
1. Risk genes increase the likelihood of developing a disease but do not guarantee it will happen. Researchers have found several genes that increase the risk of Alzheimer's. APOE-e4 is the first risk gene identified and remains the gene with strongest impact on risk. Researchers estimate that between 40-65% of people diagnosed with Alzheimer's have the APOE-e4 genes.
APOE-e4 is one of three common forms of the APOE gene; the others are APOE-e2 and APOE-e3. We all inherit a copy of some form of APOE from each parent. Those who inherit one copy of APOE-e4 from their mother or father have an increased risk of developing Alzheimer's. Those who inherit two copies from their mother and father have an even higher risk, but not a certainty. In addition to raising risk, APOE-e4 may tend to make symptoms appear at a younger age than usual.
An estimated 20-30% of individuals in the United States have one or two copies of APOE-e4; approximately 2% of the U.S. population has two copies of APOE-e4.
2. Deterministic genes directly cause a disease, guaranteeing that anyone who inherits one will develop a disorder. Scientists have found rare genes that cause Alzheimer's in only a few hundred extended families worldwide. These genes, which are estimated to account for 1% or less of Alzheimer's cases, cause familial early-onset forms in which symptoms usually develop between a person's early 40s and mid-50s. The vast majority of individuals with Alzheimer's have late-onset disease, occurring at age 65 or later [14]. 23 Chromosome Pairs; 4 Alzheimer's Genes Identified: APP PS-1 PS-2 APOE4. Amyloid precursor protein (APP),discovered in 1987, is the first gene with mutations found to cause an inherited form of Alzheimer's [14].
3. Genes that increase the risk of developing Alzheimer’s disease
The diagram below shows a number of genes associated with Alzheimer’s disease according to how common they are. Mutations in the APP, PSEN-1 and PSEN-2 genes are rare, but having a mutation gives a very high risk (or even certainty) of developing Alzheimer’s disease, so these genes are shown in the top left of the diagram.
Most people who develop Alzheimer’s don’t have familial Alzheimer’s disease, the particular reason that they develop the disease isn’t clear. It’s most likely to be due to a combination of factors such as age, environment, lifestyle and genetic risk factors. In these cases, the disease is known as ‘sporadic’ – there isn’t one clear cause.
Some of the ‘risk factor’ genes are shown in the diagram above. Having one of these genes means you’re more likely to develop Alzheimer’s disease than someone who doesn’t have the gene, but it doesn’t mean you’ll definitely develop Alzheimer’s. These genes increase your risk slightly, but some people with the genes don’t get the disease, and vice versa. There are a number of genes that are very common – lots of people have the form of the gene that increases the risk of developing Alzheimer’s disease, but they don’t have a strong effect. These are shown in the bottom right of the diagram (e.g. CR1, BIN1, and ABCA7). TREM2 on the other hand is fairly rare, but has a greater effect than those more common genes [19,20].
Early-Onset Alzheimer's Disease
What is early-onset Alzheimer disease
Alzheimer disease is the most common form of
dementia. It affects your memory, thinking, and behavior.
It often progresses to the point where it affects daily
activities and functions [21-35].
Alzheimer disease most commonly affects older adults,
but it can also affect people in their 30s or 40s. When
Alzheimer disease occurs in someone under age 65, it is
known as early-onset (or younger-onset) Alzheimer disease.
A very small number of people with Alzheimer disease have
the early-onset form. Many of them are in their 40s and
50s when the disease takes hold.
Most types of early-onset Alzheimer disease are the
same, but there are a few small distinctions:
• Common Alzheimer disease. Most people with
early-onset Alzheimer disease have the common
form of the disease. The disease progresses in
roughly the same way as it does in older people.
• Genetic (familial) Alzheimer disease. This form is
very rare. A few hundred people have genes that
directly contribute to Alzheimer disease. These
people start showing symptoms of the disease in
their 30s, 40s, or 50s.
Who’d gets early onset AD
Although AD isn’t an expected part of advancing age, you’re at increased risk as you get older. More than 32
percent of people over age 85 have AD. You may also have
an increased risk of developing AD if a parent, sibling, or
child has the disease. If more than one family member has
AD, your risk increases.
A 2016 study Trusted Source showed that African
Americans, Native Americans, and Native Alaskans are at
higher risk for developing early onset AD compared to
white people.
What causes early onset AD
The exact cause of early onset AD hasn’t been fully
determined. Many researchers believe that this disease
develops as the result of multiple factors rather than one
specific cause.
Researchers have discovered rare genes that may directly
cause or contribute to AD. These “deterministic genes” are:
• Amyloid precursor protein (APP) on chromosome
21
• Presenilin-1 (PS1) on chromosome 14
• Presenilin-2 (PS2) on chromosome 1
These genes may be carried from one generation to the
next within a family. Carrying these genes can result in
adults younger than age 65 developing symptoms much
earlier than expected.
How is early onset AD diagnosed
Talk with a doctor if you or a loved one is finding it
increasingly difficult to perform day-to-day tasks, or if you
or a loved one is experiencing increased memory loss. They
may refer you to a doctor who specializes in AD.
Especially in the case of early onset AD, symptoms may
seem to be related to other causes, like stress. There’s no one
test to diagnose AD. Your doctor may use many different
tools to arrive at a diagnosis. These include:
• Medical exam
• Neurological exam
• Cognitive tests
• Talking with family members about changes they’ve
observed
• Reviewing medical and family history
• Blood tests
• Brain imaging, such as magnetic resonance imaging
(MRI), positron emission tomography (PET) scans,
or computed tomography (CT) scan.
• Recent research has focused on blood tests that can
identify proteins associated with AD in the blood.
While these show promise, more research is needed.
Treatment
The goals of treatment are to achieve improvement in cognition and to minimize behavioral disturbances
(depression, psychosis, agitation, and insomnia).
How to choose a treatment
Your doctor will help you choose the best treatment
based on a few things about you, including:
• Your age, overall health, and medical history
• How severe your disease is
• How well a medicine or therapy will work for you
and your lifestyle
• Your preferences or those of your family or caregivers
[36]
Psychosocial treatment
Environmental manipulation, family support, and
prevention of other medical co-morbidities can improve
functioning of AD patients. In attempting to maintain
patients with AD in their homes for as long as possible, some
adjustment of a patient's environment is important. Written
daily reminders can be helpful in the performance of daily
activities. Prominent clocks, calendars, and windows are
important. Patient activities should have minimal changes.
Maintaining adequate hydration, nutrition, exercise and
cleanliness, is important. Family support is essential, since
members are at risk for depression, anxiety syndromes, and
insomnia.
Pharmacotherapy
Alzheimer’s disease is complex, and it is therefore
unlikely that any one drug or other intervention will ever
successfully treat it in all people living with the disease. Still, in recent years, scientists have made tremendous progress
in better understanding Alzheimer’s and in developing and
testing new treatments, including several medications that
are in late-stage clinical trials.
Several prescription drugs are already approved by
the U.S. Food and Drug Administration (FDA) to help
manage symptoms in people with Alzheimer’s disease.
And, on June 7, 2021, FDA provided accelerated approval
for the newest medication, aducanumab, which helps to
reduce amyloid deposits in the brain and may help slow
the progression of Alzheimer’s, although it has not yet been
shown to affect clinical symptoms or outcomes, such as
progression of cognitive decline or dementia.
Most medicines work Alzheimer’s. However, it is
important to understand that none of the medications
available at this time will cure Alzheimer’s (Tab. 1.)
| Drug Name |
Drug Type and Use |
How it Works |
Common Side Effects |
Aducanumab
(Approved on June 2021) |
Disease-'modifying
immunotherapy
prescribed to treat mild
cognitive impairment or
mild Alzheimer’s |
Removes abnormal
beta-amyloid to help
reduce the number of
plaques in the brain |
Amyloid-related imaging
abnormalities (ARIA),
which can lead to fluid
buildup or bleeding in
the brain; also headache,
dizziness, falls, diarrhea,
confusion |
| Donepezil |
Cholinesterase inhibitor
prescribed to treat
symptoms of mild,
moderate, and severe
Alzheimer's |
Prevents the
breakdown of
acetylcholine in the
brain |
Nausea, vomiting,
diarrhea, muscle cramps,
fatigue, weight loss |
| Rivastigmine |
Cholinesterase inhibitor
prescribed to treat
symptoms of mild,
moderate, and severe
Alzheimer's |
Prevents the
breakdown of
acetylcholine and
butyrylcholine (a brain
chemical similar to
acetylcholine) in the
brain |
Nausea, vomiting,
diarrhea, weight loss,
indigestion, muscle
weakness |
| Memantine |
N-methyl D-aspartate
(NMDA) antagonist
prescribed to treat
symptoms of moderate
to severe Alzheimer's |
Blocks the toxic effects
associated with
excess glutamate and
regulates glutamate
activation |
Dizziness, headache,
diarrhea, constipation,
confusion |
| Galantamine |
Cholinesterase inhibitor
prescribed to treat
symptoms of mild to
moderate Alzheimer's |
Prevents the
breakdown of
acetylcholine and
stimulates nicotinic
receptors to release
more acetylcholine in
the brain |
Nausea, vomiting,
diarrhea, decreased
appetite, dizziness,
headache |
Tab. 1. Drug name, type and use, how they work and common side effects.
Medicines to be used with caution in people with
Alzheimer's disease
There are some medicines, such as sleep aids, antianxiety
drugs, anti-convulsants, and antipsychotics, that a
person with Alzheimer’s disease should take only:
After the doctor has explained all the risks and side
effects of the medicine
After other, safer nonmedication options have not
helped treat the problem
People living with Alzheimer’s and their caregivers must
watch closely for side effects from these medications [37- 40].
Sleep aids: are used to help people get to sleep and stay
asleep. People with Alzheimer’s should not use these drugs
regularly because they make the person more confused and more likely to fall. There are lifestyle changes people can
make to improve their sleep. Learn more about getting a
good night's sleep.
Anti-anxiety drugs: are used to treat agitation. These
drugs can cause sleepiness, dizziness, falls, and confusion.
For this reason, doctors recommend they should only be
used for short periods of time.
Anti-convulsants: are drugs sometimes used to treat
severe aggression. Side effects may cause sleepiness, dizziness,
mood swings, and confusion.
Antipsychotics: are drugs used to treat paranoia,
hallucinations, agitation, and aggression. Side effects of
using these drugs can be serious, including increased risk of
death in some older people with dementia. They should only
be given to people with Alzheimer’s when the doctor agrees
that the symptoms are severe.
The future of Alzheimer's disease treatments
Alzheimer’s disease research has developed to a point
where scientists are exploring a variety of avenues to not
only treat symptoms but also address underlying disease
processes. In ongoing clinical trials, scientists are developing
and testing several possible interventions, including
immunization therapy, drug therapies, cognitive training,
physical activity, and treatments for cardiovascular disease
and diabetes [37-40].
Blueberries could be used to fight Alzheimer's,
researchers suggest
Our new findings corroborate those of previous animal
studies and preliminary human studies, adding further
support to the notion that blueberries can have a real
benefit in improving memory and cognitive function in
some older adults,” Krikorian states [41].
The deep blue coloring of blueberries is due to
compounds called anthocyanins, which are also found in
other fruits and vegetables with similar colors, such as
cranberries, red cabbage and eggplants.
Previous research has attributed protection against
cardiovascular diseases, neurodegenerative diseases and some forms of cancer to anthocyanins. It is these
anthocyanins, as well as high levels of antioxidants in the
berries, that the researchers suggest are behind the beneficial
effects they believe their studies illustrate.
According to the Alzheimer’s Association, in 2015, an
estimated 5.3 million Americans had Alzheimer’s disease,
a neurological disorder in which the death of brain cells
causes memory loss and cognitive decline. Of these people,
it is estimated that 5.1 million were aged 65 and older.
As the proportion of the US population aged 65 and
older increases, the number of people with Alzheimer’s
disease is expected to increase. The Alzheimer’s Association
predicts that by 2025, the number of people in this age
group with the disease will increase by 40%, to 7.1 million
people.
Conclusion
AD is the most common of many causes of dementia,
and its prevalence is increasing worldwide. Disease
pathology starts years before noticeable symptoms.
Neuropsychological, imaging, and spinal fluid tests can
establish the diagnosis with high accuracy. Although there
are currently no treatments that slow the disease process,
management of the cognitive and behavioral symptoms of
AD dementia can significantly improve the lives of patients
and their caregivers. AD pathology consists of -amyloid
plaques and phospho-tau neurofibrillary tangles.
AAD pathology can be present in asymptomatic,
mildly affected (MCI), or demented individuals. Although
advanced age and genetics are the predominant risk factors,
several preventable risk factors also contribute to the
likelihood of developing AD dementia. There is no perfect
diagnostic test for AD, but neuropsychological testing,
neuroimaging, and CSF analysis can substantially increase
diagnostic accuracy.
There is no cure for AD. Ideal AD management includes
a combination of symptomatic treatment for cognitive
issues, detection and judicious control of behavioral issues,
and caregiver support.
REFERENCES
- Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7(1):33.
Google Scholar, Crossref, Indexed at
- Dementia Fact sheet. World Health Organization. 2020.
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329-344.
Crossref, Indexed at
- Simon RP, Greenberg DA, Aminoff MJ. Clinical neurology (10th Ed.). [New York]: McGraw Hill. 2018;pp:111.
- Burns A, Iliffe S. Alzheimer's disease. BMJ. 2009;338:b158.
Crossref
- Todd S, Barr S, Roberts M, et al. Survival in dementia and predictors of mortality: a review. Int J Geriatr Psychiatry. 2013;28(11):1109-1124.
Google Scholar, Crossref, Indexed at
- Textbook of physiology volume 2 by AK JAIN (Avichal publishing company) 4th Edition.
- World Health Organization. Dementia – key facts. 2019.
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321-387.
- Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88(4):640-651.
Google Scholar, Crossref, Indexed at
- Kinney W, Bemiller SM, Murtishaw AS, et al. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement. 2018;4:575-590.
Google Scholar, Crossref
- De Gruyter the neuroanatomy of Alzheimer’s disease RCA persons, TPD Powell Reviews in the neuros. 1989;2(2):101-122.
- Bruen PD, McGeown WJ, Shanks MF, et al. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain. 2008;131(9):2455-2463.
Google Scholar, Crossref
- Gould RL, Brown RG, Owen AM, et al. Functional neuroanatomy of successful paired associate learning in Alzheimer's disease. Am J Psychiatry. 2005;162(11):2049-2060.
Google Scholar, Crossref, Indexed at
- Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368(2):117-127.
Google Scholar, Crossref, Indexed at
- Image supplied by Alzheimer’s Research UK in the article “Global research team discovers new Alzheimer’s risk gene. 10 early signs and symptoms of Alzheimer’s. (n.d.).
- Alzheimer’s disease facts and figures. 2019.
- Alzheimer’s disease and related dementia. 2020.
- Chen HY, Panegyres PK. The role of ethnicity in Alzheimer’s disease: Findings from the C-Path Online Data Repository. J Alzheimers Dis. 2016; 51(2):515-523.
Google Scholar, Crossref, Indexed at
- Heymann D, Stern Y, Cosentino S, et al. The association between alcohol use and the progression of Alzheimer’s disease. Curr Alzheimer Res. 2016;13(12):1356-1362.
Google Scholar, Crossref, Indexed at
- If you have younger-onset Alzheimer’s disease. (n.d.).
- Kumar A, Sidhu J, Goyal A, et al. Alzheimer disease. StatPearls. 2021.
Google Scholar, Indexed at
- Mendez MF. Early-onset Alzheimer’s disease. Neurol Clin. 2017;35(2):263-281.
Google Scholar, Crossref, Indexed at
- Reynolds S. Blood tests show promise for early Alzheimer’s diagnosis. Nat Institute Aging. 2020.
Google Scholar
- Weller J. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Res. 2018;7:1161.
Google Scholar, Crossref, Indexed at
- Alzheimer A, Stelzmann RA, Schnitzlein HN, et al. An English translation of Alzheimer's 1907 paper, "Über eine eigenartige Erkankung der Hirnrinde." Clin Anat. 1995;8(6):429-431.
Google Scholar, Crossref, Indexed at
- Alzheimer's Association, Thies W, Bleiler L. 2011 Alzheimer's disease facts and figures. Alzheimers Dement. 2011;7:208-244.
Google Scholar, Crossref
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10(9):819-828.
Google Scholar, Crossref, Indexed at
- Ballard C, Gauthier S, Corbett A, et al. Alzheimer's disease. Lancet. 2011;377(9770):1019-1031.
Google Scholar, Crossref, Indexed at
- Castillo-Carranza DL, Guerrero-Muñoz MJ, Kayed R. Immunotherapy for the treatment of Alzheimer's disease: amyloid-β or tau, which is the right target? Immunotargets Ther. 2014;3:19-28.
Google Scholar, Crossref, Indexed at
- Meeks TW, Ropacki SA, Jeste DV. The neurobiology of neuropsychiatric syndromes in dementia. Curr Opin Psychiatry. 2006;19(6):581-586.
Google Scholar, Crossref, Indexed at
- Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734-746.
Google Scholar, Crossref, Indexed at
- Savva GM, Zaccai J, Matthews FE, et al. Prevalence, correlates and course of behavioural and psychological symptoms of dementia in the population. Br J Psychiatry. 2009;194(3):212-219.
Google Scholar, Crossref, Indexed at
- Ballard CG, Gauthier S, Cummings JL, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol. 2009;5(5):245-255.
Google Scholar, Crossref, Indexed at
- McKhann G, Drachman D, Folstein M, et al. Clinical Diagnosis Of Alzheimer's Disease: Report Of The Nincds-Adrda Work Group Under The Auspices Of The Department Of Health And Human Services Task Force On Alzheimer's disease. Neurology. 1984;34(7):939-944.
Google Scholar, Crossref, Indexed at
- Harvey RJ, Skelton-Robinson M, MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatr. 2003;74(9):1206-1209.
Google Scholar, Crossref
- American Geriatrics Society 2012 Beers Criteria Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
Google Scholar, Crossref, Indexed at
- Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143-1153.
Google Scholar, Crossref, Indexed at
- Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1154-1166.
Google Scholar, Crossref, Indexed at
- Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease. Cochrane Database Syst Rev. 2006;1:CD003476.
Google Scholar, Crossref, Indexed at
- Rochon PA, Normand SL, Gomes T, et al. Antipsychotic therapy and short-term serious events in older adults with dementia. Arch Intern Med. 2008;168(10):1090-1096.
Google Scholar, Crossref












