Keywords
Alzheimer’s disease; Cerebrovascular disease; Cognitive impairment; Dementia; Prevention
Introduction
Alois Alzheimer in 1906 described a “peculiar severe disease process of the cerebral cortex” with “miliary foci” (β-amyloid plaques) and “fibrils” (neurofibrillary tangles) in a patient with dementia praecox and the condition was named “Alzheimer’s Disease” [1]. The term “Alzheimer’s Disease” is currently used in several different senses:
(a) Specifically, by neurologists, psychiatrists and others to mean the form of neurodegeneration characterized by β- amyloid plaques and neurofibrillary tangles in the brain as described by Alzheimer; the term “vascular dementia” (VaD) is used for dementia attributed to cerebrovascular disease.
(b) Loosely, to include all forms of aging-related cognitive impairment and dementia (ARCID)..
(c) Generally, in non-medical circles instead of the word “dementia”.
The different interpretations of the name “Alzheimer’s Disease” have led to misunderstanding and the meaning may only be clear from the context.
Alzheimer’s disease as first described by Alzheimer is but one of several causes of ARCID (Table 1). The commonest are AD, Cerebrovascular disease (CVD) and Lewy body disease (LBD) which frequently co-exist and result in “mixed dementia” [2]. ARCID may be regarded as a syndrome, i.e., a complex of symptoms with multiple causes, similar to other chronic diseases [3].
| Alzheimer’s disease (β-amyloid plaques, neurofibrillary tangles) |
| Cerebrovascular disease |
| Lewy Body Disease and α-synucleinopathies |
| Non-Alzheimer tauopathies (Supranuclear palsy, Pick’s disease) |
| TDP-43 proteinopathies (Fronto-temporal lobe degeneration) |
| Parkinson’s Disease |
| HIV Immunodeficiency disease |
| Prion Disease (Creutzfeldt-Jakob Disease) |
Table 1: Brain pathologies associated with cognitive impairment and dementia.
Cognitive impairment and dementia are commonly diagnosed clinically according to the 2011 criteria of the Alzheimer’s Association and the National Institute of Aging of the USA [4,5]. These are:
(a) Dementia with changes in two or more aspects of cognition or behavior interfering with day-to day function and unable to live independently; not due to delirium or a psychiatric disorder.
(b) Mild cognitive impairment (MCI) with changes in one or more cognitive domains but the ability to function independently in daily life with minimal assistance, in the absence of known systemic or brain disease.
(c) Preclinical disease with changes in biomarkers of AD in the cerebrospinal fluid, in cerebral structure or function, or in poor performance on challenging cognitive tests. Patients with preclinical disease may not necessarily progress to MCI and dementia.
Pathology of Alzheimer’s Disease, Cerebrovascular Disease and Dementia
The cerebral pathology in men and women with dementia and in those with normal cognition at the time of death has been investigated in at least four major post-mortem studies; the Religious Orders Study and Rush Memory and Aging Study, the Medical Research Council cognitive and aging study, the Vienna Trans-Danube aging study, and The National Alzheimer’s Coordinating Centre USA Study [6-9]. The findings were similar in all four studies. The main conclusions were that the changes of AD and CVD (a) frequently co-exist in late-onset dementia (b) overlap to varying degrees and have additive and synergistic effects on cognitive decline (c) are sometimes found in persons with normal cognition at the time of death [10]. The multicenter post-mortem study in the USA of 5,715 cases with neurodegenerative disease found that cerebrovascular disease was (a) a common neuropathological finding in aged subjects with dementia (b) more common in Alzheimer’s disease than in other neurodegenerative diseases, especially in younger subjects and (c) lowered the threshold for dementia due to Alzheimer’s disease and α- synucleinopathies [9]. The neurodegenerative and cerebrovascular changes associated with dementia form a spectrum from pure AD to pure CVD and are most commonly combined resulting in “mixed dementia” (Figure 1) in a recent study of 1246 cognitively normal individuals aged 30 to 95, hippocampal volume (magnetic resonance imaging) and β- amyloid (positron emission tomography) and memory performance were measure in different age groups [11]. The observation were reported as “consistent with a model of lateonset AD in which β-amyloidosis arises in later life on a background of preexisting structural and cognitive decline that is associated with aging and not with β-amyloid deposit”. “Reasonable candidates for non-AD processes associated with structural and functional decline in middle age are cerebrovascular disease and its risk factors, including primary age-related tauopathy.” The interaction between the cerebrovascular and neurodegenerative changes is believed to be of major importance in the pathogenesis of Alzheimer’s disease [12-14].
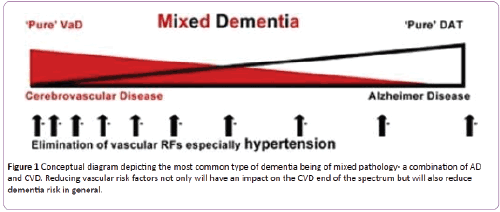
Figure 1: Conceptual diagram depicting the most common type of dementia being of mixed pathology- a combination of AD and CVD. Reducing vascular risk factors not only will have an impact on the CVD end of the spectrum but will also reduce dementia risk in general.
Cognitive reserve and Cognitive resilience
A significant proportion of men and women with normal cognition at the time of death have the neurodegenerative and cerebrovascular changes of the brain associated with dementia. The discordance between the lack of cognitive impairment and the neuropathology constitutes prima facie evidence for the role of some type of brain, neural or cognitive reserve or cognitive resilience [15]. Cognitive reserve” applies to high cognitive ability early in life, its maintenance during midlife and the consequent prevention or postponement of the development of ARCID [16]. “Cognitive resilience” refers to the prevention or delay of ARCID in spite of the development of the pathological changes of AD, CVD and LBD [6]. The occurrence of cognitive reserve and resilience is evidence that AD and ARCID are not an inevitable consequence of aging and the finding merits further investigation.
Risk Factors for Aging-Related Cognitive Impairment and Dementia
ARCID is associated with a considerable number of risk factors. A meta-analysis of 323 papers and of 93 factors considered suitable for epidemiological analysis, identified nine potentially modifiable risk factors; type-2 diabetes, obesity, hypertension, homocysteinaemia, frailty, depression, current smoking, carotid artery narrowing, low educational achievement [17]. The calculated population attributable risk combining all nine factors was 0.66 and it was claimed that two third of AD cases could be explained by these factors. Potentially modifiable risk factors had been estimated to be present in approximately 50% of individuals with AD in the USA and worldwide [18]. The seven modifiable risk factors included in these estimates were midlife hypertension, midlife obesity, diabetes mellitus, physical inactivity, smoking, depression and low education. The estimates do not take into account the non-independence of risk factors and the combined population-attributable risk factors have been estimated to be about 30% in the USA and Europe [19]. Risk Factors for ARCID may be divided into (A) Personal and Psycho-Social and (B) Cerebrovascular and Lifestyle. (Table 2) The avoidance or treatment of the known risk factors currently constitutes the best means of decreasing the incidence or delaying the onset of ARCID.
| Personal and Psycho-social |
Cerebrovascular and Life-style |
| Older age (>65) |
Cardiovascular disease and Stroke |
| Family history |
Hypertension |
| APOεE4 genotype |
Hyperlipidaemia |
| Level of education/Cognitive Activity |
Obesity |
| Depression |
Diabetes |
| Social isolation/Loneliness |
Physical Inactivity |
| Disturbed sleep |
Smoking |
| Traumatic brain injury |
Diet |
Table 2: Risk factors for aging-related cognitive impairment and dementia.
Personal and Psycho-Social Factors
Personal factors
Age, family history and the presence of the lipoprotein APOEε4 allele are in themselves non-modifiable factors but their effects can be postponed or mitigated by favorable health measures. Age is the most important factor determining the incidence and prevalence of cognitive impairment and dementia. In a prospective study in the USA the incidence of all-cause dementia increased exponentially from about 5/1,000 person-years in the 65-69 years age group to about 85/1,000 person-years in the age 90+ years [20]. Based on reviews of family and twin studies the estimates of the genetic heritability of MCI and AD vary from 50% to 80% [21,22]. The commonest genetic risk factor is the ε4 allele of the lipoprotein APOE4 which is estimated to increase the risk of AD about 3 times in heterozygotes and 15 times in homozygotes [23].
Psycho-social factors
Psycho-social factors, and protective measures that increase cognitive reserve and cognitive resilience, play an important part in the prevention of ARCID. In an analysis of more than 20 studies involving 29,000 individuals followed for a median of 7.1 years, higher brain reserve was associated with a lower risk for incident dementia OR 0.54 (0.49-0.59) [16]. The psycho social factors that have been studied include: level of education, continuing cognitive activity and cognitive interventions, social and personality factors, depression, sleep and brain injury.
Level of education, continued cognitive activity and cognitive interventions
The relative risks for low versus high education in a meta analysis of 13 cohort and 6 case-control studies were, for AD 1.80 (1.43-2.27), for non-AD 1.32 (0.92-1.88) and for all dementias 1.59 (1.26-2.01) [24]. The pooled relative risk for lower education in a meta- analysis of 31 studies with incident AD was RR 1.99 (1.30-3.04) [24]. In an analysis of 22 longitudinal studies including 21,456 individuals and 1,733 cases of dementia, the risk of dementia was lower for those with higher education OR 0.53 (0.45-0.62) [25]. The clinical trajectories before the development of AD were studied in 442 participants, 171 with low education and 271 with higher education. The first signs of cognitive decline occurred 15-16 years before dementia in higher educated subjects and 7 years before dementia in less educated subjects [26]. Higher levels of education delayed the onset of cognitive decline but was associated with a more rapid progression once decline commenced. Low level of education is one of the biggest contributors to the high prevalence of AD world-wide [16].
A systematic review of 22 cohort studies including 29,000 individuals concluded that complex patterns of mental activity in early and mid-life was associated with a significant reduction in the incidence of dementia in later life RR 0.54 (0.49-0.59) [16]. In the Rush Memory Project, after controlling for a low baseline cognitive function, past cognitive activity, socioeconomic status and current social and physical activity, frequent participation in cognitive stimulating activities was associated with less rapid decline in cognitive function and a lower incidence of AD, HR 0.58 (0.44-0.77) [27]. A Cochrane review in 2011 concluded that cognitive training interventions significantly improved immediate and delayed recall in healthy older adults [28].
Social isolation and loneliness
Social isolation and loneliness increase cognitive decline and the risk of late-life dementia and social engagement may be of benefit and protective [29,30]. Conscientiousness and purpose in life are associated with a reduced risk of ARCID [31,32]. A combined Cognitive Lifestyle Score (CLS) based on educational attainment, occupational complexity and social engagement was calculated in the MRC-CFAS Study. Those who maintained a high CLS throughout life had a 40% reduced risk of developing dementia [33].
Depression
A systematic review and meta-analysis of 20 studies and of 1,020,172 individuals found that history of depression increased risk of developing AD with a pooled OR of 2.03 (1.73-2.38) for case control studies and 1.90 (1.55-2.33) for cohort studies [34]. A clinic-pathological study of 1764 older persons found that depression was associated with cognitive decline and was independent of the neuropathological changes of AD [35]. Treatment of depression in older adults improves cognitive function but evidence on the prevention of dementia is lacking [18].
Disturbed sleep
Disturbed sleep has a significant effect on cognition. A sleep disturbance index (SDI) was measured in men and women without AD or dementia in the Survey of Health, Aging and Retirement in Europe. [36]. An increased SDI was associated with an increased risk of developing AD or dementia OR 1.23 (1.11-1.36) and remained a strong factor for dementia when overall health status was included in the analysis. A sleep fragmentation index was quantified using actigraphy in a cross-sectional study of 737 community-dwelling older adults with normal cognition followed-up for an average of 3.3 years [37]. After controlling for age, sex and education, a higher level of sleep fragmentation was associated with an increased risk of AD, HR 1.22 (1.03-1.44).
Traumatic brain injury
Moderate and severe traumatic brain injury increases the risk of cognitive decline and is estimated to increase the risk of dementia in later life two to three fold [38]. The risk of cognitive impairment and later onset of dementia is increased in military veterans who have suffered brain injuries, and also in those involved in sports such as boxing and American and Rugby football, particularly in players who have experienced multiple concussions [39,40].
Cerebrovascular and Life-Style Factors
Many cerebrovascular and lifestyle factors predispose to ARCID and are modifiable or preventable. Measures that prevent CVD are similar to those that prevent coronary heart disease and include active treatment of hypertension, hyperlipidaemia and diabetes. The cerebrovascular and neurodegenerative changes associated with aging and the development of ARCID have a long pre-clinical phase and measures to prevent or delay the onset of MCI and dementia need to be actively instituted from early or mid-life onwards.
Cardiovascular disease and stroke
Several measures of cardiovascular disease including coronary artery calcium (CAC), carotid intimal thickness, and ankle-brachial index have been associated with an increased incidence of cognitive impairment and dementia in older adults. A study of subclinical cardiovascular disease in patients 80+ years found that white women with low CAC scores had a significantly reduced risk of dementia [41]. A prospective population-based study of older adults found that those with increased carotid intima-media thickness at base-line had progressive cognitive impairment to MCI or dementia [42]. A systematic review and meta-analysis of 7 cohort studies and 2 case control studies of stroke survivors reported a significantly and independently increased risk of Alzheimer’s disease; pooled risk 1.59 (1.25-2.02) [43].
Hypertension
Mid-life, but not late-life hypertension is associated with an increased risk of AD and dementia with a calculated OR of 1.61 (1.16-2.24) [18,44]. A cohort of a random, population-based sample of 1449 individuals in Sweden was followed for an average of 21 years. Those with a raised systolic pressure in midlife (BP>160mm Hg) had a significantly higher risk of AD in later life OD 2.3 (CI1.0-5.5) after adjusting for age, body mass index, education, vascular effects, smoking and alcohol consumption [45]. A quantitative meta-analysis of 14 studies of subjects without cognitive impairment or dementia 32,658 with and 36,905 without hypertensive medication found no significant difference in the incidence of AD but those with anti-hypertensive medication has a significantly lower incidence of both vascular dementia RR 0.67 (0.52-0.87) and all-cause dementia RR 0.87 (0.77-0.96) [46]. In an analysis of 18 longitudinal studies and 11 randomised controlled trials, 7 longitudinal studies of anti-hypertensive medication found significant benefit on cognitive decline and impairment. Out of 11 longitudinal studies only 3 did not find a significant benefit of antihypertensive medication on the incidence of dementia [47]. Four of the randomized controlled trials showed antihypertensive medication was associated with a significant decreased incidence of cognitive decline and dementia.
Hyperlipidaemia
A systematic review of 18 prospective studies found a significant association between high mid-life total cholesterol (TC) and an increased risk of AD and all-cause dementia. Midlife TC but not late-life TC was associated with an increased risk of dementia [48]. A review of the effect of statins found no evidence of benefit on the risk of dementia [49]. Two separate Cochrane reviews found no association between the use of statins in late life and cognitive decline and no benefit of statins in the treatment of dementia [50,51].
Mid-life obesity
Mid-life obesity has been found to be associated with a significant increase of all-cause dementia In prospective studies and meta-analyses with a pooled estimate of RR of 1.60 (1.34-1.92) [18]. In a retrospective study from UK general practice of 1,958,191 people, those who were underweight in mid-life had a 34% increased risk of dementia and the incidence decreased with increasing BMI [52]. Very obese people had a 29% lower risk of dementia compared with those with a healthy weight. The finding of an increased incidence of dementia in those who were underweight in mid- or late- life remains to be explained.
Diabetes
A number of systematic reviews and meta-analyses have reported an increased risk of impaired cognition or dementia in association with Type-II diabetes [18]. A meta-analysis of prospective 28 observational studies found that the pooled relative risk of developing AD was 1.56 (1.41-1.73) of VaD was 2.27 (1.96-2.66) and all-cause dementia was 1.73 (1.65-1.82) [53]. Diabetes increased the risk of conversion of MCI to dementia and the risk was less in diabetics on a Mediterranean diet [54].
Smoking
A review of 37 studies found that compared with never smokers, current smokers had an increased risk of AD (RR1.40 –CI 1.13-1.73), VaD (RR 1.38 CI 1.15-1.66) and all cause dementia (RR1.30 CI 1.13-1.73) [55]. The risk of all-cause dementia increased by 34% for every 20 cigarettes smoked per day and was not increased in former smokers. In a study of a cohort of 21,123 people, heavy smoking in mid-life was associated with a more than 100% increase in AD, VaD and all– cause dementia over two decades of follow-up [56].
Physical inactivity
A review and meta-analysis of 16 prospective studies on the association between physical activity and dementia found that comparing highest vs lowest activity groups the combined RR for AD was 0.55 (0.36-0.84) and for all-cause dementia was 0.72 (0.60-0.80) [57]. These values have been reversed to reflect the risks with inactivity as 1.82 (1.19-2.78) for AD and 1.39 (1.6-1.67) for all cause dementia [18]. A review and metaanalysis of physical activity in 21 prospective cohorts comparing higher with lower levels of activity the RR on cognitive decline was 0.65 (0.55-0.76) and on dementia was 0.86 (0.76-0.97) [58]. A Cochrane analysis in 2015 found that healthy sedentary elders who begin exercise have a significant improvement in cognitive function, particularly in mental processing speed [59].
Diet
A Mediterranean diet (MeD) - high intake of vegetables, fruits, nuts and olive oil, relatively low intake of dairy products and red meat, and a moderate intake of wine with meals - has been claimed to slow cognitive decline and to lower the risk of AD in several observational studies [60]. In a prospective study of a similar “MIND” diet, high adherence was reported to be associated with a reduced risk of AD [61]. A randomized, controlled trial of MeD supplemented with olive oil and nuts has been reported to improved cognition [62]. The benefits of a MeD have been attributed to increased cerebrovascular blood flow and to the anti-oxidant and anti-inflammatory effects of specific elements in the diet.
Combined Approach to Prevention of Aging-Related Dementia
There have been few randomized, controlled studies of the effect of combined measures on ARCID. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) reported the results of a double-blind, randomized, controlled trial of 2,654 individuals aged 60-77 years assigned in a 1:1 ratio to multi-domain intervention (cognitive training, diet, exercise and vascular risk monitoring) or a control group (general health advice) [63]. The primary outcome was a change in cognition in a neuro-psychological test battery score (NTB). The difference in NTB between the two groups after 2 years was statistically significant (p=0.03). There was also a significant difference in secondary outcomes of executive functioning (p=0.04) and processing speed (0.04) but not in memory (p=0.36). It was concluded that multidomain intervention can improve cognitive function in elderly people. Nine population-based studies of dementia incidence and prevalence in England, Sweden, The Netherlands and the USA have reported a declining prevalence and age-specific incidence of dementia in recent years [64]. In the Framingham Heart Study the age and sex-adjusted hazard rates of dementia in four successive decades starting in 1975 were respectively 3.6, 2.8., 2.2 and 2.0 per 100 persons [65]. Relative to the first decade the incidence of dementia declined by 22%, 38% and 44%. The risk reduction was significant in the largest group of persons with a high school diploma or higher qualification HR 0.77 (0.67-0.88) A critical review of five studies in Western Europe concluded that the changes in overall dementia occurrence were not statistically significant. The only study however, that was specifically designed to measure changes across the generations did find a significant decrease in prevalence of dementia in the UK of 22% (P=0.03) [66]. The decrease in the incidence of dementia in recent years has been attributed to rising levels of education, healthier life-style including exercise, and better prevention and treatment of cardiovascular disease. Whether these favorable trends will continue in the face of rising levels of obesity and diabetes is uncertain. Although the age-specific incidence of dementia may be decreasing in some countries, the world population, the number living to old age, and the number with agingrelated dementia is increasing world-wide. It has been estimated that the total number of people in the world with dementia will triple from 2015 to 2050.
Conclusion
Cerebrovascular disease plays a larger part in ARCID than is often recognized and much larger part than included under the category of vascular dementia. “Mixed dementia” with varying degrees of cerebrovascular disease and Alzheimer’s disease (characterized by amyloid plaques and neurofibrillary tangle) is the commonest form of aging-related dementia. Many measures that reduce the incidence of coronary heart disease are the same as those that reduce the incidence of dementia. It is suggested that the cardiovascular measures that reduce the incidence of dementia do so primarily by reducing the occurrence or severity of cerebrovascular disease. As AD-modifying treatments are still in the early stages of development, the best hope for reducing agingrelated cognitive impairment and dementia in the immediate future lies in a broad approach; namely the promotion of physical and mental exercise and the active treatment of vascular risk factors from early and mid-life onwards, combined with better education and correction or modification of adverse psycho-social factors. The aim is to prevent or delay the onset of ARCID and enable people to live more active, healthy, longer lives and to lessen the burden on their families, their carers and society.
Conflicts of Interest
I am the sole author of this paper and there are no conflicting interests. I have received no funds or writing assistance in preparation of this paper. The permission of the authors and the publishers has been obtained for Figure 1.
Acknowledgement
Michael Valenzuela and Translational Psychiatry for permission to publish Figure 1. Clifford Jack Jr et al. and JAMA Neurology for two direct quotations from Jack CR Jr, Wiste HJ, Weigand SD et al. Age, Sex and APOE epsilon4 effects on memory, brain structure and beta-amyloid across the adult life span. JAMA Neurol 2015; 72:511-519. ORCID number 0000-0002-0904-5462. 95% confidence limits are included in brackets after all relative risks.
9406
References
- Alzheimer A (1907) Uber eine eigenartige Erkankung der Hinrninde. Allegmine Zeitschriftfϋr Psychiatrie und Psychisch-GEritliche Medizin 64:146-148.
- Valenzuela M, Esler M, Ritchie K, Brodaty H (2012) Anti-hypertensives for combating dementia?” A perspective on candidate molecular mechanisms and population-based prevention. Transl Psychiatry 2:e107.
- Larson EB, Kaffe K, Langa KM (2013) New insights into the Dementia epidemic. N Engl J Med 369:2275-2277.
- McKhann GM, Knopman DS, Chertkow H (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dement 7:263-269.
- Albert MS, Dekosky ST, Dickson D (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging - Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s. Alzheimer’s & Dement 7:270-279.
- Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE (2011) Cognition and neuropathology in aging: multidimensional perspective from the Rush Religious Orders Study and Rush Memory Aging Project. Curr Alzheimer Res 8:336-340.
- Wharton SB, Brayne C, Savva GM (2011) Epidemiological neuropathology: the MRC Cognitive Function and Aging experience. J Alzheimers Dis 25:359-372.
- Kovacs GG, Milenkovic I, Wohrer A (2013) Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol 126:365-384.
- Toledo JB, Arold SE, Raibie K (2013) Contribution of cerebrovascular disease in an autopsy confirmed neurodegenerative disease case in the National Alzheimer’s Coordinating Centre. Brain 136:2697-2706.
- Davey DA (2015) Alzheimer’s disease, cerebrovascular disease and dementia: a potentially preventable and modifiable syndrome. J Alzheimer’s Dis Parkinsonism 5:1-5.
- Jack CR Jr, Wiste HJ, Weigand SD (2015) Age, sex and APOE epsilon4 effects on memory, brain structure and beta-amyloid across the adult life span. JAMA Neurol 72:511-519.
- Kalaria RN, Akinyemi R, Ihara M (2012) Does vascular pathology contribute to Alzheimer changes? J Neurol Sci 15:141-147.
- Honjo K, Black SE, Verhoeff NP (2012) Alzheimer’s disease, cerebrovascular disease, and the β-amyloid cascade. Can J Neurol Sci 39:712-718.
- Kling MA, Trojanowski JQ, Wolk DA, Lee VM, Arnold SE (2013) Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimers & Dement 9:76-92.
- Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, Schneider JA (2014) Cognitive and social life-style: links with neuropathology in late life. Acta Neuropathol 127:137-50.
- Valenzuela MJ, Sachdev P (2006) Brain reserve and dementia: a systematic review. Psychological Medicine 36:441-445.
- Wu, Tan L, Wang HF (2015) Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry.
- Barnes D, Yaffe K (2011) The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10:819-828.
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C (2014) Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 13:788-794.
- Kukull WA, Higdon R, Bowen JD (2002) Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol 59:1737-1746.
- Wilson RS, Barral S, Lee JH (2011) Heritability of different forms of memory in the late onset Alzheimer's disease family study. J Alzheimers Dis 23:249–255.
- Gatz M, Reynolds CA, Fratiglioni L (2006) Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 63:168–174.
- Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368:387-403.
- Caamano-Isorna F, Corral M. Monyes-Matrinez A, Takkouche B (2006) Education and dementia: a meta-analytic study. Neuroepidemiology 26:226-232.
- Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zondeman AB, et al. (2014) Epidemiological studies of modifiable factors associated with cognition and dementia: systematic review and meta- analysis. BMC Public Health 14:643-676.
- Amieva H, Mokri H, Le Goff M (2014) Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: a study of 20 years of cognitive decline. Brain 137:1167-1175.
- Wilson RS, Scherrr PA, Schneider JA, Tang Y, Bennett DA (2007) Relation of cognitive activity to risk of developing Alzheimer’s disease. Neurology 69:1911-1920.
- Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F (2011) Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane database Syst Rev CD006220.
- Wilson RS, Krueger KR, Arnold SE (2007) Loneliness and risk of Alzheimer’s disease. Arch Gen Psychiatry 64:234-240.
- Fratiglioni L Paillard-Borg S, Winblad B (2004) An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol 3:343-353.
- Boyle PA, Buchman AS, Barnes LL, Bennett DA (2010) Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Arch Gen Psychiatry 67:304–310.
- Wilson RS, Schneider JA, Arnold SE, Bienas JL, Bennett DA (2007) Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry 64:1204-1212.
- Valenzuela M, Brayne C, Sachdev P, Wilcock G, Matthews F (2010) Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. Am J Epidemiol 173:1004-1012.
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D (2006) Depression and risk for Alzheimer disease: systematic review, meta- analysis, and meta-regression analysis. Arch Gen Psychiatry 63:530-538.
- Wilson RS, Capuano AW, Boyle PA (2014) Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology 83:702-709.
- Sterniczuk R, Theou O, Rusak B, Rockwood K. (2013) Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res 10:767-775.
- Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA (2013) Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep 84:1027-1032.
- Shively S, Scher AI, Perl DP, Dias-Arrastia R (2012) Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol 69:1245-1251.
- Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, et al. (2014) Traumatic brain injury and risk of dementia in older veterans. Neurology 83:312-319.
- Guskiewicz KM (2005) Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 57:719-726.
- Kuller LH, Lopez OL, Mackey RH (2016) Subclinical cardiovascular disease and death, dementia and coronary heart disease in patient 80+ years. J Am Coll Cardiol 67:1013-1022.
- Moon JH, Lim S, Han JW (2015) Carotid intima media thickness is associated with the progression of cognitive impairment in older adults. Stroke 46:10241030
- Zhou J, Yu JT, Wang HF (2015) Association between stroke and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 43:479-489
- Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, et al. (2015) Summary of the evidence on modifiable factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s & Dementia 11:718-726.
- Kivipleto M, Helkala EL, Laakso MP (2011) Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 322:1447-1451.
- Chang-Quan H, Hui W, Chao-Min W (2011) The association of antihypertensive medication use with risk of cognitive decline and dementia: a meta-analysis of longitudinal studies. Int J Clin Pract 65:1295 -1305.
- Rouch L, Cestac P, Hanon O( 2015) Anti hypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trial and meta-analyses, with discussion of potential mechanisms. CNS Drugs 29:1213-1230.
- Anstey KJ, Lipnicki DM, Low F (2008) Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry 16:343-354.
- Richardson K, Schoen M, French B (2013) Statins and cognitive function: a systematic review. Ann Int Med 159:688-697.
- McGuinness B, Craig D, Bullock R, Passmore P (2009) Statins for prevention of dementia. Cochrane Database Syst Rev 15:CD003160.
- McGuinness B, Craig D, Bullock R, Malouf R, Passmore P (2014) Statins for the treatment of dementia Cochrane DatabasSyst Rev 7:CD007514.
- Qizilbash N, Gregson J, Johnson ME (2015) BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol 3:31-36.
- Guadala K, Bansal D, Schifano F, Bhansal A (2013) Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J Diabetes Investig 4:3640-3650.
- Cooper C, Sommerland A, Lyketsos CG, Livingston G (2015) Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry 172:323-334.
- Zhong G, Wang Y, Zhang Y, Guo J, Zhao Y (2015) Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect of modifiers. PLoS One e0118333.
- Rusanen M, KivipeltoM, Quesenberry CPJr, Zhou J, Whitmen RA (2011) Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. Arch Int Med 171:333-339.
- Hamer M, Chida Y (2009) Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med 39:3-11.
- Blondell SJ, Hammersley-Mather R, Veerman J (2014) Does physical activity prevent cognitive decline and dementia? A systematic review and meta- analysis of longitudinal studies. BMC Public Health 14:510.
- Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L (2008) Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane database of systematic reviews Jul 16:CD005381.
- Lourida I, Soni M, Thompson-Coon J (2013) Mediterranean diet, cognitive function and dementia: a systematic review. Epidemiology 24:479-489.
- Morris MC, Tagney CC, Wang Y, Sacks TM, Bennett DA, et al. (2015) MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers& Dement 11:1007-1014.
- Vallas-Pedret C, Sala-Villa A, Serra-Mir M (2015) Mediterranean diet and age-related cognitive decline: A randomised clinical trial. JAMA Inter Med 175:1094-1103.
- Ngandu T, Lehtisalo J, Solomons A (2015) A 2 year multi-domain intervention of diet, exercise, cognitive training and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): randomized controlled trial. Lancet 385:2255-2263.
- Langa KM (2015) Is the risk of Alzheimer’s disease and dementia declining? Alzheimer’s Res Ther 7:34.
- Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufoil C, et al. (2016) Incidence of dementia over three decades in the Framingham heart study. N Engl J Med 374:523-525.
- Wu YT, Fratiglioni L, Mathews FE, Lobo A (2016) Dementia in Western Europe: Epidemiological evidence and implications for policy making. Lancet Neurol 15:116-124.






