Keywords
Solubility; Bioavailability; Polymers; Beta-Cyclodextrin
Introduction
Cyclodextrin (CD’s) comes under a family of cyclic oligosaccharides that are composed of α-1, 4-linkedglucopyranose subunits. It took CD’s 100 years to evolve from interesting chemical commodities to enabling pharmaceutical excipients. They were discovered in 1891, characterized in the first half of the last century but only came available as highly purified excipients during the past thirty years [1]. In the beginning CD were used to enhance aqueous solubility and chemical stability of drugs and these functionalities were related to their ability to form drug/cyclodextrin inclusion complexes. However, in recent years CD have been shown to participate in various types of noninclusion complexes with, for example, organic salts and water soluble polymers. They have also been shown to form aggregates, either alone or in combinations with other excipients. These aggregates can form dispersed drug delivery systems such as micro and nanoparticles [2]. Thus, one hundred years after their discovery CD are still regarded as novel excipients of unexplored possibilities.
Construction of calibration curve
Standard calibration curve for simvastatin in methanol
Stock solution: Accurately weighed 25 mg of Simvastatin was dissolved in 25 ml of methanol to give a concentration of 1000 mcg/ml.
Working standard: From the stock solution, working standard was prepared to give a concentration of 100 mcg/ml in methanol.
Aliquots of working standard solution (100 mcg/ml) were suitably diluted with methanol to get final concentration of 2-10 mcg/ml.
The absorbance of prepared solutions of Simvastatin in methanol measured at 237.8 nm using Shimadzu 1700 UV-Visible Spectrophotometer against reagent blank and graph was plotted. The standard calibration curve yields a straight line, which shows that drug obey’s beer’s law in the concentration range of 2-10 mcg/ml [3,4].
Standard calibration curve for simvastatin in phosphate buffer
Stock solution: Accurately weighed 25 mg of simvastatin was dissolved in 25 ml of methanol to give a concentration of 1000 mcg/ml.
Working standard: From the stock solution, working standard was prepared to give a concentration of 100 mcg/ml in phosphate buffer.
Aliquots of working standard solution (100 mcg/ml) were suitably diluted with phosphate buffer to get the final concentration range of 2-10 mcg/ml.
The absorbance of prepared solutions of Simvastatin in phosphate buffer measured at 237.8 nm using Shimadzu 1700 UV-Visible Spectrophotometer against reagent blank and graph was plotted [5]. The standard calibration curve yields a straight line, which shows that drug obeys beer’s law in the concentration range of 2-10 mcg/ml.
Methodology
Methods used in present work
Kneading method: Simvastatin with β-Cyclodextrin in different molar ratios (i.e., 1:1 M, 1:2 M) were taken. First cyclodextrin is added to the mortar, next ethanol and dichloromethane (2:1) is added with or without addition of polymers like PVP, PEG, HPMC (10%) while triturating to get slurry like consistency [6]. Then slowly drug is incorporated into the slurry and trituration is further continued for one hour. Slurry is then air dried at 25°C for 24 hours, pulverized and passed through sieve no. 100 and stored in dessicators over fused calcium chloride.
Drug evaluation studies
Estimation of simvastatin in the inclusion complex
Procedure: 25 mg of complex was accurately weighed and transferred to 25 ml volumetric flask and volume was made up to the mark with methanol [7]. From this 1 ml was taken in 10 ml volumetric flask and the volume is adjusted up to the mark with phosphate buffer (pH=6.8). The absorbance of the solution was measured at 238.7 nm using appropriate blank. The drug content of Simvastatin was calculated using calibration curve.
Dissolution characteristics
In vitro dissolution studies for simvastatin-cyclodextrin complexes
Procedure: In-vitro dissolution of Simvastatin inclusion complex was studied in USP XXIII dissolution apparatus (electrolab) employing a paddle stirrer. 900 ml of phosphate buffer of pH=6.8 was used as dissolution medium. The stirrer was adjusted to rotate at 50 rpm. The temperature of dissolution media was previously warmed to 37+0.5°C and was maintained throughout the experiment. Complex equivalent to 50 mg of simvastatin was used in each test. 5 ml of sample of dissolution medium were withdrawn by means of syringe fitted with pre-filter at known intervals of time and analyzed for drug release by measuring the absorbance at 238.7 nm after suitable dilution with phosphate buffer. The volume withdrawn at each time interval was replaced with fresh quantity of dissolution medium. Percentage amount of simvastatin released was calculated and plotted against time. For comparison, the dissolution of pure drug was studied [8,9].
Drug-excipient interaction studies
Fourier transform infrared spectroscopy: Infrared spectroscopy is one of the most powerful analytical techniques that offer the possibility of chemical identification. The IR spectra of simvastatin and their complexes were obtained by KBr pellet method [10]. The results of IR of inclusion complexes prepared by kneading method are given in Figures 1-8.
Figure 1: FT-IR spectrum of Formulation SBK1.

Figure 2: FT-IR spectrum of Formulation SBK2.
Figure 3: FT-IR spectrum of Formulation SBPVK1.
Figure 4: FT-IR spectrum of Formulation SBPVK2.
Figure 5: FT-IR spectrum of Formulation SBPEK1.
Figure 6: FT-IR spectrum of Formation SBPEK2.
Figure 7: FT-IR spectrum of Formulation SBPEK2.
Figure 8: FT-IR spectrum of Formulation SBHPK1.
Differential scanning calorimetry: The thermal behaviour of simvastatin, β-cyclodextrin and β-cyclodextrin-polymer (PVP, PEG, HPMC) inclusion complexes was studied using differential scanning calorimetry to confirm the formation of the solid complex. When guest molecules are incorporated in the cyclodextrin cavity or in the crystal lattice, their melting, boiling and sublimation points are usually shifted to a different temperature or disappear within the temperature range, where the cyclodextrin lattice is decomposed [11]. The results of DSC of inclusion complexes prepared by kneading method are shown in Figures 9-15.
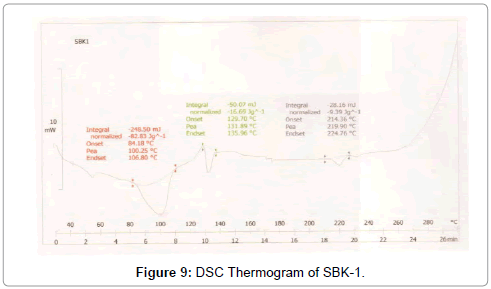
Figure 9: DSC Thermogram of SBK-1.
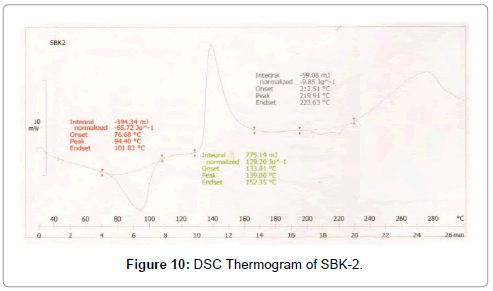
Figure 10: DSC Thermogram of SBK-2.
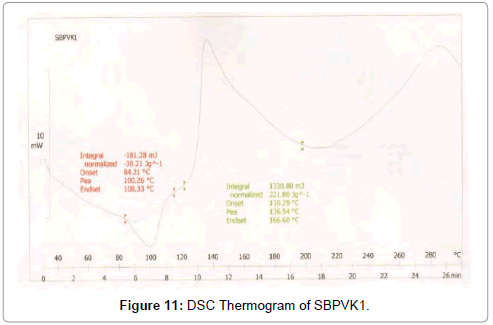
Figure 11: DSC Thermogram of SBPVK1.
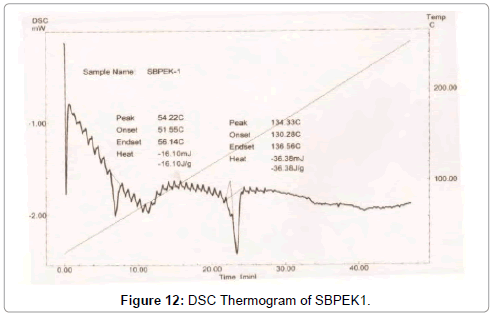
Figure 12: DSC Thermogram of SBPEK1.
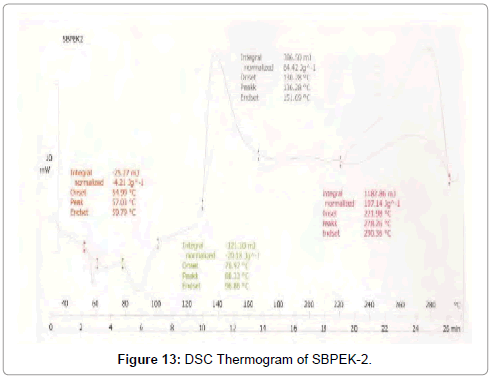
Figure 13: DSC Thermogram of SBPEK-2.
Figure 14: DSC Thermogram of SBHPK1.
Figure 15: DSC Thermogram of SBHPK2.
Results and Discussion
Simvastatin cyclodextrin complexes
The drug content of the inclusion complexes was quite uniform as can be observed from the Tables 1-3. The percent drug content of the complexes was found to be in the range of 90.15% to 97.3%.
| Simvastatin |
CiplaPharmaceuticals, Goa. |
| β-Cyclodextrin |
SA PharmachemPvt. Ltd., Mumbai |
| Methanol |
Renkem, Ranbaxy Chem., SASNagar |
| Dichloromethane |
Research Labs, Mumbai |
| Polyvinylpyrrolidone-K-30 |
West Coast Laboratories |
| Polyethylene glycol-4000 |
Genuine Chemical Co., Mumbai |
| Hydroxypropylmethyl cellulose |
SD Fine Chemicals Ltd. Mumbai |
Table 1: Materials and their sources.
| Dissolution test apparatus |
Electrolab, USP XXIII |
| UV Visible Spectrophotometer |
UV-Visible1700, Shimadzu |
| Hot Air Oven |
MCDalal and Co., Chennai |
| Digital Balance |
Shimadzu (Type, BL-2204) |
| Gyratory Flask Shaker |
Kemi Electrical |
| Sieve |
100 mesh |
| Glassware |
Borosil (A) Grade |
Table 2: Equipment’s and their Sources.
| Method |
Drug to carrier |
Drug to carrier ratio |
code |
| Kneading method |
SV:β-CD |
1:1 |
SBK1 |
| SV:β-CD |
1:2 |
SBK2 |
| SV:β-CD:PVP |
1:1 |
SBPVK1 |
| SV:β-CD:PVP |
1:2 |
|
| SV:β-CD:PEG |
1:1 |
SBPVK2 |
| SV:β-CD:PEG |
1:2 |
|
| SV:β-CD:HPMC |
1:1 |
SBPEK1 |
| SV:β-CD:HPMC |
1:2 |
SBPEK2 |
Table 3: Different formulations of simvastatin with β-cyclodextrin along with hydrophilic polymers.
FTIR study
The FTIR of SV and inclusion complexes of SV: β-CD and with hydrophilic polymers like PVP, PEG, HPMC prepared by kneading method in different molar ratios were recorded in a KBr pellet using Shimadzu FTIR-8700 (Japan) spectrophotometer. The powders of pure drug and prepared solid inclusion complexes were studied at 500 to 4000 cm-1.
FTIR patterns of SV-β-CD and SV-β-CD-Hydrophilic polymers are represented in Figures 1-8 respectively.
FTIR studies of SV shows very strong peak at 3549 cm-1 corresponding to hydroxyl moiety attached to cyclic lactone. The broad C=O peak corresponding to C=O of lactones is noticed at 1722 and 1699 cm-1. The C- H ring system exhibited peaks at the 3009, 2955, 2928 and 1699 cm-1. IR spectra of β-CD shows strong broad absorption peak due to OH group is noticed at 3376 cm-1 [12]. In PVP molecule along with the expected C-H peaks the broad hump is noticed at 3568 cm-1 and 3161 cm-1 corresponding to polymeric nature of PVP. The cyclic C=O peak found to exhibit absorption at 1693 cm-1 which is a normal place of absorption of cyclopentanone molecule. The IR spectra of HPMC give a broad absorption peak from 3500 cm-1 to 3215 cm-1 which is the characteristic property of polyhydroxyl molecule of polymers [13]. IR spectra of PEG showed the presences of characteristic absorption peak, due to hydroxyl functional group and C-H functions.
In all the cases neither the characteristics functional group of the drug molecule nor the polymer are affected in the range of absorption. The intensity of their absorption has not affected. This fact suggests that during the process of formulation followed in kneading method no chemical reaction is taken place between the drug and polymers used. All the above corresponding peaks indicate weak interaction between drug and β-CD.
DSC studies
Differential scanning calorimetry thermograms of the drug, β-CD, hydrophilic polymers like PVP, PEG, and HPMC and prepared binary and ternary systems were recorded on the Perkin-Elmer thermal analysis calorimetry. Samples (2-5 mg) were sealed in aluminium pans and scanned at a rate of 10°C/min over a temperature range of 30°C to 300°C under nitrogen gas stream.
DSC was used to characterize the SV-β-CD and SV-β-CD along with hydrophilic polymers PVP, PEG, and HPMC complexes. The thermal behaviour of the cyclodextrin inclusion complexes was studied using DSC to confirm the formation of solid inclusion complexes. The DSC thermograms of various products are shown in Figures 9-15. The DSC thermogram of SV exhibit an endothermic peak at 138.32°C corresponding to its melting point, the endothermic peak of β-CD showed a broad peak at 91.82°Cwhich may be attributed to a dehydration process [14,15].
The DSC thermogram of HPMC exhibited endothermic peak at 189.09°C, the DSC thermogram of PVP-K-30 exhibited peak at 59.44°C and DSC thermogram of PEG-4000 exhibited peak at 59.13°C. The DSC thermograms of SV with β-CD 1:1 prepared by kneading method showed a peak at 131.89°C and drug β-CD 1:2 prepared by kneading method showed peak at 139°C.
The DSC thermograms of drug: β-CD along with PVP 1:1 ratio prepared by kneading method showed peak at 136.54°C and drug: β-CD with PVP 1:2 showed peak at 133.8°C. The DSC thermograms of drug: β-CD along with PEG 1:1 prepared by kneading method showed peak at 134.33°C and that of drug: β-CD: PEG 1:2 prepared by kneading method 130.28°C. The DSC thermograms of drug: β-CD along with HPMC 1:1 prepared by kneading method showed peak 131.12°C and that of drug: β-CD: HPMC 1:2 Kneading method showed peak at 138.13°C. The slight shifting peaks indicate interaction of SV with β-CD.
In-vitro dissolution
In-vitro dissolution studies for pure SV and inclusion complexes prepared were carried out in 900 ml of phosphate buffer of (pH=6.8) using USP XXIII dissolution rate test apparatus (Electrolab) with a paddle stirrer. The dissolution data of SV, SV-β-CD and SV-β-CDhydrophilic polymer systems are given in Tables 4-8. The dissolution rate of SV from various inclusion complexes was found to be 80.9 to 100% approximately in 120 minutes, when compared to pure drug which exhibited only 12 to 39% of drug in 120 minutes.
| Code |
Percent drug content |
| SBK1 |
90.15 |
| SBK2 |
93.71 |
| SBPVK1 |
99.90 |
| SBPVK2 |
99.92 |
| SBPEK1 |
91.62 |
| SBPEK2 |
95.93 |
| SBHPK1 |
96.42 |
| SBHPK2 |
97.33 |
Table 4: Drug content estimation of Simvastatinin the inclusion complexes.
| S.No. |
Time (HR) |
Percent drug release (%) |
| SBK1 |
SBK2 |
Pure Drug |
| 1 |
5 |
25.0 |
23.4 |
11.8 |
| 2 |
10 |
29.1 |
31.1 |
12.0 |
| 3 |
15 |
33.0 |
37.9 |
16.1 |
| 4 |
30 |
36.7 |
42.7 |
22.8 |
| 5 |
45 |
39.9 |
49.5 |
26.9 |
| 6 |
60 |
44.3 |
56.6 |
28.2 |
| 7 |
75 |
53.3 |
63.4 |
32.0 |
| 8 |
90 |
63.1 |
71.7 |
34.2 |
| 9 |
105 |
71.5 |
85.9 |
34.5 |
| 10 |
120 |
80.9 |
88.0 |
39.9 |
Table 5: Dissolution Studies of SV Complexes (SBK1, SBK2 & Pure drug) in Phosphate Buffer (pH=6.8).
| S.No. |
Time (HR) |
Percent drug release (%) |
| SBPVK1 |
SBPVK2 |
Pure Drug |
| 1 |
5 |
30.5 |
29.5 |
11.8 |
| 2 |
10 |
38.5 |
41.1 |
12.0 |
| 3 |
15 |
44.8 |
50.1 |
16.1 |
| 4 |
30 |
52.8 |
57.4 |
22.8 |
| 5 |
45 |
56.6 |
67.1 |
26.9 |
| 6 |
60 |
63.8 |
79.3 |
28.2 |
| 7 |
75 |
70.0 |
91.6 |
32.0 |
| 8 |
90 |
79.7 |
100.4 |
34.2 |
| 9 |
105 |
91.6 |
100.7 |
34.5 |
Table 6: Dissolution Studies of SV Complexes (SBK1, SBK2 & Pure drug) in Phosphate Buffer (pH=6.8).
| S.No. |
Time (HR) |
Percent drug release (%) |
| SBPVK1 |
SBPVK2 |
Pure Drug |
| 1 |
5 |
29.1 |
27.6 |
11.8 |
| 2 |
10 |
3607 |
35.5 |
12.0 |
| 3 |
15 |
44.8 |
42.2 |
16.1 |
| 4 |
30 |
51.8 |
51.4 |
22.8 |
| 5 |
45 |
59.1 |
59.6 |
26.9 |
| 6 |
60 |
67.3 |
68.2 |
28.2 |
| 7 |
75 |
77.1 |
73.4 |
32.0 |
| 8 |
90 |
77.5 |
79.7 |
34.2 |
| 9 |
105 |
87.6 |
86.2 |
34.5 |
| 10 |
120 |
90.1 |
91.0 |
39.9 |
Table 7: Dissolution Studies of SV Complexes (SBPEK1, SBPEK2 & Pure drug) in Phosphate Buffer (pH=6.8).
| S.No. |
Time (HR) |
Percent drug release (%) |
| SBHPK1 |
SBHPK2 |
Pure Drug |
| 1 |
5 |
28.5 |
29.1 |
11.8 |
| 2 |
10 |
37.9 |
41.5 |
12.0 |
| 3 |
15 |
48.9 |
50.1 |
16.1 |
| 4 |
30 |
51.7 |
53.9 |
22.8 |
| 5 |
45 |
57.4 |
63.2 |
26.9 |
| 6 |
60 |
65.6 |
72.7 |
28.2 |
| 7 |
75 |
70.0 |
81.3 |
32.0 |
| 8 |
90 |
78.0 |
85.2 |
34.2 |
| 9 |
105 |
86.0 |
93.8 |
34.5 |
| 10 |
120 |
95.8 |
98.1 |
39.9 |
Table 8: Dissolution Studies of SV Complexes (SBHPK1, SBHPK2 & Pure drug) in Phosphate Buffer (pH=6.8).
Inclusion complexes of SV prepared with β-CD by kneading method exhibited release of 80.9 to 88.0% of SV from SBK1, SBK2 in 120 minutes, using phosphate buffer (pH=6.8) as dissolution medium. Inclusion complexes of SV prepared with β-CD along with hydrophilic polymers showed release 91.6%, 100%, 90.1%, 91.0%, 95.8%, 98.1% in 120 minutes for SBPVK1, SBPVK2, SBPEK1, SBPEK2, SBHPK1, SBHPK2. In 120 minutes using phosphate buffer as a dissolution media respectively. Thus, the dissolution study suggests that complexes prepared by kneading method exhibited a faster dissolution when compared to pure drug dissolution data.
The dissolution data were evaluated based on dissolution efficiency parameter at 30 minutes in phosphate buffer and results are given in Table 9. The dissolution efficiency values were compared to 30 min for pure drug and for the formulations SBK1, SBK2, SBPVK1, SBPVK2, SBPEK1, SBPEK2, SBHPK1, SBHPK2 prepared by kneading method the values are found to be 29.2, 24.0, 39.6, 42.8, 38.8, 37.4, 40.3, 41.9 whereas pure drug is having only 15.08 as dissolution efficiency value.
| S.No. |
Formulation code |
Dissolution efficiency at 30 min (DE30%) |
| 1 |
Pure drugs (SV) |
15.08 |
| Kneading method |
| 1 |
SBK1 |
29.25 |
| 2 |
SBK2 |
24.07 |
| 3 |
SBPVK1 |
39.67 |
| 4 |
SBPVK2 |
42.88 |
| 5 |
SBPEK1 |
38.89 |
| 6 |
SBPEK2 |
37.45 |
| 7 |
SBHPK1 |
40.39 |
| 8 |
SBHPK2 |
41.97 |
Table 9: Dissolution efficiency (DE 30%).
A marked improvement in dissolution rates of SV were observed with SBK1 and SBK2 prepared by kneading method. Also, highest dissolution rates were observed with SBPVK1, SBPVK2 for kneading method. Thus, inclusion of hydrophilic polymers in CD complexes markedly enhanced complexation and yielded several times higher dissolution rates than those of SV and its complexes with β-CD alone [16]. The dissolution 30 and T70 values of pure drug and its complexes with β-CD and along with polymers are given in Table 10. Least dissolution T30 and T70 values are found for SBPVK2 (T30=5 min and T70=48 min).
| Code |
T30 (in min) |
T70 (in min) |
| Pure drug |
65 |
- |
| SBK1 |
11 |
103 |
| SBK2 |
9 |
88 |
| SBPVK1 |
5 |
75 |
| SBPVK2 |
5 |
48 |
| SBPEK1 |
5 |
75 |
| SBPEK2 |
6 |
66 |
| SBHPK1 |
6 |
75 |
| SBHPK2 |
6 |
56 |
Table 10: Dissolution T30 and T70 values of pure Simvastatin and SV complexes with β-CD and with hydrophilic polymers.
Conclusions
From the present study, the following conclusions can be drawn:
1. Cyclodextrins like β-CD and hydrophilic polymers can be used to prepare inclusion complexes of SV with improved solubility of drug. Phase solubility studies of SV with β-CD and hydrophilic polymers like PVP, PEG, HPMC illustrate the solubility enhancement of β-CD and hydrophilic polymers.
2. FTIR and DSC studies indicated the formation of true complexes of SV: β-CD in 1:1 ratio. Also, the formation of 1:1 inclusion complexation of SV: β-CD: hydrophilic polymers are confirmed by FTIR and DSC studies.
3. The dissolution of SV from inclusion complexes prepared by kneading method in case of β-CD (1:1M) and (1:2M) were found to be higher than the pure drug. Other SV-β-CD Complexes prepared along with hydrophilic polymers showed higher dissolution than SV-β-CD Complex. The order of hydrophilic polymers enhancing dissolution rate of β-CD complexes was PVP>HPMC>PEG.
4. The dissolution efficiency (DE) was higher for SV: β-CD inclusion complexes when compared to pure drug. SV: β-CD: POLYMER inclusion complexes are having still higher dissolution efficiencies. Among the three polymers used PVP is having highest dissolution efficiency in 1:2 proportion.
5. The inclusion complexes of SV prepared by kneading method are subjected to short term accelerated stability studies has shown no appreciable change in its physical appearance, drug content value [17]. Hence from the above results it can be concluded that β-CD and hydrophilic polymers can be used to formulate fast releasing formulations of SV.
17935
References
- Charumanee S, Okonogi S, Sirithunyalug J, Wolschann P, Viernstein H (2016) Effect of Cyclodextrin Types and Co-Solvent on Solubility of a Poorly Water Soluble Drug. Sci Pharm 84:694-704.
- Onnainty R, Schenfeld EM, Petiti JP, Longhi MR, Torres A (2016) Permeability profiles and intestinal toxicity assessment of hydrochlorothiazide and its inclusion complex with ß-cyclodextrin loaded into chitosan nanoparticles. Mol Pharm (In press).
- Alakhali KM (2013) Method Validation for Analysis of Simvastatin in Human Plasma Using Liquid Chromatography Tandem Mass Spectrometry (LC-MS-MS). J ClinDiagn Res 7:2739-2743.
- Wang D, Qin F, Chen L, Hao Y, Zhang Y (2008) Determination of simvastatin in human plasma using ultra-performance liquid chromatography-tandem mass spectrometry. Se Pu 26:327-330.
- Ito T, Saito M, Uchino T, Senna M, Iafisco M (2012) Preparation of injectable auto-forming alginate gel containing simvastatin with amorphous calcium phosphate as a controlled release medium and their therapeutic effect in osteoporosis model rat. J Mater Sci Mater Med 23:1291-1297.
- Ghareeb MM, Abdulrasool AA, Hussein AA, Noordin MI (2009) Kneading Technique for Preparation of Binary Solid Dispersion of Meloxicam with Poloxamer 188. AAPS PharmSciTech 10: 1206-1215.
- Jun SW, Kim MS, Kim JS, Park HJ, Lee S (2007) Preparation and characterization of simvastatin/hydroxypropyl-β-cyclodextrin inclusion complex using supercritical antisolvent (SAS) process. European Journal of Pharmaceutics and Biopharmaceutics 66: 413-421.
- Shiralashetti SS, Patil AS, Patil JS (2010) Influence of method of preparation on solubility, physicochemical properties and invitro release profile of simvastatincyclodextrin inclusion complexes: a comparative study. Int J Curr Pharm Res 2: 7-12.
- Patil JS, Shiralashetti SS (2010) Influence of method of physicochemical properties and invitro release profile of Nimodipinecyclodextrin inclusion complexes: a comparative study. Int J Phar Pharmaceutical Sci 2: 71-81.
- Nortia T, Kontas E (1973) An improved potassium bromide pellet method for the measurement of i.r. spectra of hygroscopic compounds. Spectrochimica Acta Part A: Molecular Spectroscopy 29: 1493-1495.
- Ledei I (2015) Selection of solid-state excipients for simvastatin dosage forms through thermal and nonthermal techniques. J Therm Anal Calorim 121: 1093.
- Yamashita T, Takatsuka K (2007) Hydrogen-bond assisted enormous broadening of infrared spectra of phenol-water cationic cluster: an ab initio mixed quantum-classical study. J Chem Phys 126:074304.
- Avalle P, Pygall SR, Gower N, Midwinter A (2011) The use of in situ near infrared spectroscopy to provide mechanistic insights into gel layer development in HPMC hydrophilic matrices. Eur J Pharm Sci 43: 400-408.
- Gill P (2010) Differential Scanning Calorimetry Techniques: Applications in Biology and Nanoscience. J Biomol Tech 21: 167-193.
- Grochowska-Niedworok E, Kardas M, Muc-Wierzgo M, Skóra A (2014) Changes of Physical Properties of Rapeseed Oil with Added Vitamin A and E Studied Using the Differential Scanning Calorimetry Method (Dsc). J Food Process Technol 5: 405.
- Dhall M, Madan AK (2015) Studies on urea co-inclusion complexes of simvastatin for improvement of pharmaceutical characteristics. J Incl Phenom MacrocyclChem 81: 105.
- Hill SW, Varker AS, Karlage K, Myrdal PB (2009) Analysis of drug content and weight uniformity for half-tablets of 6 commonly split medications. J Manag Care Pharm 15:253-261.





















