Özyılmaz Ö* and Pirimoglu ZM
University of Health Sciences, Dr. Lütfi Kırdar Kartal Training and Research Hospital, Istanbul, Turkey
- *Corresponding Author:
- Özlem Özyılmaz
University of Health Sciences
Dr. Lütfi Kırdar Kartal Training and Research Hospital
Istanbul, Turkey
Tel: 00905322044404
E-mail: drozlemsarici81@gmail.com
Received date: August 27, 2018; Accepted date: September 19, 2018; Published date: September 24, 2018
Citation: Özyılmaz Ö, Pirimoglu ZM (2018) An Approach to Managing Pelvic Organ Prolapse in Gynecologic Cancer Survivors: A Case Report. Arch Med Vol No:10 Iss No:5:4 doi: 10.21767/1989-5216.1000285
Copyright: © 2018 Özyılmaz Ö, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords:
Gynecologic oncology; Pelvic organ prolapse; vaginal hysterectomy
Introduction
POP is a common health problem in the women both in premenopausal and postmenopausal periods. Prolapse of intraabdominal organs from vagina can be detected at any level of pelvic floor. Gynecologic oncologic diseases can coexist with POP such as endometrial cancers, postmenopausal endometrial polyps, endometrial hyperplasias, myomas, adnexal masses and cervical precancerous conditions [1]. This present case provides an approach to managing mild POP coexisting with postmenopausal endometrial polyp in which endometrial cancer was not excluded clearly, two critical located myomas causing postmenopausal bleeding, and performing BSO vaginally although anatomical and technical difficulties.
Case
A 62-year-old woman in menopausal period with active sex life, gravida 5, para 5 was admitted to the hospital with bleeding for six months and bulging mass from vagina for two years. In her family history there was a strong family risk because of her mother related to ovarian cancer. She has not undergone any operation in the past, except tissue samplings from lung masses which were reported as benign. Her medical history has been including hypertension. Transvaginal ultrasound demonstrated the uterus 90 × 70 × 60 mm in size with two myomas fundus located 50 mm in diameter and cervix located 40 mm in diameter, and a single endometrial polyp 25 mm in diameter was detected at the same time. Endometrial tissue sampling was performed before the operation which was reported as benign endocervical tissues. As a result of pathological evaluation before the operation, endometrium cancer has not been excluded yet and made confusion because of absence of endometrial tissue in the report which was given by pathologists after probe curettage. The single polyp 25 mm in diameter still has been detected by transvaginal ultrasound after probe curettage, so it can be defined as insufficient material due to the cervical stenosis in the postmenopausal patient. All tumor markers were negative. The patient diagnosed with Stage 2 POP according to the Pelvic Organ Quantification System (POP-Q) including anterior vaginal wall prolapse with paravaginal defect, posterior vaginal wall prolapse with rectocele and desensus uteri with elongatio colli. We managed this case by vaginal way including hysterectomy, prophylactic BSO, colposuspension and vaginal remodeling with no posterior repair in addition to protect from postoperative de novo dyspareunia. The uterus was measured 160 × 80 × 70 mm in size, myomas were measured 50 mm and 40 mm in diameter and cervix was measured 100 mm in lenght just before the frozen section evaluation. The size of uterus can be measured in different values when compared with preoperative examination by ultrasound and postoperative examination by surgeons and pathologists related to the position of the patient and situation of intraabdominal organs. The frozen section was reported as benign. Postoperative examination was performed in the third month after the operation. Objective cure defined as Stage 0 according to POPQ and subjective cure defined as negative response to all questions on Pelvic Organ Prolapse Distress Inventory 6 (POPDI-6). Three different secondary acquisitions were obtained such as no complication, no symptom and high quality of sex life according to Female Sexual Function Index (FSFI).
Discussion
Uterine malignancy coexists with POP is a rare health problem although isolated POP is common, a range of 0.2%-1.2% risk of detecting uterine malignancy after POP operations [1]. Can it be related to insufficient examination of pelvic floor before cancer surgery? Is there a classic idea that suggests performing cancer surgery by abdominally because of anatomical and technical difficulties in cases with mild or severe prolapse of pelvic floor? This present case is asking these questions to the gynecologic oncology as a title “why don’t we give us a chance for performing nearly all surgeries including oncology by vaginally?” The patients diagnosed with POP, in cases who complain of postmenopausal bleeding probe curettage should be performed for excluding endometrial cancer or precancerous conditions which coexist with POP [2]. POP can be described as bulging of intraabdominal organs to the vaginal walls at any level of pelvic floor, which effects to quality of life including quality of sex life which makes serious social problem. Bulging of urethra described as urethrocele, bulging of bladder described as cystocele, bulging of rectum described as rectocele, bulging of uterus described as uterine prolapse. Prolapse of pelvic floor staged according to POP-Q including mild and severe defects. The patients with POP complain of bulging mass, urinary symptoms including incontinence which makes another serious social problem like decreased quality of sex life, bleeding, chronic pelvic pain, constipation, sexual dysfunction. POP has been increasing in the past two decades and 20% of women in the United States were affected by pelvic floor disorders , and accounting for over 200.000 surgeries on pelvic floor performed in every year [3-5]. Cancer surgery can be performed by laparotomy, laparoscopy, laparoscopy assisted vaginally, robotic or vaginally. Vaginal hysterectomy is effective and reliable surgical treatment in patients with POP. On the other hand, abdominal surgery provides surgical staging exactly, which is one of the main determinant factor of treatment and prognosis of endometrial cancer. However, patients who diagnosed with endometrial cancer, especially with Stage 1 and Stage 2A diseases, coexist with prolapse of pelvic floor, can be operated with vaginal hysterectomy with BSO including peritoneal cytologic sampling by vaginal way with vaginal remodeling cosmetically and functionally [1,2]. There is no consensus related to the surgery of advanced stage endometrium cancer in patients with POP, this subject is still controversial [6]. Endometrial polyps can coexist with uterine malignancy; especially in postmenopausal period causing bleeding can be detected with endometrial cancers. Benign myomas can turn into leiomyosarcomas especially in postmenopausal period with complain of bleeding, diagnosis of malignany in myoma can be certain after removing of myoma or uterus. Vaginal hysterectomy can be performed even in bening myomas causing symptom in postmenopausal period. Vaginal way is the best way for all surgeries [7-10]. Only a good plan before the operation, and to be good at pelvic floor anatomy is required, then it is possible to do vaginal hysterectomy, BSO, colposuspension and vaginal remodeling even in complicated cases with severe or mild POP (Figures 1-5). Except BRCA Mutations there is no clear predictor factor of ovarian cancer, prophylactic BSO can be added if there is strong family history independently of BRCA Mutations even in mild prolapse cases [11,12].
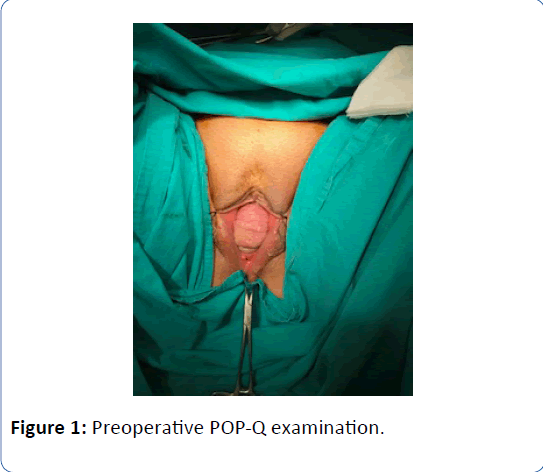
Figure 1: Preoperative POP-Q examination.
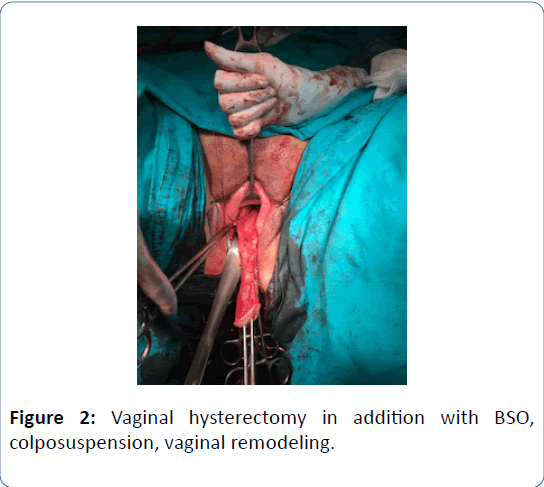
Figure 2: Vaginal hysterectomy in addition with BSO, colposuspension, vaginal remodeling.
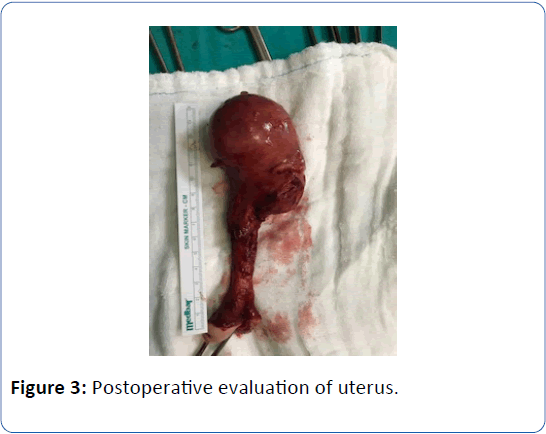
Figure 3: Postoperative evaluation of uterus.
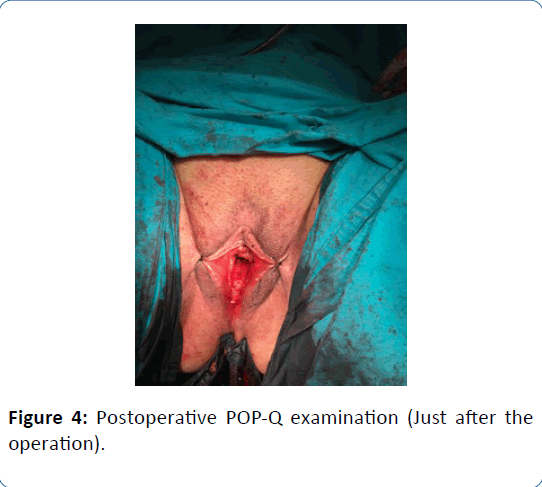
Figure 4: Postoperative POP-Q examination (Just after the operation).
Figure 5: Postoperative POP-Q examination (Three months after the operation).
Conclusion
In fact, pelvic floor disorders (PFDs) are prevalent in gynecologic oncology, if we add literal POP-Q examination before the cancer surgery in routine, this is an important area and future studies are urgently needed.
23390
References
- McPencow AM, Erekson EA, Guess MK, Martin DK, Patel DA, et al. (2013) Cost-effectiveness of endometrial evaluation prior to morcellation in surgical procedures for prolapse. Am J Obstet Gynecol 209: 22e1-22e9.
- Cliby WA, Dodson MK, Podratz KC (1995) Uterine prolapse complicated by endometrial cancer. Am J Obstet Gynecol 172: 1675-1683.
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson FM (2014) Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol 123: 1201-1206.
- Boyles SH, Weber AM, Meyn L (2003) Procedures for pelvic organ prolapse in the United States, 1979-1997. Am J Obstet Gynecol 188: 108-115.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89: 501-506.
- Chan JK, Lin YG, Monk BJ, Tewari K, Bloss JD, et al. (2001) Vaginal hysterectomy as primary treatment of endometrial cancer in medically compromised women. Obstet Gynecol 97: 707-711.
- Sasaki LMP, Andrade KRC, Figueiredo ACMG, Wanderley MDS, Pereira MG (2018) Factors associated with malignancy in hysteroscopically resected endometrial polyps: A systematic review and meta-analysis. J Minim Invasive Gynecol 25: 777-785.
- Pennant S, Manek S, Kehoe S (2008) Endometrial atypical hyperplasia and subsequent diagnosis of andometrial cancer: A retrospective audit and literature review. J Obstet Gynaecol 28: 632-633.
- Modaffari P, D'alonzo M, Garbagnati M, Pecchio S, Menato G (2018) Unexpected uterine leiomyosarcoma in a woman with multiple myomas treated with ulipristal acetate: case report and literature review. Gynecol Endocrinol 34: 192-194.
- Nemeth G (2014) Indications and methods of hysterectomy. Orv Hetil 155: 1152-1157.
- Braverman M, Kamisan Atan I, Turel F, Friedman T, Dietz HP (2018) Does Patient Posture Affect the Ultrasound Evaluation of Pelvic Organ Prolapse? J Ultrasound Med In Press.
- Straub MM, Podoll M, David SN, Wiesner GL, Desouki M (2018) Subsequent breast and high grade serous carcinomas after risk-reducing salpingo-oophorectomy in BRCA mutation carriers and patients with history of breast cancer. Ann Diagn Pathol 36: 28-30.











