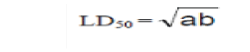Key words
|
| |
| Antidiabetic, Diabetes, Panicum maximum, hypoglycaemic |
| |
INTRODUCTION
|
| |
| Panicum maximum.Jacq ( poacace) is a perennial, tuft grass with a short, creeping rhizome regarded as the most valuable fodder plant and extensively used to make hay. The stem of this robust grass can reach a height of up to 2m. the leaf sheath are found at the bases of the stems and are covered in fine hairs. It is widely distributed in Africa where it originates and almost all tropical parts of the world [1]. The plant (leaf) is use traditionally by the Ibibios of Akwa Ibom State, Nigeria in the treatment of various ailments such as rheumatism pain, inflammation and diabetes. Ethnopharmacological and scientific reports on this plant is scarce. In this study , we investigated the effect of ethanolic leaf extract of Panicum maximum on alloxan induced diabetic rats during single and repeated administrations to observe acute and chronic effects of the extract on blood glucose levels of the diabetic rats in bids to confirm its ethnobotanical uses in the management of diabetes. |
| |
MATERIALS AND METHODS
|
| |
|
Plant materials
|
| |
| Fresh leaves of Panicum maximum were collected in September, 2008 at a farmland in University of Uyo, Uyo, Nigeria. The plant was identified and authenticated by Dr. Margaret Bassey, a taxonomist in the Department of Botany, University of Uyo, Uyo. Nigeria. Herbarium specimen (FPH. 321) was deposited at Faculty of Pharmacy Herbarium. The fresh leaves (2kg) of the plant were dried on laboratory table for 2 weeks and reduced to powder. The powder 100g was macerated in 95% ethanol (300ml) for 72 hours. The liquid filtrate obtained was concentrated in vacuo at 40oC. The yield was 4.83% w/w. The extract was stored in a refrigerator at 4oC until used for experiment reported in this study. |
| |
|
Phytochemical Screening
|
| |
| Phytochemical screening of the crude leaf extract was carried out employing standard procedures and tests [2,3], to reveal the presence of chemical constituents such as alkaloids, flavonoids, tannins, terpenes, saponins, anthraquinones, reducing sugars, cardiac glycosides among others. |
| |
|
Animals
|
| |
| Albino wistar rats (125 – 168g) and albino Swiss mice (20-25g) of either sex were obtained from the University of Uyo animal house. They were maintained on standard animal pellets and water ad libitum. The animal were handled in accordance with the NIH guidelines (NIH publication no. 80 – 23; revised 1978). Permission and approval for animal studies were obtained from the College of Health Sciences Animal Ethics committee, University of Uyo. |
| |
|
Determination of median lethal dose (LD50)
|
| |
| The median lethal dose (LD50) of the extract was estimated using albino mice by intraperitoneal (i.p) route using the method of Lorke [4]. This involved the administration of different doses of the extract to groups of three mice each .The animals were observed for manifestation of physical signs of toxicity such as writhing, decreased motor activity, decreased body/limb tone, decreased respiration and death. The number of deaths in each group within 24 hours was recorded. The LD50 was calculated as geometrical means of the maximum dose producing 0% (a) and the minimum dose producing 100% mortality (b). |
| |
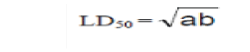 |
| |
|
Evaluation of antidiabetic activity of the extract
|
| |
|
Induction of diabetes
|
| |
| The animals (male rats) were fasted overnight and diabetes was induced by a single intraperitoneal injection of a freshly prepared solution of Alloxan monohydrate (150mg/kg) in ice cold 0.9% NaCl saline solution. The animals were given 5% dextrose solution to drink immediately after induction to overcome the drug induced hypoglycemia. Control rats were injected with normal saline alone. 72 hours later rats with blood glucose level (BGL) above 200mg/dl were considered diabetic and selected for the experiment. |
| |
| The animals were divided into five groups of 6 rats each and treated as follows: |
| |
| Group I: Diabetic rats administered P. maximum extract (47mg/kg/day) acqeous solution orally for 14 days. |
| |
| Group II: Diabetic rats given P. maximum extract (95mg/kg/day) in acqeous solution orally for 14 days. |
| |
| Group III: Diabetic rats administered orally with P. maximum extract (190 mg/kg/day) acqeous solution orally for 14 days. |
| |
| Group IV: Diabetic rats given Glibenclamide (10mg/kg/day) for 14 days orally. |
| |
| Group V: Diabetic control rats. |
| |
| The body weight gain and fasting BGL of all the rats were recorded at regular intervals during the experimental period. For acute study, the BGL was monitored after 1, 3, 5 and 7h of administration of a single dose of the extract and at the end of 1, 3, 5, 7 and 14 days for prolonged treatments. The BGL was monitored in the diabetic rats by tail tipping method. The blood was dropped on the dextrostix reagent pad. This was inserted into microprocessor digital blood glucometer and the readings were noted [5]. |
| |
|
Statistical analysis and data evaluation
|
| |
| Data obtained from this work were analyzed statistically using Students’ t- test and ANOVA (One- or Two - way) followed by a post test (Tukey - kramer multiple comparison test ). Differences between means will be considered significant at 1% and 5% level of significance i.e P ≤ 0.01and 0.05. |
| |
RESULTS
|
| |
|
Phytochemical screening
|
| |
| Phytochemical screening of the ethanolic leaf extract of Panicum maximum revealed that the leaf extract contains terpenes, tannins, phlobatanins, cardiac glycosides and anthraquinones. Flavonoids, saponins and alkaloids were however absent. |
| |
|
Acute toxicity
|
| |
| The extract (100-1000mg/kg) produced physical signs of toxicity such as writhing, gasping, palpitation, decreased respiratory rate, body limb and death. These effects were dose- dependent. All the mice treated with 500mg/kg and above died, while the mice treated with 450mg/kg of the extract survived. The intraperitoneal medium lethal dose (LD50) of the extract in mice was calculated (as the geometrical mean of the two doses i.e 450 and 500mg/kg ) to be 474.34mg/kg. |
| |
|
Antidiabetic activity
|
| |
| A dose-dependent reduction in BGL was observed in alloxan-induced diabetic rats treated with ethanolic leaf extract of P. maximum. After a single dose of the extract on the alloxan-induced diabetic rats, there was a significant (P<0.01 – 0.001) reduction in BGL of the diabetic rats within the period of acute study compared to control with the maximum effect at 7h with the highest dose of the extract (190.0 mg/kg). However, the effect was less than that of the standard drug, glibenclamide (Table 1). |
| |
| During prolonged study (14 days), the extract produced a sustained significant (P<0.001) reduction in BGL of the diabetic rats compared to control(Table 2). The effect of the highest dose of the extract was comparable to that of standard drug, glibenclamide, 10mg/kg on day 15. |
| |
Discussion
|
| |
| Evaluation of antidiabetic activity of P.maximum leaf extract was carried out in alloxan induced diabetic rats. The extract which showed moderate toxicity was observed to demonstrate significant antidiabetic activity in alloxan diabetic rats. Some phytochemical compounds such as polysaccharides [6], terpenes and tannins [7] and steroids [8], have been implicated in the antidiabetic activities of plants. Phytochemical studies of the leaf extract revealed the presence of terpenes, tannins , phlobatannins and anthraquinones. These constituents may in part be responsible for the observed significant activity of this extract either singly or in synergy with one another. Sulphonylureas cause hypoglycemia by stimulating insulin secretion from the pancreas and these compounds are potent in mild alloxan induced diabetes and inactive in intense alloxan induced diabetes whereby nearly all â –cells have been destroyed [9]. The observed reduction in BGL of the diabetic rats by glibenclamide in this study portrays an insevere state of diabetes. In this study, continous treatment with the leaf extract of P. maximum for a period of 2 weeks caused significant decrease in BGL of treated rats compared to untreated diabetic rats. Diabetes is characterised by a severe loss in body weight due to loss or degradation of structural proteins [10]. This condition was alleviated by the treatment of the diabetic rats with leaf extract of P. maximum as the treated rats were healthy and agile at the end of the study. Some plants’ extracts are reported to exert hypoglycemic action by potentiating the insulin effect, either by increasing the pancreatic secretion of insulin from the cells of islets of langerhans or its release from bound insulin [11]. While others act through extra pancreatic mechanisms by inhibition of hepatic glucose production [12] or corrections of insulin resistance [13]. This extract could have utilized one of the above mechanism in exerting its antidiabetic effect. |
| |
Conclusion
|
| |
| The results of this study show that ethanolic leaf extract of P. maximum possessed antidiabetic properties as shown in its ability to reduce blood glucose level of alloxan induced diabetic rats. This confirmation justifies its use in ethnomedical medicine for the treatment of diabetes. |
| |
Acknowledgment
|
| |
| The authors are grateful to Mr. Nsikan Malachy of Pharmacology and Toxicology Dept, University of Uyo, Uyo, Nigeria for his technical assistance. |
| |
Conflict of Interest
|
| |
| NONE |
| |
Source of Support
|
| |
| NIL |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |