Ramadoss Karthikeyan1*, Sai Koushik O1, Srinivasa Babu P1 and Jayendra Chunduru2
1Vignan Pharmacy College, Vadlamudi, Guntur (Dist.)-522 213, Andhra Pradesh, India
2Department of Chemistry, Texas A&M University, Commerce, USA
- Corresponding Author:
- Ramadoss Karthikeyan
Assistant professor, Vignan Pharmacy College, Vadlamudi, Guntur (Dist.) -522 213. Andhra Pradesh, India
Tel: 91-9966847127
E-mail: rkcognosy@gmail.com
Received date: March 30, 2016; Accepted date: May 09, 2016; Published date: May 13, 2016
Citation: Karthikeyan R, Koushik OS, Babu PS, et al. Anti-Inflammatory Activity of Ethanolic Extract of Flowers Hymenocallis Littoralis (Jacq.) Salisb. By Hrbc Membrane Stabilization Method. Transl Biomed. 2016, 7:2.
Keywords
Hymenocallis littoralis (Jacq.); Salisb; Ethanolic extract; HRBC membrane stabilization method; Phago burst assay; Anti-inflammatory activity
Introduction
Hymenocallis littoralis (Jacq.) Salisb (Amryllidaceae) is native to coastal regions of Central America, southern Mexico and also along the west coast of Florida [1]. The only part of the plant used for wound healing is the bulb. Mixture of crushed bulbs and oil applied on face to treat blemishes and freckles. Throughout the history of H. littoralis, from its bulb several alkaloids had been discovered. Lycorine the first alkaloid was proven to have antiviral, antineoplastic and cytotoxic properties. H. littoralis is pharmacologically well known plant (Figure 1). Besides H. littoralis, other Hymenocallis species also have been used widely as traditional remedies. The bulbs are commonly employed as an ornamental plant and used in cosmetic preparations also [2]. The genus was first phytochemically studied in 1920 and resulted in isolation of lycorine [3]. Since 1920, there are several alkaloids that have been discovered from H. littoralis bulbs; such as littoraline, trazettine, o-methyllycorinine, pretazettine, macronine, lycorine.homolycorine, demethylmaritidine, haemanthamine, vittatine, and 5,6-dihydrobicolorine [4], hippeastrine, 11- hydroxyvittatine and two flavonoids quercetin3’-O-glucoside and rutin were isolated. In addition, Abou-Donia and coworkers 2008 identified 26 known volatile constituents from H. littoralis flowers. The primarily isolated lycorine alkaloid from H. littoralis was proven to have antineoplastic, cytotoxicity and antiviral properties [5]. Pancratistatin from H. littoralis has been proven to be effective against 60 human cancer cell lines including melanoma, brain, colon, lung and renal cancers by U.S. National Cancer Institute’s panel [6,7]. In healthcare practices the traditional uses of medicinal plants are providing clues to new areas of research and hence its importance is now well recognized [8,9]. In this study the antiinflammatory activity was attempted to prove the efficacy of flower of H. littoralis.

Figure 1: Hymenocallis littoralis (Jacq.) Salisb. Flower.
Materials and Methods
Collection, authentification extraction of plant material
The plant material was collected from the plant H. littoralis (Jacq.) Salisb. Which are collected during the month of December at Vadlamudi, Guntur (Dist.) of Andhra Pradesh. Then it was authentified by Dr. P. Satyanarayana Raju, professor, Department of Botany and Microbiology, Acharya Nagarjuna University, Nagarjuna nagar, Guntur. The flowers were extracted with soxhlet apparatus using ethanol as solvent (yield 3.7%). The samples were prepared and used for antiinflammatory activity.
Chemicals and instruments
Sodium chloride, Sodium citrate, Dextrose, Citric acid and Buffer tablet were purchased from S.D fine chemicals, Bangalore. Ethanol was procured from national scientific with the brand of qualigen. Reference standard Diclofenac sodium obtained as a gift sample from Symed Pharm. Pvt. Ltd, Hyderabad. Systronics 220 (Double beam) spectrophotometer was used for the estimation of anti-inflammatory activity.
Preliminary phytochemical screening
Preliminary phytochemical screening was performed by using standard protocol [10].
Physicochemical Constants
Determination of Physicochemical constants is performed as per the standard protocol followed in the Ayurvedic Pharmacopoeia [11].
Preparation of plant extract
Ethanol extract: This extract was prepared by using cold maceration method. About 100 g of fresh flowers were minced and were extracted with 300 ml ethanol solvent at room temperature until the color of the plant part becomes pale. The extracts obtained were filtered separately using Whatmann No. 1 filter paper. This was repeated for 2-3 times and similarly pooled together and the filtrate was collected and concentrated at low temperatures on heating mantle. This concentrated ethanol extract is used for further studies. The residual extracts were weighed and stored in desiccator in China dish. Percent extractive values were calculated by the following formula:
Percent extracts = Weight of dried extract/Weight of flower material
Anti-inflammatory activity by HRBC membrane stabilization method
The anti-inflammatory activity of flower extract of H. littoralis was determined by HRBC membrane stabilization method. Blood was collected from healthy volunteers. The collected blood was mixed with equal volume of (2% dextrose, 0.8% sodium citrate, 0.05% citric acid and 0.42% sodium chloride in water). The blood was centrifuged at 300 rpm and packed cells were washed with isosaline (0.85%, pH 7.2) and 10% v/v suspension was made with isosaline. The assay mixture contained the drug. 1 ml phosphate buffer (0.15 M, pH 7.4), 2 ml of hyposaline (0.36%)% 0.5 ml of HRBC suspension. Diclofenac was used as the reference drug. Instead of hyposaline, 2 ml of distilled water was used as control. All the assay mixtures were collected at 37°C for 30 minutes and centrifuged. The hemoglobin content in the supernatant solution was estimated using colorimeter at 560 nm. The percentage heamolysis was calculated by assuming the heamolysis produced in the presence of distilled water as 100%. The percentage of HRBC membrane stabilization or protection was calculated using the following formula [12].
% Protection = 100-Optical density of drug sample/Optical density of control
Anti-inflammatory activity using phagoburst assay: Two hundred micro liters of cells (Raw 264.7 macrophages) was transferred to a coverslip in a multiwell plate. The cells were incubated at 37°C, 5% CO2 overnight, to allow cells to attach. Cells were treated with 100 μL and 500 μL of plant extracts (100/500 μg/mL of ethanol) and 10 mg/mL of LPS for 24 hours. Two microliters of PMA was also added for 30 minutes. After incubation, the medium was aspirated. Cells were stained with 50 μL of 10-5 M of hydrophilic alcohol dihydro dichlorofluorescein (H2DCF-DA) and incubated for 20 minutes in the dark. Cells were stained for the second time with 50 μL of 20 μg/mL, 4′,6-diamidine-2-phenylindole (DAPI) and further incubated for 20 minutes in the dark. Staining solutions were removed and 3.7% paraformaldehyde was added to a coverslip to fix the cells. Coverslips were mounted to a microscope slide and examined under fluorescence microscope [13].
Statistical analysis
The calculation parts in these studies were calculated by EXCEL sheet. Remaining all other calculations were done with specific formulas mentioned in the respective part of the study.
Results and Discussion
Physicochemical studies revealed the presence of total ash value; 7.1%, Acid insoluble ash value; 2.4%, water soluble extractive; 6.2% and Sulphated ash value; 11.12%. The foreign matter adulterated in one gram powder was found to be 1%. The moisture content of the powdered drug was found to be 0.1%. The ethanol soluble extractive value found to be 2.7%. The values are tabulated in Table 1. Assurance of safety, quality and efficacy of medicinal plants is of utmost importance. To establish purity and identity, criteria such as physical constants, contaminants, ash content, solvent residues and moisture have to be checked. The level of cleanness and high values represents Ash value, may be during the sample collection process and due to unsuitable handing measures. Phytochemical screening of the plant is preliminary and important aspect of the process of establishing herbal medicine quality. Preliminary phytochemical analysis is helpful in determining the chemical constituents of plant materials. They are also useful in locating the source of pharmacologically active chemical compounds. Preliminary phytochemical study showed that the presence of steroid, flavonoids, cardiac glycosides, saponin, carbohydrates, tannins and phenolic compound are depicted in Table 2. Successively the crude ethanol extracts have shown potent antiinflammatory activity with 83.46% and 84.72% for 100 and 500 μg/ml, respectively. Table 3 showed the % haemolysis and % protection of heamolysis produced by hyposaline in vitro. The crude ethanol extract showed dose dependent protection of heamolysis and significant activity from the HRBC membrane. The preliminary phytochemical investigation revealed the presence of flavonoid and steroids compounds in the polar extracts of the plant. Due to the presence of these constituents, anti-inflammatory activity of the extract of H. littoralis may be observed. Flower extracts of H. littoralis have been reported to have anti-inflammatory activity by phago burst assay. The results of this showed in Table 4. High level of reactive oxygen species tends to attack macromolecules and this facilitates cells to undergo oxidative stress and inflammatory response. Antioxidant compounds scavenge the free radical molecules by donating one electron or proton to a molecule. High intensity of green colour is the indication of high level of free radical molecules which can lead cells to undergo oxidative stress and inflammation. Reactive oxygen species act as a mediator to regulate cytokines production through activation of the transcription factors, such as NF-κB. This suggests the direct link between ROS and other cytokines to initiate inflammatory response [14,15]. Ethanol extracts were observed to reduce the level of ROS formation in cells while. Ethanol extracts of H. littoralis have been reported to contain flavonoid, a constituent that has been reported as the anti-inflammatory agent of medicinal plants [16]. The possibility that active constituent present in the flower may be responsible for observed activity (Figure 2).
| ASH Values |
Units |
| Total ash value |
7.1% |
| Acid insoluble ash value (dil. HCl) |
2.4% |
| Sulphated ash value(H2SO4) |
11.12% |
| Water soluble ash value (H2O) |
6.2% |
| Moisture content |
1% |
| Foreign matter |
0.1g |
| Ethanol soluble extractive value |
2.7% |
Table 1: Different ash values of H. littoralis
| Name of the phytoconstituent |
n-Hexane |
Ethyl acetate |
Diethyl ether |
Ethanol |
| Molish’s test(Carbohydrates) |
+ |
+ |
+ |
+ |
| Test for gums |
+ |
+ |
- |
+ |
| Test for mucilage |
- |
- |
- |
+ |
| Biuret’s test (Proteins) |
- |
- |
- |
- |
| Ninhydrin test (Amino acids) |
- |
- |
- |
+ |
| Salkowski test (Steroids) |
- |
- |
- |
+ |
| Legal’s test(Cardiac glycosides) |
- |
- |
- |
+ |
| Shinoda test (Flavonoids) |
- |
- |
- |
+ |
| Dragendorff’s test (Alkaloids) |
+ |
+ |
+ |
+ |
| 5%FeCl3 solution (Tannins and phenolic compounds) |
- |
+ |
- |
+ |
| Lead acetate solution (Tannins and phenolic compounds) |
- |
+ |
- |
+ |
| Test forVitamin A |
- |
- |
- |
- |
| Test for Vitamin D |
- |
- |
- |
- |
| Test for Vitamin C |
- |
- |
- |
- |
Table 2: Preliminary phytochemical screening of flowers of H. littoralis.
| S. No. |
Name of
the drug |
Concentration
(µg/ml) |
Absorbance
(560 nm) |
% Haemolysis |
% Protection |
| 1 |
Control |
- |
2.526 |
100% |
0% |
| 2 |
Ethanolic extract |
100 |
0.418 |
16.54% |
83.46% |
| 500 |
0.386 |
15.28% |
84.72% |
| 3 |
Diclofenac sodium |
100 |
0.593 |
23.47% |
86.54% |
| 500 |
0.362 |
14.33% |
95.67% |
Table 3: Evaluation of Anti-inflammatory activity of ethanolic extract of flowers of H. littoralis by HRBC membrane stabilization method.
| Preparation |
100(µg/ml) |
500(µg/ml) |
| Ethanol extract |
45% |
68% |
| Diclofenac sodium |
61% |
87% |
Table 4: Percentage of macrophages bursted and stained during the drug treatment.
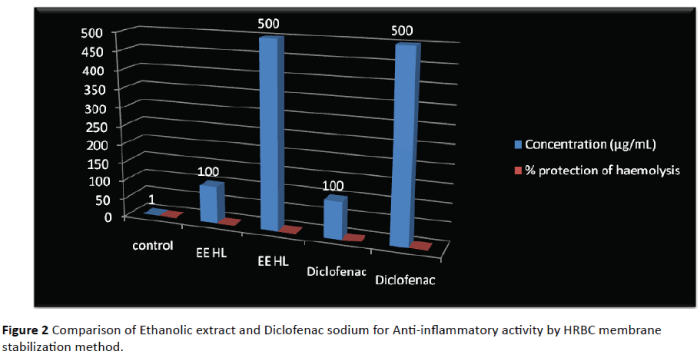
Figure 2: Comparison of Ethanolic extract and Diclofenac sodium for Anti-inflammatory activity by HRBC membrane stabilization method.
Preliminary phytochemical screening
Preliminary phytochemical screening was performed for establishing the profile of the flower extracts for its chemical composition by using standard procedures.
Conclusion
These results show that ethanol extract of H. littoralis has anti-inflammatory properties and can reduce inflammatory injury and tissue damage. The study serves as a scientific proof for the use of H. littoralis flowers in traditional medicine for treatment of inflammatory related diseases. Further studies are required to isolate anti-inflammatory compounds. The active constituents responsible for this activity are to be isolated and also for elucidating the exact mechanism of action is also required. The details of results of preliminary and detailed phytochemical analysis established in the present study will facilitate in preparation of monographs of this plant and recognizing the original drug.
Conflict of Interest
The authors are not showing any conflict of interest to publish this paper.
Acknowledgement
The authors are thankful to management of Vignan Pharmacy College, Vadlamudi for providing facilities to do this tiny research work.
9546
References
- Subramaniam S, Rosli N, Sumathy V, Vikneswaran M (2012) Screening of potential antimicrobial activity from Hymenocallislittoralis (Jacq.) Salisb. Pharmacology online SILAE 1: 93-98.
- Ingrassia L, Rouzeau S, Ribaucour F, Thomas S, Roland I, et al. (2007) The AmaryllidaceaeIsocarbostyrilNarciclasine Induces ApoptosisBy Activation of the Death Receptor and/or MitochondrialPathways in Cancer Cells But Not in Normal Fibroblasts. Neoplasia 9: 766-776.
- Chen N, Zhu H, Ji Y, Ling N, Li W, et al. (2014) Alkaloids from Beach Spider Lily (Hymenocallislittoralis) induce apoptosis of HepG-2 cells by the Fas-signaling pathway. Asian Pacific Journal of Cancer Prevention 15: 9319-9325.
- Yew CK, Balakrishnan B, Sundarasekar J, Subramaniam S (2010) The effect of cytokinins on in vitro shoot lengths and multiplication of Hymenocallis littoralis. J Med Plants Res 4: 2641-2646.
- Abou-Donia AH, Toaima SM, Hammoda HM, Shawky E, Kinoshita E, et al. (2008) Phytochemical and Biological Investigation of Hymenocallis littoralisSalisb. Chem Biodivers 5: 332-340.
- Lewis J (1998) Amaryllidaceae and Sceletium alkaloids. Natural Product Reports 15: 107-110.
- Ioset JR, Marston A, Gupta MP (2001) Hostettmann K. A methylflavan with free radical scavenging properties from Pancratiumlittorale. Fitoterapia 72: 35-39.
- Backhaus RA, Pettit III GR, Huang DS, Pettit GR, Groszek G, et al. (1992) Biosynthesis of the antineoplastic pancratistatin following tissue culture of Hymenocallis littoralis (Amaryllidaceae). Acta Hortic 306: 364-366.
- Pettit GR, Pettit III GR, Backhaus RA, Boyd MR, Meerow AW (1993) Antineoplastic agents, 256. Cell growth inhibitory isocarbostyrils from Hymenocallis. J Nat Prod 56: 1682-1687.
- Doss A (2009) Preliminary phytochemical screening of some Indian medicinal plants. Ancient science of life 29: 12-16.
- Kokate CK, Purohit AP, Gokhale SB (2005) Pharmacognosy(39thedn), NiraliPrakashan, Pune 607-611.
- SaiKoushik O, Himaja V, SrinivasaBabu P, RamadossKarthikeyan (2015) Anti - Inflammatory Activity of Flowers of Nymphaea alba by HRBC Membrane Stabilization Method Plant Biology 5: 18-20.
- Divya S, Rahul N, Tripta S, Gautam RK (2013) In vitro anti-inflammatory and antiarthritic activity of hydroalcoholic extract of Pongamiapinnata (L.) Pierre seed. International Journal of Pharma Research & Review 2: 20-25.
- Ilavarasan R, Mallika M, Venkataraman S, (2006) “Anti-inflammatory and free radical scavenging activity of Ricinuscommunis root extract,” Journal of Ethnopharmacology 103: 478-480.
- Saini AK, Goyal R, Gauttam VK, Kalia AN (2010) “Evaluation of anti-inflammatory potential of Ricinuscommunis Linn leaves extracts and its flavonoids content in Wistar rats,” Journal of Chemical and Pharmaceutical Research 2: 690-695.
- Taur DJ, Waghmare MG, Bandal RS, Patil RY (2011) “Antinociceptive activity of Ricinuscommunis L. leaves,” Asian Pacific Journal of Tropical Biomedicine 1; 139-141.







