Key words
|
| |
| Everniastrum cirrhatum (Fr.) Hale, TLC, Agar well diffusion, MIC, Pheretima pasthuma, Larval mortality |
| |
INTRODUCTION
|
| |
| Lichens are symbiotic organisms composed of a fungus (mycobiont) and an alga (photobiont). They produce characteristic secondary metabolites, lichen substances, which seldom occur in other organisms [1]. A wide range of secondary metabolites of lichens were characterized. According to their chemical structure, most lichen substances are phenolic compounds, dibenzofuranes, usnic acids, depsidones, depsones, lactones, quinines and pulvunic acid derivatives [2]. In various systems of traditional medicine worldwide, including the Indian system of medicine, these lichen species are said to effectively cure dyspepsia, bleeding piles, bronchitis, scabies, stomach disorders, and many disorders of blood and heart [3-5]. Everniastrum cirrhatum (Fr.) Hale belongs to the family Parmeliaceae is a foliose lichen in which phycobiont is a chlorolichen alga. It usually grows on the barks of trees in temperate regions [6,7]. It grows luxuriantly in tropical Himalayas, central India and higher altitudes of southern India. It is characterized by linear, laciniate lobes which are grey in color. Thallus is tapering apically, 2-6mm wide and 10cm long. Thallus is loosely attached to the substratum and pendulous in nature (Figure 1). Apothecia are laminal, margins are inflexed, cilia are black in color, and spores are large [8]. In Ayurveda, it is mentioned as astringent, resolvent, laxative, carminative and aphrodisiac. It is also useful in bleeding piles, leprosy and excessive salivation. It is used as spices in Madhya Pradesh. Whole boiled material used as vegetable in Nepal and north Sikkim [9,10]. E. cirrhatum is traditionally used as antiseptic, used to heal wound and bronchitis in Sikkim [3]. E. cirrhatum has been used as material for sacrificial fire by Gaddi tribe of Kangra valley. It is as a spice and flavoring agent for meat and vegetables by Bhaiga, Bhil, Bhilala, Gond, Korka, Muria of Madhya Pradesh. It is used as vegetables in Lepchas and Nepalese of Sakyong valley, North Sikkim. In Uttaranchal, Uttar Pradesh and Sikkim, it is commercially sold as spice [11]. The mycobiont (lichen forming fungus) of E. cirrhatum was shown to cause mycelial growth inhibition of hot pepper anthracnose pathogen, Colletortichum acutatum [1]. Ethanol extract of E. cirrhatum has shown antimycobacteral properties against Mycobacterium tuberculosis H37Rv and H37Ra strains with minimum inhibitory concentration of 500µg/ml [7]. In a study, it was reported that the whole thallus of E. cirrhatum yielded a red brown dye [12]. Although it is used as traditional medicine in Indian subcontinent, literature survey reveals that many of the bioactivities of this lichen have not yet been documented. Its ethnobotanical claim prompted us to undertake this investigation. In this study, we have investigated antimicrobial, anthelmintic and insecticidal activity of methanol extract of E. cirrhatum collected from Bhadra wildlife sanctuary, Karnataka, India. |
| |
MATERIALS AND METHODS
|
| |
|
Collection and Identification of Lichen
|
| |
| The lichen E. cirrhatum, growing on barks of trees, was collected from the Bhadra wildlife sanctuary, Karnataka, India, during August 2010. The lichen specimen was identified by morphological, anatomical, chemical tests [13]. The voucher specimen of the lichen (KU00703) was deposited in the University herbaria, Department of PG Studies and Research in Botany, Shankaraghatta-577451, Karnataka, India for future reference. |
| |
|
Detection of Secondary Metabolites by Thin Layer Chromatography (TLC)
|
| |
| The shade dried powdered lichen material was extracted with methanol, spotted on the silica plate and developed with solvent A (180 ml toluene: 60 ml 1-4, dioxine: 8 ml acetic acid) to detect secondary metabolites using standard protocols [14, 15]. |
| |
|
Preparation of Extract Using Methanol
|
| |
| The lichen was dried at room temperature under shade. After drying, the lichen material was ground to fine powder and extracted by soxhlet apparatus using methanol as solvent. The extract was filtered using Whatman filter paper no. 1 and concentrated at 40oC under reduced pressure. The condensed methanol extract was stored at 4oC until use [16]. |
| |
|
Qualitative Phytochemical Analysis of Methanol Extract
|
| |
| The extract obtained after solvent evaporation was subjected to standard tests for detection of alkaloids (Dragendorff’s reagent and Mayer’s reagent), tannins (ferric chloride test), saponins (frothing test and hemolysis test), glycosides (Salkawski test and Keller- Kiliani test), sterols (Burchard test), flavonoids (Shinoda test) and terpenoids (Salkowski test) [17,18]. |
| |
|
Antibacterial Activity of Methanol Extract of E. cirrhatum
|
| |
| The antibacterial efficacy of methanol extract of E. cirrhatum was tested against Staphylococcus aureus, Bacillus cereus, Streptococcus epidermindis, Pseudomonas aeruginosa and Escherichia coli by agar well diffusion method [19]. Briefly, 24 hours old Muller- Hinton broth cultures of test bacteria were swabbed on sterile Muller-Hinton agar plates using sterile cotton swab followed by punching wells of 6mm with the help of sterile cork borer. The standard drug (Tetracycline, 1mg/ml of sterile distilled water), methanol extract (20mg/ml of 10% DMSO) and control (10% DMSO) were added to respectively labeled wells. The plates are allowed to stand for 30 minutes and were incubated at 37oC for 24 hours in upright position and the zone of inhibition was recorded. Experiment was carried in triplicate and average values were recorded. |
| |
|
Determination of Minimum Inhibitory Concentration (MIC)
|
| |
| MIC was determined by micro-dilution method using serially diluted (2 folds) extract (0 to 20000µg/ml) in nutrient broth tubes. Briefly, 0.1ml of standardized inocula (107cfu/ml) of test bacteria was added in each tube. The tubes were incubated at 37°C for 24 hours. Two control tubes (not containing extract) were maintained for each organism. The lowest concentration (highest dilution) of the extract that produced no visible growth (no turbidity) when compared with the control tubes were regarded as MIC [20]. |
| |
|
Antifungal Activity of Methanol Extract of E. cirrhatum
|
| |
| The antifungal efficacy of methanol extract of E. cirrhatum was tested against two fungal species of Aspergillus namely A. niger and A. fumigatus. The spore suspensions of the test fungi were prepared in 0.85% sterile normal saline containing 0.01% Tween 80 detergent [21]. The antifungal activity was carried using agar well diffusion method [19]. The spore suspension of test fungi was swab inoculated aseptically on the sterile sabouraud dextrose agar plates followed by making wells of 6mm diameter using sterile cork borer. Fluconazole was used as standard antifungal antibiotic. The extract (1-20mg/ml), standard (1mg/ml) and control (10% DMSO) were added to respectively labeled wells. The plates are allowed to stand for 30 minutes and then incubated at room temperature for 72 hours. After incubation, the zone of inhibition formed around the wells was measured with the ruler. The experiment was carried in triplicates to get concordant reading. |
| |
|
nthelmintic Activity of Methanol Extract of E.cirrhatum
|
| |
| The assay was performed on adult Indian earthworm Pheretima pasthuma due to its anatomical and physiological resemblance with the intestinal roundworm parasite of human beings. Standard drug (Piperazine citrate, 1%) and different concentrations of methanol extract (0.1-20mg/ml) were prepared in 0.85% normal saline and poured into respective labeled petriplates (50 ml). A saline control was kept. Six worms of nearly equal size were introduced into each of the plates. Observations were made for the time taken to paralysis and death of individual worm. Paralysis was said to occur when the worms were not able to move even in normal saline. Death was concluded when the worms lost their motility followed with fading away of their body colors. Death was also confirmed by dipping the worms in slightly warm water. The mortality of parasite was assumed to have occurred when all signs of movement had ceased [22, 23]. |
| |
|
Insecticidal Activity of Methanol Extract of E. cirrhatum
|
| |
| The insecticidal efficacy of methanol extract of E. cirrhatum was determined against second and third instar larvae of Aedes aegypti. Briefly, twenty larvae were placed separately into beakers containing different concentrations of methanol extracts (0.1-20mg/ml). A beaker containing 10% DMSO (without extract) serves as control. The larvicidal effect of the extracts was determined by counting the number of dead larvae after 24 hours. Dead larvae were identified when they failed to move after probing with a needle in siphon or cervical region. The test was repeated thrice and the percentage of larval mortality for each concentration of extract was calculated [24]. |
| |
|
Statistical Analysis
|
| |
| All data were expressed as mean ± SD of the number of experiments (n = 3). Past software version 1.92 was used. |
| |
RESULTS AND DISCUSSION
|
| |
|
Secondary Metabolites Detected in E. cirrhatum
|
| |
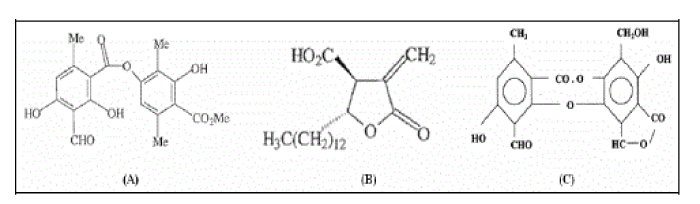
| Preliminary phytochemical analysis of methanol extract of E. cirrhatum was determined by chemical tests. Phytoconstituents namely alkaloids, saponins, tannins and terpenoids were detected in the extract (Table-1). TLC in solvent A showed the presence of Atranorin, Salazinic acid and Protolichesterinic acid in the lichen material and the structures of these are shown in Figure 2. |
| |
|
Antibacterial Activity of Methanol Extract of E. cirrhatum
|
| |
| The result of antibacterial activity of methanol extract is shown in Table 2. In this study, antibacterial activity was determined by agar well diffusion technique. Results were recorded as presence or absence of zones of inhibition around the well. The inhibitory zone around the well indicated the absence of bacterial growth and it as reported as positive and absence of zone as negative. Among bacteria, marked inhibition was observed in case of gram positive bacteria than gram negative bacteria. Methanol extract caused more inhibition of S. epidermidis than other bacteria. Least inhibition was observed in case of P. aeruginosa. Standard antibiotic caused more inhibition of test bacteria than methanol extract. No inhibition of test bacteria was observed in case of control i.e., 10% DMSO. It appears that overall the bacteria were found to be sensitive to extract. |
| |
|
Minimum Inhibitory Concentration
|
| |
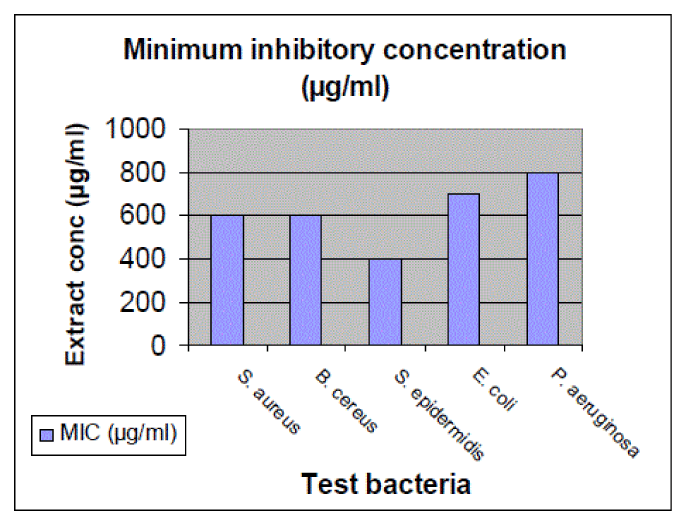
| Figure 3 shows minimum inhibitory concentration of extract (µg/ml) against test bacteria. The lowest concentration of the extract that produced no visible growth (no turbidity) when compared with the control tubes were regarded as MIC. Among bacteria, S. epidermidis has been found to get inhibited at low extract concentration (400 µg/ml) followed by S. aureus (600µg/ml), B. cereus (600µg/ml), E. coli (700µg/ml) and P. aeruginosa (800µg/ml). It is evident from the results that the Gram positive bacteria were inhibited at low extract concentration as compared to inhibition of Gram negative bacteria. |
| |
|
Antifungal Activity of Methanol Extract of E.cirrhatum
|
| |
| The result of antifungal activity of methanol extract of E. cirrhatum is shown in Table 3. Results were recorded as presence or absence of zones of inhibition around the well. The inhibitory zone around the well indicated the absence of growth and it as reported as positive and absence of zone as negative. Marked inhibition of test fungi was observed in a dose dependent manner. Overall, A. niger was shown to be more sensitive than A. fumigatus. No inhibition of test fungi was observed in case of extract concentration 2.5mg/ml and lesser and control i.e., DMSO. The inhibitory activity of extract was found lesser than that of standard. Infectious diseases caused by bacteria, fungi, viruses, and parasites remain a major threat to public health, despite tremendous progress in human medicine. Their impact is particularly great in developing countries because of the relative unavailability of medicines and the emergence of widespread drug resistance [27]. Interest in plants with antimicrobial properties has revived as a result of current problems associated with the use of antibiotics [28]. Numerous lichens were screened for antibacterial activity in the beginning of the antibiotic era in the 1950s [29]. Burkholder et al. [30] reported for the first time the presence of antibiotic substances in lichens. Several lichen metabolites were found to be active against Gram-positive organisms [31]. The antimycobacterial activity of lichen compounds was reported against nontubercular species of Mycobacterium [32]. Gupta et al. [7] investigated antimycobacterial activity of nine lichens collected from Narayan Asharam, Pitthoragarh District, Uttranchal, India. A marked inhibition of gram positive bacteria (as compared to gram negative bacteria) was observed in this study. The higher resistance of gram negative bacteria to extract has previously been documented and related to thick murein layer in their outer membrane, which prevents the entry of inhibitor substances into the cell. Gram negative bacteria contain a lipopolysaccharide layer at the exterior, followed underneath by a thin layer of peptidoglycan. This might have accounted for greater resistance of gram negative bacteria in this study [33]. In this study, the preliminary phytochemical analysis of the methanol extract of E. cirrhatum showed the presence of tannins, alkaloids, saponins and terpenoids. Antimicrobial activities of tannins, flavonoids, saponins, terpenoids and alkaloids have been documented [34-38]. The antimicrobial activity of extract could be chiefly due to the presence of these constituents. |
| |
|
Anthelmintic Activity of Methanol Extract of E.cirrhatum
|
| |
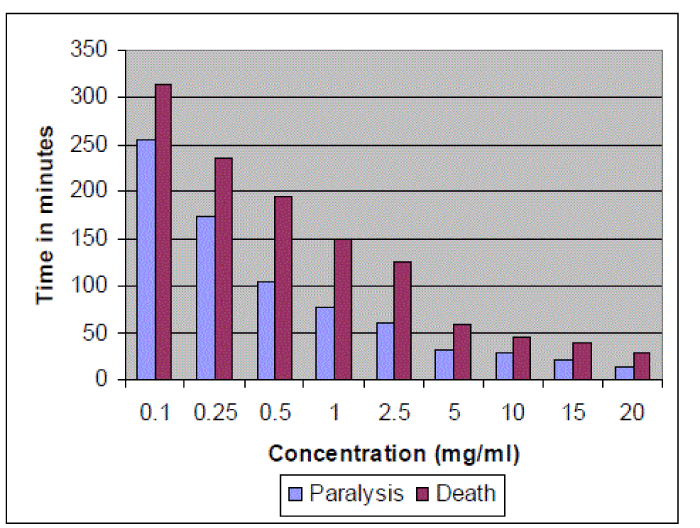
| Anthelmintic activity of methanol extract of E. cirrhatum was determined in adult Indian earthworm model due to their anatomical and physiological resemblance with intestinal round worms. Paralysis and death time were determined for different concentrations of methanol extract (0-20mg/ml). The results have revealed dose dependent efficacy of extract i.e., higher the dose, shorter was the time taken to cause paralysis and death of worms. Piperazine citrate was used as reference anthelmintic. It caused paralysis and death of worms at 38 and 64 minutes respectively. At concentration 5mg/ml and higher, the anthelmintic effect of extract was higher than that of reference drug (Figure 4). |
| |
| Helminthes are recognized as a major problem to livestock production throughout the tropics. Parasitic helminthes affect human being and animals by causing considerable hardship and stunted growth. Most diseases caused by helminthes are of a chronic and debilitating in nature [39]. During the past few decades, despite numerous advances made in understanding the mode of transmission of the parasites and the treatment of diseases, there are still no efficient products to control certain helminthes and the indiscriminate use of some drugs has generated several cases of resistance. Furthermore, it has been recognized recently that anthelmintic substances having considerable toxicity to human beings are present in foods derived from livestock, posing a serious threat to human health. Consequently, the discovery and development of new chemical substances for helminth control is greatly needed and has promoted studies of traditionally used anthelmintic plants, which are generally considered to be very important sources of bioactive substances [40]. The traditional medicines hold a great promise as a source of easily available effective anthelmintic agents to the people, particularly in developing countries, including India. It is in the context that people consume several plants or plant derived preparations to cure helminthic infections [23]. In view of this several workers have undertaken studies pertaining to testing of lichens for their proclaimed anthelmintic activity. Reported anthelmintic effect of tannins is that they can bind to free proteins in the gastrointestinal tract of host animal or glycoprotein on the cuticle of the parasite and may cause death [41, 42]. Preliminary phytochemical analysis revealed the presence of tannins in the methanol extract which could be responsible for the anthelmintic effect of extract. The result of the present study is suggestive that the extract could be used in the control of round worm infections such as Ascariasis, hookworm infections etc as the worms used in the study are in anatomical and physiological resemblance with the intestinal round worms. |
| |
|
Insecticidal Activity of Methanol Extract of E.cirrhatum
|
| |
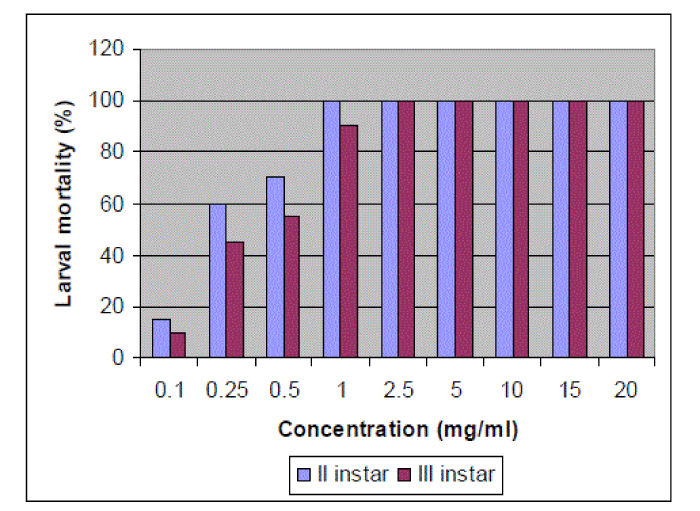
| The insecticidal efficacy of different concentrations of methanol extract of E. cirrhatum was evaluated against 2nd and 3rd instar larvae of A. aegypti. The mortality of the larvae by the extract was found to be concentration dependent. Among larvae, 2nd instar larvae were shown to be more susceptible than 3rd instar larvae. The mortality of 2nd instar larvae was recorded 100% at extract concentration of 1mg/ml and higher whereas mortality of 3rd instar larvae was 100% at extract concentration of 2.5mg/ml and higher (Figure 5). |
| |
| Mosquitoes are the most important single group of insects acting as vector for many tropical and subtropical diseases such as dengue fever, yellow fever, malaria, filariasis, Japanese encephalitis and others [43]. Killing larvae of mosquitoes is a successful way of minimizing mosquito densities in breeding grounds before they reach adult stage. It largely depends on the use of synthetic chemical insecticides. But their repeated use has caused environmental problems and widespread development of resistance. Natural products offer an alternative source of insect control agents because they contain a range of bioactive chemicals, many of which are selective and have little or no harmful effect on non target organisms and the environment. It is observed that the carbohydrates, saponins, phytosterols, phenols, flavonoids and tannins are having mosquito larvicidal activity [24]. Prenylated xanthones, tetracyclic phenols and saponins are reported to be effective in controlling mosquito A. aegypti, the vector of yellow fever [44]. The larvicidal effect of methanol extract of E. cirrhatum makes it a suitable source for bioactive compounds which can be used against insect vectors such as Aedes aegytpi etc to control arboviral diseases such as chickungunya, dengue etc. |
| |
| India, being a hotspot for lichen diversity, made considerable progress in taxonomic and floristics studies and represents about 2450 species with high degree of endemism. The Western Ghats in South India and the Eastern Himalayas with mostly tropical climate possess maximum diversity of lichens (949 and 759 spp. respectively), whereas the Western Himalayas (550 spp.) with temperate climate has luxuriant growth of lichen [13,45-47]. The availability of diverse climate, vegetation, topography and substratum in particular, makes India one of the lichen-rich countries with ecologically and physiologically interesting mycota [6]. Bhadra reserve area is 75°15'-75°5°' E and 13°25'-13°5°' N latitude. The area comprises the forests of Western Ghats and its fringes. Sanctuary being situated in the south interior Karnataka, with cool climate throughout the year and affords pleasant days during the hot months. Several studies have been carried out on bioactivities of lichens collected from Bhadra reserve area [48-51]. E. cirrhatum is one of the well known lichens used traditionally in India for various purposes. Its use has been mentioned in Ayurveda. In this study, we scientifically explored the antimicrobial, anthelmintic and insecticidal property of this lichen. This is the first report on these potentials of E. cirrhatum from Bhadra wildlife sanctuary, Karnataka. |
| |
CONCLUSION
|
| |
| The results of bioactivities of E. cirrhatum are promising. The activities of the extract may be attributed to the presence of various secondary metabolites. The lichen thus forms a potential candidate for drug discovery and development. Further studies on isolation of active principles from the extract and their bioactivities are under investigation. |
| |
ACKNOWLEDGEMENTS
|
| |
| Authors express their sincere thanks to HOD, Dept. of Microbiology and Principal, Sahyadri Science College (Autonomous), Shivamogga for all the facilities provided and for their moral support. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
|
|
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |
| |











