Research - (2022) Volume 13, Issue 1
Antipsychotic-induced Changes in Blood Levels
of Leptin and Ghrelin in Schizophrenia: A Short-term
Prospective Study
Waleed H Osman1*,
Nadia YS Morcos1,
Safeya M Effat2 and
Naglaa S Sherif1
1Department of Biochemistry, Faculty of Science, Ain Shams University, Egypt
2Department of Neuro-psychiatry, Faculty of Medicine, Ain Shams University, Egypt
*Correspondence:
Waleed H Osman, Department of Biochemistry, Faculty of Science, Ain Shams University,
Egypt,
Tel: +00201020701025,
Email:
Received: 20-Oct-2021, Manuscript No. ipjnn-21-12103;
Editor assigned: 22-Oct-2021, Pre QC No. P-12103;
Reviewed: 21-Jan-2022, QC No. Q-12103;
Revised: 24-Jan-2022, Manuscript No. R-12103;
Published:
31-Jan-2022
Abstract
Background: Schizophrenia (SZ) is a psychotic disorder with a complex pathophysiology. It requires long term treatment with antipsychotics (APs) which are associated with metabolic syndrome.
Aim of the study: To assess the effect of three widely-used APs, namely olanzapine (OLZ), quetiapine (QUET), and risperidone (RIS) on body weight, and its relation with appetite hormones, metabolic markers, and oxidative stress, in SZ AP-naïve men.
Subjects and Methods: 25 Patients were recruited and investigated in a functional follow-up analysis for 8 weeks. Critical changes in body weight as well as in leptin, ghrelin, glucose, lipid profile and oxidative stress markers were examined as a function of duration of AP exposure. Fifteen healthy men were included as controls.
Results: At baseline, there were no major differences between SZ patients and controls. Treatment with APs caused a significant increase in body weight, leptin, glucose, cholesterol, triglycerides, LDL, VLDL, and malondialdehyde, which were negatively correlated with ghrelin, HDL, and catalase. These changes were more profound in the OLZ group, but negligible in the RIS one. However, not all patients in each treatment group were affected equally.
Conclusion: The role of leptin in AP-induced weight gain is supported. However, not all patients respond to an AP similarly. Tailored SZ medications and thorough biochemical markers assessment must be incorporated in clinical treatments. Further studies with larger number of patients and longer periods of follow-up are recommended.
Keywords
Critical differences; Olanzapine; Quetiapine; Risperidone; Oxidative
stress; Antipsychotic-induced weight gain; Treatment outcome
Introduction
Schizophrenia (SZ) is a chronic, severe mental disorder that
affects how a person thinks, feels, and behaves. It can cause
hallucinations, delusions, and other mental problems that make
it seem like a person has lost touch with reality. It affects about
1 in 100 people, and tends to run in families [1]. Recent studies
have identified genetic factors that confer an increased risk of SZ
and participate in the disease etiopathogenesis [2].
Atypical antipsychotics, an effective treatment for schizophrenia,
have a range of pharmacologic properties leading to differences
in tolerability as well as heterogeneity in treatment response [3].
Antipsychotic-induced Weight Gain (AIWG) is a serious side-effect
of AP medication leading to metabolic syndrome and increased
cardiovascular morbidity, which contributes to poor treatment
adherence and significant morbidity [4]. Unfortunately, the
mechanisms responsible for these complications are not
completely understood. According to various studies, leptin has
been associated with AIWG [5,6].
Leptin is considered one of the best markers of total body fat in
animals and humans. It is secreted by adipose tissue and regulates
energy homeostasis, neuroendocrine function, metabolism,
immune function and other systems through its effects on the
central nervous system and peripheral tissues. A hyperleptinemic
state has been observed in obese subjects, which appears to
indicate a loss of leptin’s ability to stop overeating behavior
(leptin resistance) [5]. Thus far, results on the relation between
leptin and AIWG have been mixed such that some studies
reported increased leptin levels [7,8] while others reported
no change [6,8]. This inconsistency could be due an impact of
genetic variation in the genes encoding leptin and leptin receptor,
but results have not been conclusive [3,9]. Consequently,
individual patient characteristics must be considered when
making treatment choices, especially from an adverse event or
tolerability perspective.
Accordingly, the aim of the present work was to assess individual
changes in body weight and BMI after the start of a specific AP
(during 8 weeks of treatment). Also, to correlate these changes
with the critical changes (clinical relevant changes) in different
metabolic markers.
Methodology
Study design and population
This is a prospective cohort study. It included 25 male drug-naïve
schizophrenic patients, aged between 18 and 47 years (27.8 ±
8.3). The patients were selected among inpatients in the institute
of psychiatry-neuropsychiatry department, Ain Shams University
Hospitals in Cairo, Egypt, who were medically indicated for
initiation of treatment with olanzapine (10mg/day), quetiapine
(600 mg/day), or risperidone (6 mg/day). The diagnosis of
schizophrenia was performed following the criteria of the
International Classification of Diseases-10 (ICD-10). All patients
received electroconvulsive therapy (ECT) (6–8 sessions). Fifteen
healthy males without current or past psychiatric disorders were
included and served as controls. Blood samples were collected,
and body weights recorded before the commencement of
treatments (baseline, all subjects), and repeated after 2, 4 and
8 weeks for all patients. The study protocol was approved by the
ethics committee of Faculty of Medicine, Ain Shams University.
A written informed consent was obtained from the patients and
control groups involved in the study. It was taken in a private
setting after full discussion of the study rational. Participants
in the study were informed that the study is totally free and
voluntary, and that it does not imply a direct benefit for him/her,
although data obtained could be used for the benefit of other
patients. They also were told that they have the right to withdraw
from the study at any time without giving any justification.
Exclusion criteria:
1) Patients who had a substance-related disorder or other
physical illness, including hypertension or hyperlipidemia
that might affect their appetite or glucose metabolism;
2) Significant weight loss/gain ± 1 kg in the past 8 weeks.
Blood collection and assessment
Venous blood was collected in the morning after 12 hours of
fasting, into two tubes: A part of the blood was taken on sodium
fluoride for the determination of plasma glucose (10). Another
part was left to coagulate to obtain serum after centrifugation at
1000 g for 15 minutes. The serum was then stored at -20°C to be
thawed only once on demand for the remaining analysis.
Routine laboratory investigations were carried using commercial
kits (Spectrum diagnostics, Egypt). These included: Fasting blood
glucose (FBS) [11], total cholesterol [12], triglycerides (TG) [13],
LDL- cholesterol [14], HDL- cholesterol [15], and VLDL- cholesterol [16].
Serum leptin was measured using double-antibody enzyme
immunoassay method of [17] using an ELISA kit (BioVendo, Czech
Republic). Serum ghrelin was determined by the double-antibody
enzyme immunoassay method [18] using an active ghrelin ELISA
kit, according to the manufacturer’s instructions (BioVendo, Czech
Republic). Both lipid peroxidation as malondialdehyde (MDA) and
catalase enzyme activity were measured using commercial assay
kits (Biodiagnostics, Egypt) according to the colorimetric methods
described by Sinha AK [19] and Yoshioka T, et al. [20] respectively.
Statistical analysis
Statistical analysis was performed using SPSS/PC software
program (version 21; IBM SPSS statistics, USA). Data were tested
for normal distribution using the Kolmogorov–Smirnov test,
and were found to be normally distributed at baseline, hence
represented as mean ± standard deviation. The study design and
complex aim call for different ways of statistical analysis that allow
to compare not only effects of APs, but also to compare these
effects with respect to the baseline of each subject individually,
stratifying for duration of AP use.
The corresponding parameters in the groups at baseline (healthy
and SZ patients) were compared by means of independentsamples
T-test. Results after 8 weeks of treatment were
compared with their own baseline values (paired-sample t-tests).
Correlations between the changes (as percentage) in the different
parameters with body weight were carried by Pearson correlation
coefficient analysis. The level of statistical significance was set
at P < 0.05.
Critical differences: To assess the significance of differences
(true change) between consecutive results obtained in each
patient, the reference change value (RCV) was calculated. RCV
is defined as the critical differences that must be exceeded
between two sequential results for a significant (or true) change
to occur [21]. The RCV is calculated as percentage change from
the median value of each marker according to Ricos C, et al. [22]
and Westgard QC [23], and does not depend on the method of
measurement used. In schizophrenic drug-naïve patients a 7%
change in body weight was considered clinically relevant weight
gain [8] RCV values for leptin, ghrelin, MDA and catalase were
determined by the sample quartiles (percent change of highest
quartile from lowest quartile). The results were computed by the
crosstabs procedure in two-way tables, and presented in stacked
column charts, in which columns represent percent of patients
who achieved marked differences (critical differences) (Figure 1). The legend under each column represents RCV value for each
analyte.
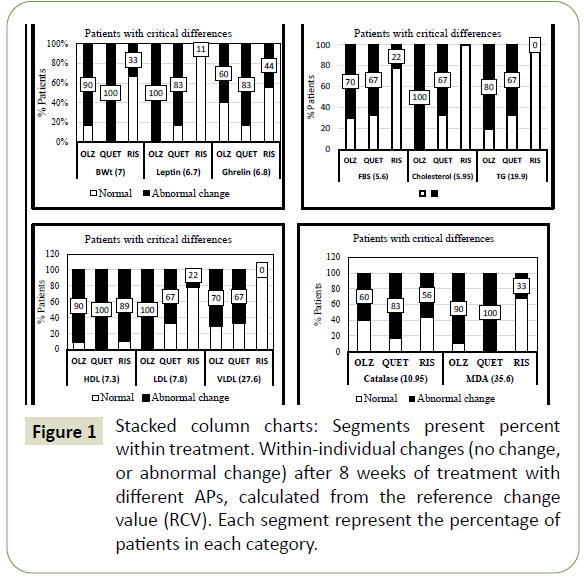
Figure 1: Stacked column charts: Segments present percent within treatment. Within-individual changes (no change, or abnormal change) after 8 weeks of treatment with different APs, calculated from the reference change value (RCV). Each segment represent the percentage of patients in each category.
Results
Baseline characteristics of the patients and controls are shown
in Table 1. Cases were similar to controls with regard to baseline
characteristics, with the following exceptions cholesterol; LDL
and catalase were lower in patients group.
| Variables |
Control (n=15) |
Patients (n=25) |
P |
| Mean ± SD |
Mean ± SD |
| Body weight |
71.5 ± 9.41 |
68.0 ± 8.6 |
NS |
| BMI |
23.9 ± 4.2 |
22.7 ± 3.1 |
NS |
| Leptin ng/mL |
2.99 ± 0.22 |
3.05 ± 0.22 |
NS |
| Ghrelin pg/mL |
44.9 ± 2.3 |
44.5 ± 2.1 |
NS |
| FBS mg/dL |
79.8 ± 9.4 |
79.5 ± 9.2 |
NS |
| Cholesterol mg/dL |
170.7 ± 10.6 |
163.0 ± 9.8 |
0.05 |
| Triglycerides mg/dL |
80.7 ± 15.7 |
76.0 ± 9.1 |
NS |
| HDL mg/dL |
56.7 ± 4.7 |
58.9 ± 5.8 |
NS |
| LDL mg/dL |
98.1 ± 10.9 |
88.9 ± 11.3 |
0.01 |
| VLDL mg/dL |
16.1 ± 3.2 |
15.2 ± 1.8 |
NS |
| Catalase U/L |
468.1 ± 125.5 |
387.1 ± 148.8 |
0.05 |
| MDA nmol/mL |
3.68 ± 1.7 |
3.53 ± 1.5 |
NS |
Table 1 Descriptive characteristics of schizophrenia cases and controls at baseline (Independent-Samples T Test).
The changes in each AP treated group after 2, 4, and 8 weeks
of treatment presented as percent change from baseline are
illustrated in Figures 2a and 2b. In both the olanzapine (OLZ) and
quetiapine (QUET) treated groups the degree of change amplified
by time, reaching maximum after 8 weeks. The changes were
more pronounced in the OLZ treated patients compared to those
receiving QUET. Patients treated with risperidone (RIS) showed
little or no changes through the treatment period. Results
revealed an increase in body weight, glucose, leptin, cholesterol,
triglycerides, LDL, VLDL, and MDA, while a decrease in ghrelin,
HDL, and catalase was observed.
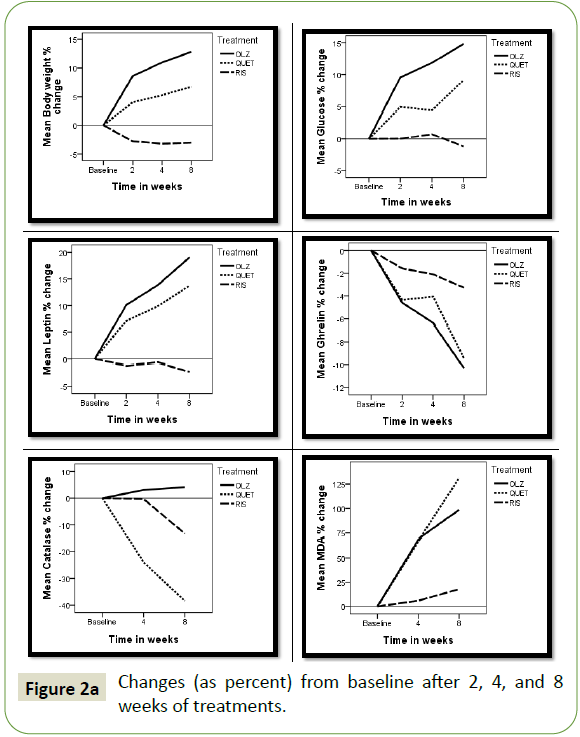
Figure 2a: Changes (as percent) from baseline after 2, 4, and 8 weeks of treatments.
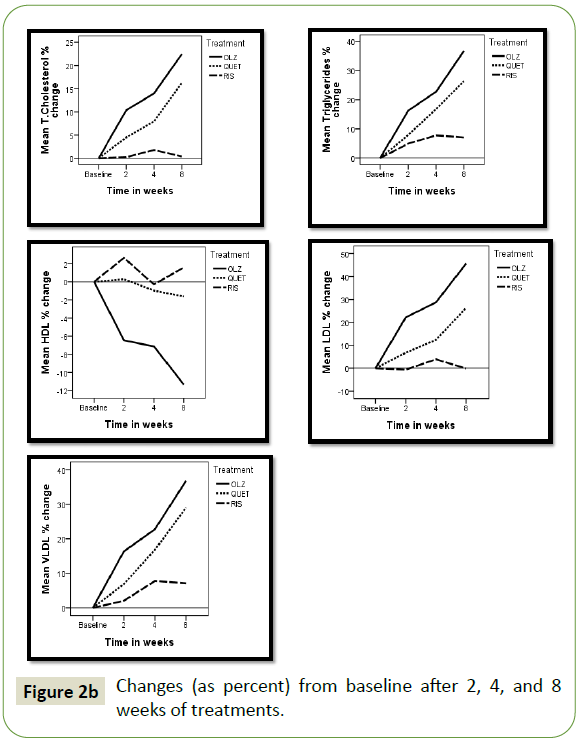
Figure 2b: Changes (as percent) from baseline after 2, 4, and 8 weeks of treatments.
To test the significance of changes after 8 weeks of treatment
compared to baseline, paired-sample t-test was applied on all the
parameters for each AP treatment (Table 2).
<
|
Treatment (n=number of patients) |
|
OLZ (n=10) |
QUET (n=6) |
RIS (n=9) |
| Weeks |
0 |
8 |
0 |
8 |
0 |
8 |
| Body weight Kg |
65.8 ± 9.9 |
73.9 ± 9.3 |
67 ± 4.6 |
71.5 ± 5.8 |
71.2 ± 8.3 |
69 ± 8.5 |
| p |
0.001 |
0.010 |
NS |
| BMI |
22.0 ± 3.7 |
24.7 ± 3.4 |
22.34 ± 1.9 |
23.8 ± 2.3 |
23.6 ± 3.2 |
22.9 ± 3.0 |
| p |
0.001 |
0.012 |
NS |
| Leptin ng/mL |
3.06 ± 0.2 |
3.64 ± 0.2 |
3.05 ± 0.1 |
3.5 ± 0.3 |
3.03 ± 0.3 |
2.96 ± 0.3 |
| p |
0.001 |
0.020 |
NS |
| Ghrelin pg/mL |
45.0 ± 2.2 |
40.3 ± 2.4 |
44.1 ± 2.3 |
40.0 ± 3.3 |
44.2 ± 2.1 |
42.7 ± 2.1 |
| p |
0.001 |
0.004 |
NS |
| FBS mg/dL |
77.0 ± 8.4 |
87.6 ± 4.6 |
81.1 ± 8.4 |
88.0 ± 4.8 |
81.1 ± 10.9 |
79.2 ± 6.4 |
| p |
0.004 |
0.010 |
NS |
| Cholesterol mg/dL |
162.0 ± 11.2 |
198.2 ± 16.2 |
162.2 ± 9.0 |
188.5 ± 18.8 |
164.7 ± 9.5 |
164.9 ± 3.6 |
| p |
0.001 |
0.010 |
NS |
| TG mg/dL |
75.5 ± 8.0 |
103.0 ± 14.8 |
82.2 ± 10.4 |
103.6 ± 17.0 |
72.5 ± 8.0 |
77.2 ± 6.3 |
| p |
0.001 |
0.010 |
NS |
| HDL mg/dL |
62.4 ± 5.5 |
55.0 ± 3.9 |
55.5 ± 2.9 |
54.4 ± 3.5 |
57.3 ± 6.0 |
57.7 ± 3.9 |
| p |
0.002 |
NS |
NS |
| LDL mg/dL |
84.5 ± 10.4 |
122.6 ± 15.8 |
90.3 ± 8.6 |
113.5 ± 18.1 |
93.0 ± 12.9 |
91.7 ± 5.9 |
| p |
0.001 |
0.030 |
NS |
| VLDL mg/dL |
15.1 ± 1.6 |
20.6 ± 3.0 |
16.4 ± 2.1 |
21.1 ± 3.8 |
14.5 ± 1.6 |
15.4 ± 1.3 |
| p |
0.001 |
0.020 |
NS |
| Catalase U/L |
364.9 ± 165 |
291.2 ± 124 |
467.0 ± 172 |
271.8 ± 89 |
358.5 ± 106 |
299.0 ± 149 |
| p |
NS |
0.024 |
NS |
| MDA nmol/mL |
4.11 ± 1.8 |
7.18 ± 1.9 |
2.16 ± 0.6 |
5.03 ± 2.0 |
3.81 ± 0.9 |
4.34 ± 1.3 |
| p |
0.010 |
0.010 |
NS |
Table 2 Changes after 8 weeks of treatment compared to baseline (Paired-Sample t-test).
In the OLZ group all the studied parameters were significantly
(p<0.01 – p<0.001) altered, except catalase enzyme, which showed
non-significant change. QUET treatment caused less significant
changes than the OLZ group (p<0.05 – p<0.01), showing that the
decrease in HDL was not significant. None of the parameters was
significantly changed in the RIS treated group.
Correlations between the changes (as percentage) in the different
parameters with body weight for all patients through the followup
period were carried by Pearson correlation coefficient analysis
(Table 3). Changes in body weight were significantly correlated
with the changes in all the studied parameters. The strongest
correlations were those with leptin and total cholesterol (r=0.874
and 0.819 respectively). The changes in leptin were positively
correlated with total cholesterol, LDL, VLDL, and MDA in a
descending order, while an inverted correlation was observed
between the changes in leptin with ghrelin, HDL, and catalase.
| |
Glucose |
Leptin |
Ghrelin |
Catalase |
MDA |
Chol. |
TG |
HDL |
LDL |
VLDL |
| B.wt |
0.587 |
0.874 |
-0.519 |
-0.343 |
0.654 |
0.819 |
0.599 |
-0.436 |
0.761 |
0.615 |
| Glucose |
- |
0.476 |
-0.414 |
-0.341 |
0.499 |
0.613 |
0.360 |
NS |
0.515 |
0.328 |
| Leptin |
- |
- |
-0.616 |
-0.310 |
0.577 |
0.778 |
0.701 |
-0.487 |
0.745 |
0.706 |
| Ghrelin |
- |
- |
- |
0.382 |
-0.552 |
-0.542 |
-0.534 |
0.383 |
-0.500 |
-0.519 |
| Catalase |
- |
- |
- |
- |
-0.599 |
-0.343 |
-0.287 |
0.465 |
-0.363 |
-0.290 |
| MDA |
- |
- |
- |
- |
- |
0.660 |
0.582 |
-0.290 |
0.612 |
0.587 |
| Chol. |
- |
- |
- |
- |
- |
- |
0.742 |
-0.365 |
0.918 |
0.730 |
| TG |
- |
- |
- |
- |
- |
- |
- |
NS |
0.602 |
0.985 |
| HDL |
- |
- |
- |
- |
- |
- |
- |
- |
-0.585 |
NS |
| LDL |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0.586 |
All correlations were highly significant (p<0.001). Shaded squares: significant (p< 0.05).
NS: Not significant
Table 3 Pearson correlation coefficients (r) relating strength of correlations between the changes (percentage) in the studied parameters for all patients through the 8 weeks of follow-up.
The number of patients who showed marked adverse effects
(critical differences) after treatment with the different APs
(within-individual change) were calculated from the reference
change value (RCV) by cross-tabulation analysis. Results revealed
that body weight increased clinically, i.e., more than 7% from the
baseline level (RCV=7) in 90% of patients treated with OLZ, 100%
of the patients receiving QUET, and only 11% in the RIS treated
group. Leptin was elevated (RCV= 6.7) in 100%, 83%, and 11%
of patients treated with OLZ, QUET, and RIS respectively, these
changes paralleled with 60%, 83%, and 44% of patients with a
critical decrease in ghrelin (RCV= 6.8). Fasting blood sugar (FBS)
was clinically elevated (RCV= 5.6) in 70%, 67%, and 22% of patients
receiving OLZ, QUET, and RIS respectively. Total cholesterol
(RCV=5.95) and triglycerides (RCV=19.9) increased in both the
OLZ (100% & 80%) and QUET (67% & 67%) groups respectively,
but none of the RIS treated group showed altered levels. The
decrease in HDL (RCV=7.3) and increase in LDL (RCV=7.8) and VLDL (RCV=27.6), were recorded in 90%, 100%, and 70% of the
OLZ group, in 100%. 67%, and 67% in the QUET group, and in
89%, 22% and zero % in the RIS group respectively. A decrease in
catalase (RCV= 10.95) and an increase in MDA (RCV=35.6) were
recorded in 60% and 90% of the OLZ group, 83% and 100% of the
QUET group, and in 56% and 33% of the RIS group respectively.
Discussion
It is now well known that use of the atypical antipsychotics is
perhaps the most effective treatment we currently have for
schizophrenia and other serious mental illnesses. Unfortunately
due to their associated risk for metabolic syndrome, use of these
medications may be placing these individuals at greater risk for
several comorbidities, resulting in an accumulation of life years
lost due to cardiovascular disease [3,24].
The present study assessed absolute changes in body weight and BMI as well as the proportion of subjects with more than 7%
increase in body weight (or clinically relevant weight gain) after
the start of three APs, namely olanzapine (10 mg/day), quetiapine
(600 mg/day), and risperidone (6 mg/day), in APs naïve patients.
We aimed also to discover whether metabolic complications of
schizophrenia (SZ) are present in before treatment, and to what
extend these complications are intensified during treatment.
These changes were followed for 8 weeks, allowing for assessment
of possible progress with duration of AP treatment.
The present study presents several outcomes:
(1) At baseline SZ patients’ characteristics did not differ
significantly from controls.
(2) OLZ and QUET caused increase in body weight and BMI
with increased duration of AP use, which was not observed
in the RIS treated patients.
(3) The changes in body weight were strongly correlated with
increase in leptin.
(4) Hyperleptinemia was accompanied by an increase in the
risk of metabolic syndrome (increase in FBS, cholesterol,
triglycerides, LDL, VLDL, and MDA, and a decrease in HDL,
catalase, and ghrelin levels).
(5) The above changes differed among patients in each treated
group. Hence, individual patient characteristics must be
considered when making treatment choices, especially
from an adverse event or tolerability perspective.
Weight gain is a major side effect of antipsychotics (APs),
which contributes to poor treatment adherence and significant
morbidity. The mechanisms involved in AP-induced weight gain
are incompletely understood. Studies in drug-naïve patients are
more informative than switch studies, as weight outcomes are
not influenced by the level of overweight due to a previous AP,
thus allowing for assessment of an effect that can be attributed
to a specific AP [8]. At baseline the body weight and BMI of SZ
patients did not differ from controls. After treatment with OLZ
and QUET both showed a significant increase, which correlated
with duration of treatment, and the proportion of patients
gaining more than 7% weight (or clinically relevant weight gain)
expanded with duration of AP. Many reports have suggested that
olanzapine and quetiapine induce weight gain [5,6,8,9,25-27].
However, the mechanism behind this weight gain remains unclear
[6]. Eating disorders was one of the suggested causes [28].
Appetite and body weight are controlled by complex hypothalamic
neurocircuitry, in which leptin and ghrelin are crucial elements
of this control system [9]. Both leptin and ghrelin were within
the normal ranges before treatment, this is in line with previous
studies [29], conversely others suggested that schizophrenia is
associated with increased blood leptin [30]. During treatment with
OLZ and QUET, we found a significant increase in leptin, which was
negatively correlated with ghrelin level, while patients receiving
RIS showed normal leptin and ghrelin levels, which is supported
by previous reports [9,31]. It was suggested that hyperleptinemia
in schizophrenia is likely to represent a secondary effect related
to AP-induced weight gain, signifying that leptin acts as a negative feedback signal in the event of fat increase [9]. Leptin resistance
is characterized by decreased availability of leptin to the brain
despite normal or even higher plasma levels. Mechanisms of
leptin resistance are complex including genetic mutation of
leptin receptor, failure of self-regulation of hypothalamic centers,
limited transport of leptin through blood-brain barrier, and
intracellular molecular mechanisms [32]. Consequently, it was
projected that ghrelin may be downregulated as a consequence
of body weight gain induced by APs, showing a normalizing effect
on energy homeostasis and metabolic change induced by b the
antipsychotics [2,25].
Within-individual changes in leptin revealed that all OLZ treated
patients had clinical increase in their leptin levels (an increase
>6.7% from base line), corresponding to 83% of the QUET treated
group. None of the patients treated with RIS showed abnormal
leptin level. Ghrelin decreased critically (RCV=6.8) in 60%,
83%, and 44% of the patients treated with OLZ, QUET, and RIS
respectively. To our knowledge this is the first study on the critical
differences of leptin and ghrelin in APs treated patients.
The etiology of the cardiometabolic disorders in schizophrenia
is multifactorial and includes oxidative stress [2,28,33],
conventional risk factors such as genetic and lifestyle factors, and drug side effects [2,34]. In addition, as in the general population,
eating behaviors and eating disorders are crucial in determining
the etiology of cardiometabolic disorders in patients with
schizophrenia [28]. In the present work at baseline fasting blood
sugar was within normal range, but increased significantly after
8 weeks of treatment with OLZ and QUET (paired-sample t-test).
Results on the pre-treatment glucose levels have been mixed
such that some studies report increased levels [35], while others
report no change [36]. Recent genetic studies have provided
suggestive support for the involvement of glucose metabolism
cascades in the genetic risk of SZ, which could explain these
diversities. The present results concur with this conclusion,
since critical difference analysis showed that glucose was not
altered equally in patients treated with a specific APs. In the OLZ
group 72% of the patients developed hyperglycemia (RCV=5.6),
corresponding to 67% in the QUET group, and 22% in the RIS
group. Thus, genetic factors modulate these changes [2].
At baseline total cholesterol and LDL levels were lower in patients
compared to controls. After 8 weeks of treatment, body weight
gain and increased leptin levels were positively correlated with
dyslipidemia (elevation in cholesterol, triglycerides, LDL, and
VLDL, with a decrease in HDL). The severity of the metabolic
disturbances were maximum in patients treated with OLZ,
followed by those treated with QUET. Patients treated with RIS
showed minimum changes. Critical analysis confirmed these
results, which concur with the conclusions presented by Potvin
S, et al. [9], that some APs such as olanzapine and quetiapine
(less so) are associated with significant metabolic side effects,
while others, such as risperidone have mild adverse effects. The
effect of APs on metabolic markers was previously studied in SZ
patients under different conditions, as reported in recent reviews
[2,9,37,38]. Antipsychotics have been shown to disrupt lipid
homeostasis, they inhibit cholesterol biosynthesis and impair the
intracellular cholesterol trafficking, leading to lipid accumulation
in the late endosome/lysosome compartment. These effects
could underlie some of the metabolic adverse effects induced
by prolonged treatment with antipsychotic [38], however, the
mechanisms responsible for metabolic side effects associated
with APs are not completely understood.
Catalase and malondialdehyde were studied as parameters of oxidative stress. Before treatment catalase activity was lower in
patients compared to control, but MDA showed no significant
difference, which is in line with previous meta-analysis [39,40].
After treatment for 8 weeks catalase was reduced only in the
QUET treated group (paired sample t-test). These results do
not represent the true effect of APs on catalase, as seen from
the critical difference analysis, which revealed that catalase
was reduced clinically (RCV=10.95) in 60%, 83% and 56% of
patients treated with OLZ, QUET, and RIS respectively. Thus we
recommend that within-individual variations should be taken
into consideration when following the effect of drugs on patients
[3,4]. This would aid in the effort to personalize mental illness
pharmacotherapy and optimize treatment. We did not find
similar analysis in the literature, nonetheless our results concur
with previous studies on the important role of genetic factors on
patient’s outcome [41].
Lipid peroxidation marker MDA was elevated significantly (p<0.01)
after treatment with both OLZ and QUET, and was inversely
correlated with the catalase level. This could be expected, since
the decreased catalase would shunt the conversion of hydrogen
peroxide from water and oxygen toward hydroxyl radical
production. Increase hydroxyl radicals would result in increased
lipid peroxidation and consequently increase MDA [40,42].
Conclusion
In summary, metabolic cascades are altered in SZ patients
treated with APs, which include changes in the levels of leptin,
ghrelin, glucose, and lipid profile. Genetic factors are known to
modulate these changes, but it remains elusive whether and how
a genetic predisposition to metabolic disturbance has a primary
role in SZ pathology. Clinicians should focus on preventing
initial cardiometabolic risk because subsequent reduction in
this risk is more difficult to achieve, either through behavioral
or pharmacologic. In order to prevent well studied long-term
consequences of the metabolic risk factors a careful individual
decision for an antipsychotic, a close monitoring for metabolic side
effects of the chosen antipsychotic, switching the antipsychotic
therapy if appropriate and focusing on how to improve physical
activity of the individual patient are essential.
REFERENCES
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, et al. (2016) Schizophrenia Working Group of the Psychiatric Genomics Consortium, Daly MJ, Carroll MC, Stevens B, McCarroll SA. Schizophrenia risk from complex variation of complement component. Nature 4.
- Landek-Salgado MA, Faust TE, Sawa A (2016) Molecular substrates of schizophrenia: Homeostatic signaling to connectivity. Mol Psychiatry 21: 10-28.
Google Scholar, Crossref, Indexed at
- Citrome L, Eramo A, Francois C, Duffy R, Legacy SN, et al. (2015) Lack of tolerable treatment options for patients with schizophrenia. Neuropsychiatr Dis Treat 11: 3095-3104.
Google Scholar, Crossref, Indexed at
- Brandl EJ, Frydrychowicz C, Tiwari AK, Lett TA, Kitzrow W, et al. (2012) Association study of polymorphisms in leptin and leptin receptor genes with antipsychotic-induced body weight gain. Prog Neuropsychopharmacol Biol Psychiatry 38: 134-141.
Google Scholar, Crossref, Indexed at
- Park HK, Ahima RS (2015) Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metabolism 64: 24-34.
Google Scholar, Crossref, Indexed at
- Stip E, Potvin S (2015) The 10th Anniversary of the Eli Lilly Chair of Schizophrenia from the University of Montreal. Can J Psychiatry 60: 1-4.
Google Scholar, Indexed at
- Hosojima H, Togo T, Odawara T, Hasegawa K, Miura S, et al. (2006) Early effects of olanzapine on serum levels of ghrelin, adiponectin and leptin in patients with schizophrenia. J Psychopharmacol 20: 75-79.
Google Scholar, Crossref
- Bak M, Fransen A, Janssen J, Van OSJ, Drukker M (2014) Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One 9: e94112.
Google Scholar, Crossref, Indexed at
- Potvin S, Zhornitsky S, Stip E (2015) Antipsychotic-induced changes in blood levels of leptin in schizophrenia: A meta-analysis. Can J Psychiatry 60: 26-34.
Google Scholar, Indexed at
- Mikesh LM, Bruns DE (2008) Stabilization of glucose in blood specimens: Mechanism of delay in fluoride inhibition of glycolysis. Clinical Chemistry 5: 930-932.
Google Scholar, Crossref
- Trinder P (1969) Enzymatic colorimetric determination of glucose. Ann Clin Biochem 2: 24-27.
- Kaplan LA, Pesce AJ (1984) Clinical Chemistry: Theory, Methods and Practice. St. Louis, MO: C.V. Mosby Co. 1032-1036.
- Bucolo G, David H (1973) Quantitative determination of serum triglycerides by use of enzymes. Clin Chem 19: 476-482.
Google Scholar, Indexed at
- Okada M, Matsui H, Ito Y, Fujiwara A, Inano K (1998) Low-density lipoprotein cholesterol can be chemically measured: A new superior method. J Lab Clin Mad 132: 195-201.
Google Scholar, Crossref, Indexed at
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR (1977) High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 62: 707-714.
Google Scholar, Crossref, Indexed at
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 409-502.
Google Scholar, Crossref, Indexed at
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, et al. (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292-295.
Google Scholar, Crossref, Indexed at
- Grosch M, Uhr M, Kraus T (2004) Evaluation of comparability of commercial ghrelin assays. Clin Chem 50: 457-465.
Google Scholar, Crossref, Indexed at
- Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47: 389-394.
Google Scholar, Crossref, Indexed at
- Yoshioka T, Kawada K, Shimada T, Mori M (1979) Lipid peroxidation in maternal and cord blood and protective mechanism against activated oxygen toxicity in the blood. Am J of Obst Gynecol 135: 372-376.
Google Scholar, Crossref, Indexed at
- Smellie WS (2008) What is a significant difference between sequential laboratory results? J Clin Pathol 61: 419-425.
Google Scholar, Crossref
- Ricos C, Perich C, Minchinela J, Álvarez V, Simón M, et al. (2009) Application of biological variation: A review. Biochem Med 19: 250-259.
Google Scholar, Crossref
- Westgard QC. Biologic variation database, the 2012 update.
- Hrdlicka M, Dudova I (2015) Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat 11: 907-913.
Google Scholar, Crossref, Indexed at
- Kim BJ, Sohn JW, Park CS, Hahn GH, Koo J, et al. (2008) Body weight and plasma levels of ghrelin and leptin during treatment with olanzapine. J Korean Med Sci 23: 685-690.
Google Scholar, Crossref, Indexed at
- Tarricone I, Ferrari Gozzi B, Serretti A, Grieco D, Berardi D (2010) Weight gain in antipsychotic-naive patients: A review and meta-analysis. Psychol Med 40: 187-200.
Google Scholar, Crossref, Indexed at
- Tomasik J, Schwarz E, Lago SG, Rothermundt M, Leweke FM, et al. (2016) Pretreatment levels of the fatty acid handling proteins H-FABP and CD36 predict response to olanzapine in recent-onset schizophrenia patients. Brain Behav Immun 52: 178-186.
Google Scholar, Crossref
- Kouidrat Y, Amad A, Desailloud R, Diouf M, Fertout E, et al. (2013) Increased advanced glycation end-products (AGEs) assessed by skin autofluorescence in schizophrenia. J Psyc Res 47: 1044-1048.
Google Scholar, Crossref
- Neelamekam S, Nurjono M, Lee J (2014) Regulation of interleukin-6 and leptin in schizophrenia patients: A preliminary analysis. Clin Psychopharmacol Neurosci 12: 209-214.
Google Scholar, Crossref, Indexed at
- Stubbs B, Wang AK, Vancampfort D, Miller BJ (2016) Are leptin levels increased among people with schizophrenia versus controls? A systematic review and comparative meta-analysis. Psychoneuroendocrinol 63: 144-154.
Google Scholar, Crossref
- Sentissi O, Epelbaum J, Olie JP, Poirier MF (2008) Leptin and ghrelin levels in patients with schizophrenia during different antipsychotics treatment: A review. Schizophr Bull 34: 1189-1199.
Google Scholar, Crossref, Indexed at
- Kantorová E, Čierny D, Zeleňák K, Sivák Š, Stančík M, et al. (2015) The Intricate network of adipokines and stroke. Int J Endocrinol 2015: 967698.
Google Scholar, Crossref, Indexed at
- Boke O, Aker S, Sarisoy G, Saricicek EB, Sahin AR (2008) Prevalence of metabolic syndrome among inpatients with schizophrenia. Int J Psychiatry Med 38: 103-112.
Google Scholar, Crossref
- de Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen DA, et al. (2011) Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 10: 52-77.
Google Scholar, Crossref, Indexed at
- Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH (2007) Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet Med 24: 481-485.
Google Scholar, Crossref
- Holmes E, Tsang TM, Huang JT, Leweke FM, Koethe D, et al. (2006) Metabolic profiling of CSF: evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS Med 3: e327.
Google Scholar, Crossref, Indexed at
- Papanastasiou E (2013) The prevalence and mechanisms of metabolic syndrome in schizophrenia: A review. Ther Adv Psychopharmacol 3: 33-51.
Google Scholar, Crossref
- Canfrán-Duque A, Pastor O, Reina M, Lerma M, Cruz-Jentoft AJ, et al. (2015) Curcumin mitigates the intracellular lipid deposit induced by antipsychotics in vitro. PLoS One 10: e0141829.
Google Scholar, Crossref, Indexed at
- Zhang M, Zhao Z, He L, Wan C (2010) A meta-analysis of oxidative stress markers in schizophrenia. Sci China Life Sci 53: 112-124.
Google Scholar, Crossref, Indexed at
- Flatow J, Buckley P, Miller BJ (2013) Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry 74: 400-409.
Google Scholar, Crossref
- Chowdari KV, Bamne MN, Nimgaonkar VL (2011) Genetic association studies of antioxidant pathway genes and schizophrenia. Antioxid Redox Signal 15: 2037-2045.
Google Scholar, Crossref
- Sarandol A, Sarandol E, Acikgoz HE, Eker SS, Akkaya C, et al. (2015) First-episode psychosis is associated with oxidative stress: Effects of short-term antipsychotic treatment. Psychiatry Clin Neurosci 69: 699-707.
Google Scholar, Crossref, Indexed at
Citation: Osman WH, Morcos NYS, Effat SM and Sherif NS (2022) Antipsychoticinduced Changes in Blood Levels of Leptin and Ghrelin in Schizophrenia: A Short-term Prospective Study. J Neurol Neurosci Vol.13 No.1:406.








