Keywords
Pancreatic cancer; Peptides; Proteomics
Introduction
Pancreatic Cancer, or Pancreatic Ductal Adenocarcinoma (PDAC), is one of the deadliest neoplasms in the United States and across the globe. Even with rapid advancements in modern medicine, less than 5% of pancreatic cancer patients survive past five years from their diagnoses date [1]. The high mortality rate of pancreatic cancer can be attributed to three main factors: difficult surgical access and resection, extraordinary chemo-resistance, and the common presence of multiple comorbidities within patients.
Pancreatic cancer arises due to a build-up of mutations in the ductal epithelium, with patients demonstrating aberrant expression in one or multiple pathways, including but not limited to KRAS, CDKN2A (Cyclin-dependent Kinase Inhibitor 2A), TP53 (Tumor Antigen P53) and DPC4 (Deleted in Pancreatic Cancer locus 4) [2]. One or several of the following genetic alterations is commonly seen in dysplastic PDAC cells in purple. These include the upregulation of the KRAS protooncogene, downregulation of the CDKN2A tumor suppressor gene, as well as knockouts of the regulators TP53 and DPC4 [2].
See Figure 1 for an overview of common genetic alterations and mutations seen during the dysplastic change of pancreatic ductal cells. The tumor itself is characteristic in demonstrating a desmoplastic reaction, where pancreatic stellate cells induce invasive dense fibrous tissue growth as well as angiogenesis [3]. Critically, certain pancreatic cancer cells have demonstrated stem-cell qualities, which explains the high recurrence rate of the neoplasm even after extensive surgery, chemotherapy and radiation [4]. Even if a small population of these cancer stem-cells remain viable after treatment, the tumor could rapidly proliferate via asymmetric division [5]. Considering the aggressive and chemo-resistant nature of this neoplasm, the best “treatment” is often early detection and diagnosis.
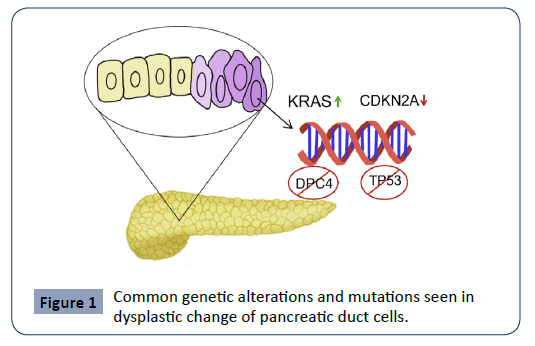
Figure 1: Common genetic alterations and mutations seen in dysplastic change of pancreatic duct cells.
Traditionally, for earlier-staged patients, the treatment of pancreatic cancer has involved the Whipple Procedure or a radical pancreatoduodenectomy with extensive lymph node resection in conjunction with chemotherapy [6]. The pharmacological agents that have shown the best efficacy towards pancreatic cancer are the cytotoxic pyrimidine analog, Gemcitabine, and the small molecule epidermal growth factor (EGFR) inhibitor, Erlotinib [7]. The combination of these agents yielded small survival improvements but also resulted in significant increase in toxicity, especially in gastrointestinal-related distress [8].
In this review, we seek to explore a new direction in the diagnosis and management of Pancreatic cancer. The recent advancements in proteomics research has propelled development of novel methods in improving diagnostic sensitivity and accuracy [9]. Various peptides are conjugated to traditional chemotherapeutic agents to enhance their delivery and efficacy. Peptide-based cancer treatment vaccines are being evaluated for their immunomodulatory effects [10]. Furthermore, antimicrobial [11], anti-angiogenic [12] and anti-metalloproteinase [13] peptides are being explored for their antineoplastic properties. With these new tools in hand and combined with traditional treatment, clinicians may find more effective ways to combat pancreatic cancer. This review aims to illuminate this path forward.
Peptide-enhanced imaging and progression monitoring
Current diagnostic tools for PDAC include imaging modalities such as CT, MRI or endoscopic ultrasound. Contemporary evidence points to endoscopic ultrasound as having superior diagnostic accuracy [14]. Unfortunately, over 80% of patients with PDAC will have progressed to locally advanced cancer or distant metastasis before detection, leading to poor prognosis [15]. PDAC is often considered a “silent killer” because the neoplasm can often diffusely spread in the retroperitoneal space without producing obvious symptoms [16]. Thus, new and improved diagnostic tools can be revolutionary in bettering the prognosis of PDAC. Radiolabeled peptides and conjugated Quantum Dots are examples of novel modalities being investigated for their use in diagnosis and continual monitoring of PDAC.
PDAC cells often contain unique types of surface peptides that differentiate them from normal neighboring cells. Thus, radiolabeled peptides can be a useful tool in enhancing the visualization of PDAC on imaging [17]. Mucins are a class of heavily glycosylated glycoproteins with a thick peptide core that anchors them to pancreatic epithelium. Mucins are heavily involved in the chemotherapeutic resistance of PDAC, which is a topic that will be discussed in depth in a later section [18-20]. Well known mucins include CA 19-9, MUC2, MUC5AC, MUC5B, MUC6, MUC7, MUC8, MUC9 and MUC19, MUC1, MUC3A/B, MUC4, MUC11, MUC12, MUC13, MUC15, MUC16, MUC17, MUC20, MUC21 and MUC22. All mucins above have been found to be elevated in patients with PDAC. Specifically, MUC1 is a key focus of several studies [21]. For example, in one study, PAM4-reactive MUC1 was a unique biomarker present in 87% of PDAC patients that helped surgeons track tumor activity post-pancreatectomy [16,22]. However, while these biomarkers are useful in tracking PDAC progression, their usefulness in early detection of the disease is yet to be explored. Additionally, CA 19-9 can be falsely elevated due to other issues, such as biliary tract infection [23]. Bombesin is another small peptide being studied for its use in diagnostic imaging. Radiopharmaceuticals that bind to Bombesin receptors have shown promise in non-invasive diagnosis and radiotherapy of GRP receptor positive pancreatic tumors [24].
Quantum dots (QDs) are nanoparticles that target cell-surface integrins. Researchers have conjugated QDs to arginine-glycineaspartic (RGD) acid, which bind with high affinity to specific integrins on cancer cells [25]. PDAC cells frequently express integrin receptors αvβ6 and ανβ3 at high levels [26-29], thus making them not only good targets for diagnostic development but also precision for chemotherapy.
Peptide conjugates for precision therapy
Standard PDAC treatment protocol: Traditional treatment of PDAC is dependent upon the stage. For earlier-staged and resectable tumors, which make up less than 20% of diagnosed cases, the standard treatment is surgery and adjuvant chemotherapy (fluorouracil and folinic acid, gemcitabine, or the mFOLFIRINOX regimen) [30,31]. The 5-year survival for cases treated with surgery and adjuvant chemotherapy is 20-30% [31,32]. Unfortunately, more than half of cases are diagnosed at advanced stages, for which only palliative care is indicated [33]. The 5-year survival for these advanced staged cases is 6-13% [32]. Thus, alternative therapeutic approaches are indicated to improve the prognosis of both surgically-resectable and advanced cases of PDAC.
Peptide therapy is one such novel approach, which involves conjugating drugs to cell-penetrating and/or nuclear localization signal peptides [34]. This method capitalizes on the increased density of peptide receptors on malignant cells to deliver therapy preferentially to targeted organelles. Peptide applications have been investigated in numerous animal and human cell models and show promising results for use in both tumor imaging and diagnostics, as well as in therapy [17].
General mechanism of peptide-drug conjugates: While many different PDAC cell receptors have been targeted by peptidedrug conjugates, the integrin receptor (αvβ6 and ανβ3) has been widely successful in animal model experiments [26-29]. These integrin receptors are absent in normal cells but are highly expressed in PDAC cells due to their role in regulating tumor growth, angiogenesis, and metastasis [7,9]. Reader et al. found that over 80% of 491 PDAC specimens studied expressed the αvβ6 integrin receptor. These cancers also retained expression of αvβ6 during metastasis, further substantiating this receptor’s potential for therapeutic targeting [35].
Additionally, the peptide sequence arginine-glycine-aspartic acid (RGD) has been effective in delivering drugs and therapies via targeting the αvβ6 receptor [27,28]. The drugs and therapies that have been tagged to integrin receptor peptides include targets for photodynamic therapy [26-28] and ProAgio [29]. Turaga et al. utilized the conjugated ProAgio to target integrin ανβ3 and induce apoptosis of pancreatic stellate cells. The complex was also capable of opening collapsed tumor vessels, enabling Gemcitabine delivery into the tumor [29]. In a mousemodel, this regimen demonstrated enhanced survival rate over the use of ProAgio or Gemcitabine alone [29]. Studies have also demonstrated effective and preferential binding of the peptide to the integrin receptor regardless of the type of drug conjugate.
Receptor-mediated endocytosis is the mechanism behind the internalization of the drug-peptide conjugate. The integrin receptor predominantly activates clathrin-dependent endocytosis, where the binding of RGD to the integrin receptor stimulates the creation of a clathrin-coated pit [36], which is then hydrolyzed from the cell membrane via the dynamin GTPase [37]. Upon internalization, the therapy is then successfully delivered to various cytoplasmic organelles. Certain drugs may require nuclear localization sequences, which help with traversing the nuclear envelope. ProAgio, a peptide vehicle conjugated to Gemcitabine (or another chemotherapeutic), targets the conjugated ανβ3 integrin receptor to activate clathrin-dependent endocytosis [9]. Next, the binding of RGD to the integrin receptor stimulates the creation of a clathrin-coated pit [36], which is hydrolyzed from the cell membrane via the dynamin GTPase [37]. The vesicle then trafficks the therapy to an appropriate cytoplasmic organelle or in the case of Gemcitabine, to the nucleus, where it exerts its chemotherapeutic effects inhibiting DNA synthesis. See Figure 2 for a demonstration of this mechanism.
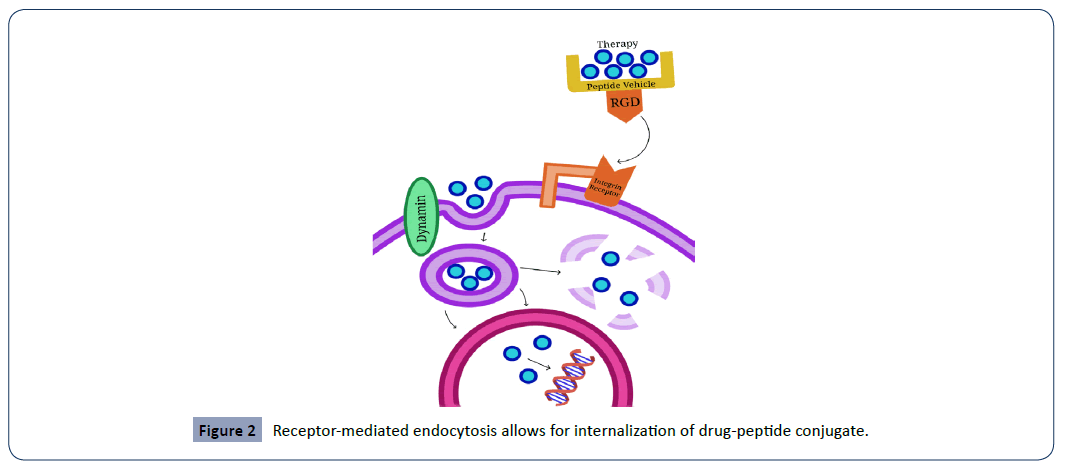
Figure 2: Receptor-mediated endocytosis allows for internalization of drug-peptide conjugate.
Despite the successful therapeutic use of the integrin receptor in animal studies, integrin receptor peptide-drug conjugate therapy has only been investigated in a few phase II clinical trials for PDAC patients, with limited scope. One such study analyzed Volociximab (an anti-α5β1 antibody) with gemcitabine in 16 PDAC patients and showed a partial response in one patient and only half of patients showed short-term stable disease (SD) with a median survival of only 9.6 months [38]. Due to its small sample size and short SD, this study needs further substantiation to draw a useful conclusion of therapeutic efficacy in PDAC patients [38]. However, phase II clinical trials with inhibitors targeting ανβ3 to treat highly angiogenic glioblastomas have shown some therapeutic benefits [39] and promise in patients with late-stage glioblastomas [40]. Thus, further investigation should be conducted to assess its potential use for peptide-drug conjugation in pancreatic cancer patients. A 2015 review concluded that anti-ανβ3 or anti-αν integrin agents may have improved efficacy in PDAC patients and should be pursued further [41]. Table 1 provides a condensed overview of common peptide-drug conjugates currently under clinical trial investigation for cancer treatment.
Table 1 Common Antineoplastic Peptide-Drug Conjugates and Associated Clinical Trials.
| Peptide Vehicle |
Targeted Receptor |
Conjugation to Chemotherapy Drug(s) |
Current Clinical Trials |
Clinical Trial Phase |
| RGD |
Integrin ανβ3 [64] |
CPT [64] |
- |
- |
| iRGD |
Integrin ανβ3/ ανβ5 [64], NRP-1 [65] |
PTX [65] |
- |
- |
| octreotide |
SSTR2/5 [64] |
PTX [64] |
- |
- |
| D-Lys6-LHRH |
LHRH-R [64] |
Dox (SM), CPT [64] |
Advanced LHRH-receptor-expressing solid tumors [66] |
3 |
| Angiopep-2 |
LRP-1[64] |
PTX (SM) |
Metastatic breast cancer [67] |
2 |
| GE11 |
ErbB1 (EGFR) [64] |
Se NPs [68], PEG [69] |
- |
- |
| GnRH |
Type II GnRH-R [64] |
Sunitinib [70] |
- |
- |
| SST |
SSTR1-5 [64] |
CPT [64] |
- |
- |
| CNGRCG |
CD13 receptor [64] |
hTNFα (Protein) [64] |
Malignant pleural mesothelioma [71] |
3 |
| Polyglutamic acid |
- |
PTX (SM) [64] |
Non-small cell lung cancer [72] |
3 |
| LHRH |
LHRH-H [64] |
CLIP71 (lytic peptide) [64] |
Advanced, LHRH-receptor-expressing solid tumors [73] |
1 |
| DRDDS (spacer) |
Folate receptor [64] |
DAVBLH (SM) [64] |
Epithelial ovarian cancer [74] |
3 |
| D-γ-E-γ-E-γ-E-E (masking moiety) |
PSMA [64] |
12ADT-Asp [64] |
Advanced solid tumors [75] |
2 |
Other notable peptide receptors: Additional small peptides are also noteworthy for their potential therapeutic uses in PDAC cells, including Somatostatin, Bombesin, and Neurotensin. These small peptides can penetrate tumors faster than monoclonal antibodies, but have a short biological half-life and lose binding affinity upon coupling with a chelator [24]. Additionally these peptides can be utilized in diagnostic imaging as radiopharmaceuticals that bind to Bombesin receptors have shown promise in non-invasive diagnosis and radiotherapy of GRP receptor positive pancreatic tumors [24]. Valkema et al. conducted a promising study where 58 gastroenteropancreatic neuroendocrine tumor patients were treated with escalating doses of a radiolabeled somatostatin analog; 57% of patients showed some degree of response to therapy and benefitted from a longer overall survival [42]. In sum, more clinical trials must be conducted to evaluate the use of these peptides in PDAC patients.
Peptides’ role in immunomodulation
In addition to enhancing the delivery and efficacy of traditional chemotherapeutics via conjugation, peptides and proteins can also be targeted to condition and modify the body’s intrinsic immune response to combat pancreatic cancer.
The development of therapeutic vaccines for human cancers is not a new concept. The most well-known studies in this area have come from breakthroughs in the treatment of human papillomavirus (HPV) related cervical cancers [43]. For example, the fusion protein vaccine TA-CIN was designed to target the E7 protein, an important structural and oncogenic component of HPV. TA-CIN does so by eliciting a robust host CD8+ T cell response [44]. It is important to note that these therapeutic vaccines are of different nature than preventive vaccines. Preventive vaccines for human cancers are currently only available for HPV-related cervical cancer [45] and HBV-related hepatocellular carcinoma [46]. Our discussion today will be focused on the development of therapeutic vaccines for pancreatic cancer.
One of these regimens currently under active clinical investigation for pancreatic cancer is a dendritic cell (DC)-based vaccine targeting MUC1 [47]. MUC1, mentioned previously regarding its use in imaging, is a transmembrane mucin protein seen in over 90% of all PDAC cells. Its expression levels have also been shown to be linked directly with treatment-resistance metastatic progression via upregulation of drug efflux pumps [48,49].
The MUC1 glycosylated transmembrane mucin protein and other mucins present on the surface of PDAC cells contribute to the chemotherapeutic resistance of the tumor by upregulating p-glycoprotein or P-gp, a known drug efflux pump [49]. This leads to resistance to chemotherapy drugs (e.g. Paclitaxel-PTX) and poor prognosis [48]. DC-based vaccines are designed to elicit a CD8 T cell response specific to mucins such as MUC1, thus enhancing immune-mediated tumor killing [52]. Figure 3 demonstrates of the mechanism of the vaccine. In several phase I/II studies, this vaccine was investigated to be safe and elicited a strong MUC1-specific response, albeit without clear effects on clinical significance [50,51]. In one study conducted by Lepisto et al., twelve patients with advanced PDAC were vaccinated after surgical resection, and four out of the twelve survived to the 5th year post-surgery [52]. If such results can be replicated in larger scale studies with consistency, these DC-based vaccines could become a powerful treatment tool when used in conjunction with surgical resection and traditional chemotherapy. One other such DCbased vaccine targets mesothelin (MSLN), a surface glycoprotein involved in cellular adhesion that is often overexpressed in PDAC [53]. Miyazawa et al. investigated the vaccine and found that it activated both CD4 and CD8 T cell responses against the tumor, although further studies are needed to evaluate the vaccine’s clinical significance and long-term mortality benefit [54]. A 2018 Japanese study also investigated a DC-based vaccine targeting the Wilms’ Tumor 1 (WT1) antigen of pancreatic cancer. They determined in the phase I trial that the vaccine was safe and that seven out of eight patients demonstrated WT-1 specific CD8 T cell response [55]. Guang et al. manipulated DCs in a different manner to induce cytotoxic T cell response [56]. They configured DCs to present KRAS mutant peptides with cationic nanoparticles and found that the resulting CD8 T cells showed specific killing activity towards pancreatic cells expressing the KRAS mutant in mice.
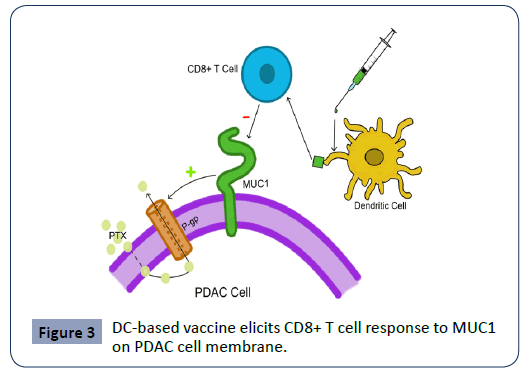
Figure 3: DC-based vaccine elicits CD8+ T cell response to MUC1 on PDAC cell membrane.
Other immunogenic peptide targets that are under investigation for therapeutic vaccine development in include telomerase peptide GV1001 [57], vascular endothelial growth factor receptor 1/2 [58] and survivin, a class of apoptosis inhibitors [10,59]. Interestingly, patient with PDAC immunized with the survivin- 2B vaccine after surgical resection lived for 12 years, an extreme rarity given the traditionally poor prognosis of the cancer. Cases like these call for further investigation into the development of therapeutic peptide vaccines for pancreatic cancer. When used in conjunction with surgical resection and traditional chemotherapy, peptide vaccines may deliver promising results and inspire new treatment regimens.
Direct peptide targeting of integral tumor functions
Peptides and proteins that are integral to the survival and proliferation of the pancreatic cancer cells can also be targeted directly to inhibit their activity and functioning.
ADAM8 is a metalloprotease disintegrin that is crucial in PDAC cell migration and invasion. Its expression is linked with poor prognosis [60]. ADAM8, once multimerized, is shown to increase metalloproteinase (MMP) activity and interact with B1 integrin to faciliatate PDAC invasion. Schlomann et al. demonstrated that BK-1361, a peptide multimerization inhibitor of ADAM8, resulted in reduced invasiveness and metastasis of the cancer cells in mice, a mechanism further elucidated in Figure 4. ADAM8 is a MMP disintegrase that becomes active via multimerization at the cell membrane [60]. ADAM8 facilitates PDAC invasion and metastasis by increasing MMP activity and interacting with B1 integrin. MMPs remodel surrounding ECM to facilitate tumor growth [63]. BK-1361 is a peptide inhibitor of ADAM8 multimerization and is shown to reduce pancreatic cancer cell invasion in mice [60]. Lu et al. also explored the topic of MMP-2 peptide inhibitors, stating that peptides MS204C4 and M205C4 inhibited invasion of pancreatic cancer cells in vitro [13].
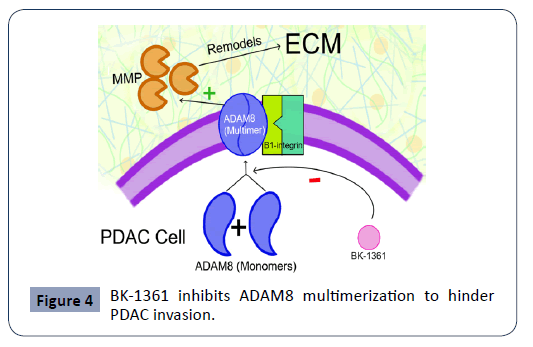
Figure 4: BK-1361 inhibits ADAM8 multimerization to hinder PDAC invasion.
Angiogenesis is another process targeted by researchers using peptide therapy. Kern et al found that Troponin I, a peptide that normally functions in muscle cells, contained an active site pTnI that had antiangiogenic properties [12]. At a dose of just 1 ug/ mL, pTnI demonstrated strong inhibition of vascular endothelial growth factor production in PDAC cells in vitro.
The loss of tumor suppressor p16, a cyclin-dependent kinase inhibitor, is observed in many human cancers, including PDAC. Hosotani et al investigated the inactivation of p16 in pancreatic cancer and designed the Trojan p16 peptide, which helped restore p16’s tumor suppressive functions. Using a BxPC-3 tumor model, they demonstrated that the Trojan p16 peptide resulted in significant apoptosis of tumor cells [61].
Certain antimicrobial peptides (AMPs) have also shown promise of anti-tumor activity in vitro. Deslouches et al. show that cationic amphipathic peptides demonstrate activity against both bacterial cell and cancer cell membranes. They posit that this mechanism is due to the high concentration of phosphtatidylserine, a negatively charged phospholipid, on the membrane of cancer cells [11]. The study of AMPs are currently more extensive than that of anti-cancer peptides, and may provide valuable prospects in the development of future peptide-based therapy for pancreatic cancer.
Closing Remarks
The application of peptides and proteomics to the diagnosis, monitoring and management of pancreatic cancer opens a world of possibilities. The versatility of peptides allows researchers to conveniently configure them for their specific needs.
Peptides can be detected as intrinsic diagnostic markers that PDAC cells produce or radiolabeled and paired with novel imaging techniques for more precise monitoring of tumor progression. Traditional chemotherapy drugs such as gemcitabine can be conjugated to peptide vehicles to enhance their permeability of the tumor tissue. These drug-peptide conjugates can be designed to enjoy higher bioavailability, potency as well as decreased side effects due to selective targeting. Therapeutic peptide vaccines are being evaluated in numerous phase I/II trials and have mostly demonstrated to be safe and immunogenic in mice and in vitro [50,51]. These vaccines are often designed to be given in conjunction with traditional chemotherapy postsurgical resection to prevent PDAC recurrence or metastasis [52]. Researchers have also looked into using peptides to directly target vital functions of PDAC cells, including escape of apoptosis [61], membrane stability [11], tissue invasion [60] and angiogenesis [12]. The ongoing COVID-19 pandemic has seen tens of millions of dollars invested into biopharmaceutical research [62-75], which may create a ripple effect that helps advance therapeutic investigations in oncology as well.
Pancreatic cancer may be one of the deadliest and most treatment-resistant neoplasms at present. However, with the advent of novel proteomic diagnostics and innovative peptidebased therapies on the horizon, clinicians will be more confident in combating PDACs. With these new tools, they will be able to catch dysplastic change earlier, closely monitor neoplastic growth and metastasis, and design more powerful and efficacious therapeutic regimens for patients.
Acknowledgements
We would greatly acknowledge the supports from Shenzhen Science and Technology Program (Grant No: KQTD20170810154011370), Xiangtan Institute of Industrial Technology Collaborative Innovation, and Xiangtan Science and Technology.
Conflicts of Interests
The authors have no conflict of interests to declare.
32673
References
- Maitra A, Hruban RH (2008) Pancreatic cancer. Annu Rev Pathol 3: 157-188.
- Chu GC, Kimmelman AC, Hezel AF, DePinho RA (2007) Stromal biology of pancreatic cancer. J Cell Biochem 101: 887-907.
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, et al. (2007) Identification of pancreatic cancer stem cells. Cancer Res 67: 1030-1037.
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, et al. (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1: 313-323.
- Shaib Y, Davila J, Naumann C, El-Serag H (2007) The impact of curative intent surgery on the survival of pancreatic cancer patients: a U.S. Population-based study. Am J Gastroenterol 102: 1377-1382.
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, et al. (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25: 1960-1966.
- Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C (2008) Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 8: 82.
- Li X, Li J, Zhang B, Gu Y, Li Q, et al. (2020) Comparative peptidome profiling reveals critical roles for peptides in the pathology of pancreatic cancer. The International Journal of Biochemistry & Cell Biology 120: 105687.
- Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, et al. (2006) Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother 55: 1294-1298.
- Deslouches B, Di YP (2017) Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget 8: 46635-46651.
- Kern BE, Balcom JH, Antoniu BA, Warshaw AL, Castillo CF (2003) Troponin I peptide (Glu94-Leu123), a cartilage-derived angiogenesis inhibitor: in vitro and in vivo effects on human endothelial cells and on pancreatic cancer. Journal of gastrointestinal surgery 7: 961-969.
- Lu G, Zheng M, Zhu Y, Sha M, Wu Y, et al. (2012) Selection of peptide inhibitor to matrix metalloproteinase-2 using phage display and its effects on pancreatic cancer cell lines PANC-1 and CFPAC-1. Int J Biol Sci 8: 650-662.
- Palazzo L, Roseau G, Gayet B, Vilgrain V, Belghiti J, et al. (1993) Endoscopic ultrasonography in the diagnosis and staging of pancreatic adenocarcinoma. Endoscopy 25: 143-150.
- McCarroll J, Teo J, Boyer C, Goldstein D, Kavallaris M, et al. (2014) Potential applications of nanotechnology for the diagnosis and treatment of pancreatic cancer. Front Physiol 5: 2.
- Gold DV, Goldenberg DM, Karacay H, Rossi EA, Chang CH, et al. (2008) A Novel Bispecific, Trivalent Antibody Construct for Targeting Pancreatic Carcinoma. Cancer Research 68: 4819.
- Fani M, Maecke HR, Okarvi SM (2012) Radiolabeled peptides: valuable tools for the detection and treatment of cancer. Theranostics 2: 481-501.
- Suh H, Pillai K, Morris DL (2017) Mucins in pancreatic cancer: biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am J Cancer Res 7: 1372-1383.
- Trehoux S, Lahdaoui F, Delpu Y, Renaud F, Leteurtre E, et al. (2015) Micro-RNAs miR-29a and miR-330-5p function as tumor suppressors by targeting the MUC1 mucin in pancreatic cancer cells. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1853: 2392-2403.
- Zhang Y, Sun L (2020) Sweetening the Deal: Glycosylation and its Clinical Applications. Journal of Biomedical Sciences 9: 3-9.
- Potjer TP, Mertens BJ, Nicolardi S, van der Burgt YEM, Bonsing BA, et al. (2016) Application of a serum protein signature for pancreatic cancer to separate cases from controls in a pancreatic surveillance cohort. Transl Oncol 9: 242-247.
- Han S, Jin G, Wang L, Li M, He C, et al. (2014) The role of PAM4 in the management of pancreatic cancer: diagnosis, radioimmunodetection, and radioimmunotherapy. Journal of immunology research 2014: 268479.
- Zhang L, Sanagapalli S, Stoita A (2018) Challenges in diagnosis of pancreatic cancer. World J Gastroenterol 24: 2047.
- Okarvi SM (2008) Peptide-based radiopharmaceuticals and cytotoxic conjugates: Potential tools against cancer. Cancer Treat Rev 34: 13-26.
- Shi X, Shi C, Ye W, Wang L, Zhan Y, et al. (2020) Targeted Fluorescence Imaging and Biological Effects of Peptide Conjugated Quantum Dots on Pancreatic Cancer Cells. Journal of nanoscience and nanotechnology 20: 1351-1357.
- Gao D, Gao L, Zhang C, Liu H, Jia B, et al. (2015) A near-infrared phthalocyanine dye-labeled agent for integrin αvβ6-targeted theranostics of pancreatic cancer. Biomaterials 53: 229-238.
- Li MM, Cao J, Yang JC, Shen YJ, Cai XL, et al. (2017) Effects of arginine-glycine-aspartic acid peptide-conjugated quantum dots-induced photodynamic therapy on pancreatic carcinoma in vivo. Int J Nanomedicine 12: 2769-2779.
- Li S, Yang J, Lei X, Zhang J, Yang H, et al. (2016) Peptide-Conjugated Quantum Dots Act as the Target Marker for Human Pancreatic Carcinoma Cells. Cell Physiol Biochem 38: 1121-1128.
- Turaga RC, Sharma M, Mishra F, Krasinskas A, Yuan Y, et al. (2020) Modulation of Cancer-associated Fibrotic Stroma by An Integrin α(v)β(3) Targeting Protein for Pancreatic Cancer Treatment. Cell Mol Gastroenterol Hepatol.
- Lambert A, Schwarz L, Borbath I, Henry A, van Laethem JL, et al. (2019) An update on treatment options for pancreatic adenocarcinoma. Ther Adv Med Oncol 11: 1758835919875568.
- Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, et al. (2016) Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 34: 2541-2556.
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, et al. (2019) SEER Cancer Statistics Review 1975-2017. National Cancer Institute.
- Ryan DP, Hong TS, Bardeesy N (2014) Pancreatic adenocarcinoma. N Engl J Med 371: 1039-1049.
- Wang S, Liu S, Zhang Y, He J, Coy DH, et al. (2020) Human Serum Albumin (HSA) and Its Applications as a Drug Delivery Vehicl. Health Science Journal 14: 1-8.
- Reader CS, Vallath S, Steele CW, Haider S, Brentnall A, et al. (2019) The integrin alphavbeta6 drives pancreatic cancer through diverse mechanisms and represents an effective target for therapy. J Pathol 249: 332-342.
- Zhao J, Santino F, Giacomini D, Gentilucci L (2020) Integrin-Targeting Peptides for the Design of Functional Cell-Responsive Biomaterials. Biomedicines 8: 307.
- Bridgewater RE, Norman JC, Caswell PT (2012) Integrin trafficking at a glance. J Cell Sci 125: 3695-701.
- Valle JW, Ramanathan RK, Glynne-Jones R, Anthoney A, Berlin J, et al. (2006) Phase II study of volociximab (M200), an α5β1 anti-integrin antibody in metastatic adenocarcinoma of the pancreas (MPC). Journal of Clinical Oncology 24: 4111-4111.
- Alday-Parejo B, Stupp R, Ruegg C (2019) Are Integrins Still Practicable Targets for Anti-Cancer Therapy? Cancers (Basel) 11.
- Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10: 9-22.
- Oron Y (2015) Integrin-based therapy of pancreatic adenocarcinoma: current status and future perspectives. Minerva Gastroenterologica E Dietologica 16: 71-86.
- Valkema R, Pauwels S, Kvols LK, Barone R, Jamar F, et al. (2006) Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 36: 147-56.
- Nayereh KG, Khadem G (2012) Preventive and Therapeutic Vaccines against Human Papillomaviruses Associated Cervical Cancers. Iran J Basic Med Sci 15: 585-601.
- de Jong A, O'Neill T, Khan AY, Kwappenberg KMC, Chisholm SE, et al. (2002) Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine 20: 3456-3464.
- Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Aranda C, et al. (2011) HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 365: 1576-1585.
- Chang MH (2009) Cancer prevention by vaccination against hepatitis B, in Cancer Prevention II. 2009, Springer. p. 85-94.
- Kajihara M, Takakura K, Kanai T, Ito Z, Matsumoto Y, et al. (2016) Advances in inducing adaptive immunity using cell-based cancer vaccines: Clinical applications in pancreatic cancer. World J Gastroenterol 22: 4446- 4458.
- Torres MP, Chakraborty S, Souchek J, Batra SK (2012) Mucin-based targeted pancreatic cancer therapy. Curr Pharm Des 18: 2472-2481.
- Jin W, Liao X, Lv Y, Pang Z, Wang Y, et al. (2017) MUC1 induces acquired chemoresistance by upregulating ABCB1 in EGFR-dependent manner. Cell Death Dis 8: e2980.
- Ramanathan RK, Lee KM, McKolanis J, Hitbold E, Schraut W, et al. (2005) Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother 54: 254-264.
- Pecher G, Häring A, Kaiser L, Thiel E (2002) Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial. Cancer Immunol Immunother 51: 669-673.
- Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, et al. (2008) A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther 6: 955-964.
- Hassan R, Thomas A, Alewine C, Le DT, Jaffee EM, et al. (2016) Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J Clin Oncol 34: 4171-4179.
- Miyazawa M, Iwahashi M, Ojima T, Katsuda M, Nakamura M, et al. (2011) Dendritic cells adenovirally-transduced with full-length mesothelin cDNA elicit mesothelin-specific cytotoxicity against pancreatic cancer cell lines in vitro. Cancer letters 305: 32-39.
- Yanagisawa R, Koizumi T, Koya T, Sano K, Koido S, et al. (2018) WT1-pulsed dendritic cell vaccine combined with chemotherapy for resected pancreatic cancer in a phase I study. Anticancer Res 38: 2217-2225.
- Tan G, Wang Z, Zhang X, Cai Z, Zhang J (2011) Induction of CTLs by DCs pulsed with K-ras mutant peptide on the surface of nanoparticles in the treatment of pancreatic cancer. Oncology reports 26: 215-221.
- Bernhardt SL, Gjertsen MK, Trachsel S, Møller M, Eriksen JA, et al. (2006) Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. British journal of cancer 95: 1474-1482.
- Suzuki N, Hazama S, Iguchi H, Uesugi K, Tanaka H, et al. (2017) Phase II clinical trial of peptide cocktail therapy for patients with advanced pancreatic cancer: VENUS‐PC study. Cancer Sci 108: 73-80.
- Shima H, Kutomi G, Satomi F, Imamura M, Kimura Y, et al. (2018) Case report: long-term survival of a pancreatic cancer patient immunized with an SVN-2B peptide vaccine. Cancer Immunology, Immunotherapy 67: 1603-1609.
- Schlomann U, Koller G, Conrad C, Ferdous T, Golfi P, et al. (2015) ADAM8 as a drug target in pancreatic cancer. Nat Commun 6: 1-16.
- Hosotani R, Miyamoto Y, Fujimoto K, Doi R, Otaka A, et al. (2002) Trojan p16 Peptide Suppresses Pancreatic Cancer Growth and Prolongs Survival in Mice. Clinical Cancer Research 8: 1271.
- Zhang Y, Nguyen A, Chun M, Lin Z, Ross J, et al. (2020) Ninety Days in: A Comprehensive Review of the Ongoing COVID-19 Outbreak. Health Science Journal 14: 1-13.
- Quintero-Fabián S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V, et al. (2019) Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front Oncol 9: 1370.
- Vrettos EI, Mezo G, Tzakos AG (2018) On the design principles of peptide-drug conjugates for targeted drug delivery to the malignant tumor site. Beilstein J Org Chem 14: 930-954.
- Simon-Gracia L, Hunt H, Scodeller P, Gaitzsch J, Kotamraju VR, et al. (2016) iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes. Biomaterials 104: 247-257.
- Engel JB, Tinneberg HR, Rick FG, Berkes E, Schally AV (2016) Targeting of Peptide Cytotoxins to LHRH Receptors For Treatment of Cancer. Curr Drug Targets 17: 488-494.
- Li F, Tang SC (2017) Targeting metastatic breast cancer with ANG1005, a novel peptide-paclitaxel conjugate that crosses the blood-brain-barrier (BBB). Genes Dis 4: 1-3.
- Pi J, Jiang J, Cai H, Yang F, Jin H, et al. (2017) GE11 peptide conjugated selenium nanoparticles for EGFR targeted oridonin delivery to achieve enhanced anticancer efficacy by inhibiting EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathways. Drug Deliv 24: 1549-1564.
- Genta I, Chiesa E, Colzani B, Modena T, Conti B, et al. (2018) GE11 Peptide as an Active Targeting Agent in Antitumor Therapy: A Minireview. Pharmaceutics 10: 2.
- Argyros O, Karampelas T, Asvos X, Varela A, Sayyad N, et al. (2016) Peptide-Drug Conjugate GnRH-Sunitinib Targets Angiogenesis Selectively at the Site of Action to Inhibit Tumor Growth. Cancer Res 76: 1181-1192.
- Gregorc V, Gaafar RM, Favaretto A, Grossi F, Jassem J, et al. (2018) NGR-hTNF in combination with best investigator choice in previously treated malignant pleural mesothelioma (NGR015): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 19: 799-811.
- Langer CJ, O'Byrne KJ, Socinski MA, Mikhailov SM, Leśniewski-Kmak K, et al. (2008) Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J Thorac Oncol 3: 623-630.
- Curtis KK, Sarantopoulos J, Northfelt DW, Weiss GJ, Barnhart KM, et al. (2014) Novel LHRH-receptor-targeted cytolytic peptide, EP-100: first-in-human phase I study in patients with advanced LHRH-receptor-expressing solid tumors. Cancer Chemother Pharmacol 73: 931-941.
- Graybill WS, Coleman RL (2014) Vintafolide: a novel targeted agent for epithelial ovarian cancer. Future Oncol 10: 541-548.
- Mahalingam D, Wilding G, Denmeade S, Sarantopoulas J, Cosgrove D, et al. (2016) Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br J Cancer 114: 986-994.









