Mercy Aparna L1*, Aparna S1, Sarada I1 and Ram D2
1Blue Peter Public Health & Research Centre (BPHRC), LEPRA Society, Cherlapally, Hyderabad, India
2National Institute of Malaria Research (ICMR) Sector -8, Dwarka, New Delhi -110077, India
Corresponding Author:
Mercy Aparna L
MARUTERU- 534122, W.G. District, Andhra Pradesh, India
Tel: +91 9654425774
E-mail: mercy_aparna@yahoo.co.in
Received Date: July 24, 2017; Accepted Date: July 26, 2017; Published Date: July 30, 2017
Citation: Mercy Aparna L, Aparna S, Sarada I, Ram D (2017) Assessment of Sputum Quality and Its Importance in the Rapid Diagnosis of Pulmonary Tuberculosis. Arch Clin Microbiol. Vol. 8 No. 4:53. doi: 10.4172/1989-8436.100052
Copyright: © 2017 Mercy Aparna L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: Sputum smear microscopy is the gold standard
method for the diagnosis of pulmonary tuberculosis. Poor
quality of sputum might result in missing the diagnosis. In
the present study, we aimed at assessing the microscopic
quality of sputum with reference to the number of pus cells
and squamous epithelial cells observed on Gram’s stained
smears as an indirect marker.
Methods: A total number of 200 sputum specimens were
collected from 91 suspected TB patients and were subjected
to ZN staining, LJ culture to out the acid fast bacilli; gram's
staining and Blood agar culture for lower respiratory tract
infectious organisms.
Results: Of 200 specimens, 59 from 28 patients were
positive for acid fast bacilli. Based on the number of pus
cells and squamous epithelial cells, specimens were
categorized into four groups. Group 1 (sputum) was
significantly associated with ZN smear positivity and Group
4 (saliva) with smear negativity (p<0.001). The specimens
with >25 pus cells per field (including group 1&2) were
significantly associated with ZN and LJ culture positivity
(p<0.001), whereas the presence of >10 SE cells was not
associated with smear negativity (p>0.1).
Conclusion: Group 1 (sputum) was associated with ZN
smear positivity and Group 4 (saliva) was associated with
smear negativity. Pus cells were proved as an indirect
marker for sputum quality assessment. Sputum samples
with more number of pus cells (regardless of the group)
were significantly associated with ZN and LJ culture
positivity. So, proper patient education before obtaining the
sputum sample and timely monitoring during collection
time can be helpful to obtain good quality sputum to
enhance the TB disease diagnostic yield.
Keywords
Tuberculosis; Sputum quality; Smear examination; Microscopy
Introduction
Tuberculosis (TB) is one of the highly infectious diseases across the world with 10.4 million incidences and 1.4 million deaths [1]. In India, 2.8 million TB cases were occurred [1]. Microscopic examination of sputum smears has been the mainstay of diagnosis in DOTS (Directly Observed Treatment Short course) programme in India. Despite of DOTS strategy for early diagnosis and improved case detection, half of the active TB cases are found to be negative by sputum smear examination [2]. Diagnosis of these cases has been done based on either clinical or radiological symptoms. Moreover, sputum smear results form a critical base for diagnosis and treatment follow-up of TB including the decision of cure. However, microscopy has been known to be less sensitive as compared to other techniques such as culture due to inherent reasons, and the presence of few bacilli too less to detect or inadequate specimen quality. Hence, it is essential to improve the sensitivity of this simple and important technique. In the current DOTS guidelines, specimen quality is being judged based on macroscopic observation of the sputum [3]. Previous reports on the utility of microscopic examination for judging the sputum quality was meant for cultures [4]; more so, while diagnosing lower respiratory tract infections such as pneumonia [5]. These studies were based on the number and proportion of inflammatory/pus cells (PC) and squamous epithelial cells (SEC) as sputum quality indicators. This quantification has been shown to provide an estimate of the oropharyngeal contamination of sputum as a surrogate marker for the specimen quality [6]. However, there is limited information on applying such criteria during sputum smear microscopy for TB [4]. Hence our study aimed to assess the microscopic quality of sputum on the smear microscopy results under a DOTS clinic setting.
Methods
Sputum specimens collected from suspected TB patients attending a semi-urban DOTS Clinic (Designated Microscopy Centre-DMC) at Hyderabad, between July and Oct 2007, were studied. Three consecutive samples from each patient (spot, home and spot: sputum sample collected at the clinic is referred to as the spot sample and the early morning sputum sample collected at home on 2nd day called as home sample) were collected over two days as per the Revised National Tuberculosis Control Programme (RNTCP) guidelines [3]. All the samples were subjected to routine Ziehl Neelsen (ZN) staining and culturing on Lowenstein Jensen’s (LJ) media as recommended [3]. In addition to this, Gram’s staining was carried out separately to enumerate the presence and proportion of PC and SEC as indicators to assess sputum quality and to find out the organisms involved [7]. All sputum specimens were also subjected to Blood agar medium to find out the presence of gram positive, gram negative and fastidious microorganisms as well. In brief, Gram’s stained sputum smears were examined under the 10X objective; the average number of PC and SEC from three consecutive fields was recorded [8]. Sputum smear having an average number of <10 SEC and >25 PC/ field was considered as good quality and smear with >10SEC and <25 PC/ field was treated poor quality. SPSS software (Version 20) was used for data analysis to find out the correlation and significance.
Results
A series of 200 sputum specimens were collected from 91 suspected TB patients (39 patients provided three samples, 31 patients provided two samples and 21 provided only one sample). Out of 200 specimens, 67 were from home and 133 were spot samples. Out of 200, 59 sputum samples from 28 patients were positive for acid fast bacilli. Of 59 positives, 20 were home (34%) and 39 from spot specimens (66%). All ZN smear positives were also positive on LJ culture.
Based on the number of PC and SEC, specimens were categorized into four groups ranging from sputum (Group 1) to saliva (Group 4) as described in Table 1. Of 200 samples, 56 were from Group 1 and among them 25 (43%) were ZN as well as LJ culture positive and remaining samples were negative. In Group 2 (MEMP), out of 61 specimens, 23 showed ZN smear and culture positivity. Thirty three lies in group 3 (LELP) and of them only five were positive to ZN and the rest were negative. Group 4 (saliva) results revealed that out of 50 samples, six were ZN positive and 44 were ZN negative. Group 1 considered to be good quality sputum and Group 4 as poor quality of sputum (Figure 1). The statistical analysis revealed that Group 1 was significantly associated with ZN smear positivity, whereas Group 4 with smear negativity (p<0.001). The specimens with >25 pus cells per field (group 1&2) were significantly associated with ZN and LJ culture positivity (p<0.001), whereas the presence of >10 SE cells was not associated with smear negativity (p>0.1) (Table 2). There was no association found between smear positivity and site of collection (p>0.1).
| Group |
No. of specimens N=200 |
ZN positive |
ZN negative |
| 1 |
56 |
25 |
31 |
| 2 |
61 |
23 |
38 |
| 3 |
33 |
5 |
28 |
| 4 |
50 |
6 |
44 |
| Total |
200 |
59 |
141 |
Group 1- Sputum (Smears with average no of<10 SEC and >25PC),
Group 2- MEMP (More SEC and more PCs, >10 SEC and>25 PC),
Group 3- LELP (Low SEC and PC, <10 SEC and < 25PC),
Group 4- Saliva ( smears having >10 SEC and<25 PC)
Table 1: Categorization of sputum quality in context of ZN smear microscopy.
| N=164 |
ZN Positive N=55 |
ZN Negative N=109 |
Grand Total |
| Number of Pus cells (PC)(p<0.001) |
| <25 |
11 |
47 |
58 |
| >25 |
44 |
62 |
106 |
| Squamous epithelial cells (p>0.1) |
| <10 |
28 |
40 |
68 |
| >10 |
27 |
69 |
96 |
>25PC: Associated with ZN smear & culture positivity
>10SEC: Not associated with ZN smear & culture negetivity
Table 2: Correlation of PCs and SECs with ZN smear positivity.
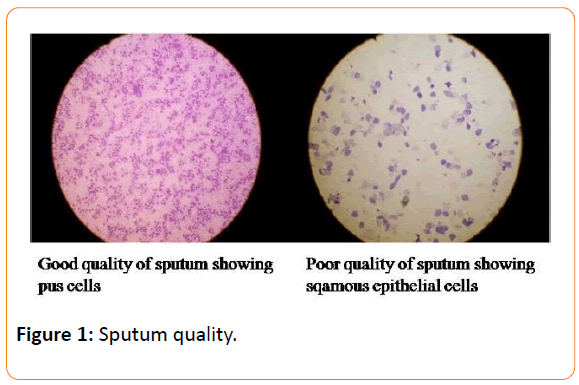
Figure 1: Sputum quality.
Moreover, gram staining results revealed that the presence of mixture of gram positive, gram negative cocci or bacilli. All the sputum samples were further cultured on blood agar media and out of 200 specimens, 195 were grown on blood agar culture. Blood agar culture has revealed the presence of the mixture of different bacteria with various morphological types i.e., streptococcus, staphylococcus, diplococcus, and some hemolytic streptococcus. Interestingly, gram positive coccobacilli were significantly associated with group 1 and 2 (p<0.001).
Discussion
Sputum smear microscopy is rapid and cost effective method for diagnosis of pulmonary TB and the accuracy of this test lies in the quality of the sputum provided by the suspected TB patient. In a recent systemic review on sputum quality assessment has revealed that sputum quality assessments are urgently needed to enhance the diagnostic yield and as well as to prevent the wasteful testing’s [9,10]. In the present study, we assessed the sputum quality with regard to smear positivity. Current guidelines follow the microscopic observation of specimen quality as part of the routine sputum examination [3]. However, this exercise vowing to its subjective nature may not reflect the actual specimen quality required for detection of acid fast bacilli. There are some existing reports on the sputum quality and bacteriological yield in the diagnosis of lower respiratory infections such as pneumonia [5]. According to them, the number and proportion of PC and SEC on Gram stained smears had considered as the lower respiratory tract secretions and oropharyngeal contamination respectively. Murray and Washington described the criteria for good sputum quality based on the number of SEC [6]. However, Van Scoy considered the criterion of more than 25 leucocytes regardless the number of epithelial cells per LPF (low power field) to specify the sputum quality [8]. Present study adapted both the criteria to evaluate the sputum quality for microscopic examination of acid fast bacilli. Our findings are in conformity with those of Van Scoy [8].
Furthermore, enriched medium like blood agar medium has revealed that gram positive coccobacilli were significantly associated with Group 1 and 2; these two groups have more than 25 pus cells. Similarly, a case study from India reported that the presence of gram positive coccobacilli in a person with cough and fever [9]. We did not go for further identification of the organism because it deviate the study objectives (Figure 1).
In the current study, a considerable number of patients were negative to ZN smear microscopy. If they are smear negative symptomatic cases, they might have missed the microscopic diagnosis probably due to the bad quality of sputum. Apart from this, the current nucleic acid amplification tests such as Xpert MTB/RIF, Line Probe assay etc. are sputum based tests used for rapid TB diagnosis and detection of multi drug resistant TB (MDR TB) patients [10]. It indicates that macroscopic assessment of sputum quality during specimen collection time could facilitate to obtain good sputum quality by repeating the sputum collection at that moment.
Moreover, specimens from middle age group yielded good quality sputum as compared to those from both the extremes (data not shown). There was no significant difference in the positivity between the home and spot collected specimens. It is noteworthy that all ZN smear positives were also positive on LJ culture, and it indicates that the maintenance of standard operational procedure (SOP) would have helped to get accurate results.
Conclusion
In conclusion, Group 1 (good quality sputum) was associated with ZN smear positivity and group 4 (saliva) was associated with smear negativity. Pus cells were proved as an indirect marker for sputum quality assessment than squamous epithelial cells. Sputum samples with more number of PC (regardless of the group) field were significantly associated with ZN and LJ culture positivity. There was no significant association was found between SEC and smear negativity. So, this study emphasizes the need of proper patient education before obtaining the sputum sample and timely monitoring during collection time can be helpful to obtain good quality sputum to enhance the diagnostic yield of sputum based molecular diagnostic tests to facilitate timely treatment which is the main goal of TB control program.
Acknowledgements
I acknowledge Blue Peter Public Health & Research Center (BPHRC), LEPRA Society, Hyderabad for providing patient’s samples and funding this study.
Authors Contributions
M.A.L.L. contributed in patient sample collection, clinical testing’s, data analysis, interpretation and writing the article. A.S. contributed the study design and manuscript correction. S.I. cross-checked the microscopic slides. R.D. contributed in critical reviewing of the article.
19954
References
- Deun AV, Roorda FA, Chambugonj N, Hye A, Hossain (1999) Reproducibility of sputum smear examination for acid-fast bacilli: practical problems met during cross-checking. Int J Tuberc Lung Dis 3: 823–829.
- Manual for laboratory technicians (2005) Revised National Tuberculosis control Programme (RNTCP). Central TB division 121.
- Curione CJ, Kaneko GS, Voss JL, Hesse F, Smith RF (1977) Gram stain evaluation of the quality of sputum specimens for mycobacterial culture. J ClinMicrobiol 5: 381-382.
- Tebbutt GM, Coleman DJ (1978) Evaluation of some methods for the laboratory examination of sputum. J ClinPathol 31: 724-729.
- Martin RS, Sumarah RK, Robart EM (1978) Assessment of expectorated sputum for bacteriological analysis based on polymorphs and squamous epithelial cells: six-month study. J ClinMicrobiol 8: 635-637.
- Forbes BA, Sahm DF, Weissfeld AS (2002) Infections of the lower respiratory tract. In: Diagnostic microbiology. Bailey and Scott (eds) St Louis Mosby 12: 896.
- Wong LK, Barry AL, Horgan SM (1982) Comparison of six different criteria for judging the acceptability of sputum specimens. J ClinMicrobiol 16: 627-631.
- SupriyaKoyal, Urvi Patel, Pinkson GR, Rodriguez M (2007) 40-Year-Old Man with Cough and Fever. Clin Infect Dis 44: 1518-1519.
- Pai M, Schito M (2015) Tuberculosis diagnostics in 2015: Landscape, priorities, needs, and prospects. J Infect Dis 211: S21-S28.






