Keywords
Lichen, Cladonia ochrochlora, Parmotrema nilgherrensis, Parmotrema sancti-angelii, Secondary compounds, Bactericidal activity
Introduction
Resistance of microorganisms to many standard antibiotics pose serious threats to human health and creates huge medical problem in the treatment of infectious diseases. Therefore, new sources of bioactive substances have been searched for, such as medicinal herbs, fungi and lichens [1]. Some of the most successful antibiotics ever been discovered to combat disease are the small molecules or secondary compounds. Lichen is a self-supporting symbiotic organism composed of a fungus and an algal partner. Lichens have been used for medical purposes since ancient times and are known to produce unique secondary compounds. These secondary compounds are depsides, depsidones, dibenzofurans and pulvinic acid derivatives having low molecular weight [2]. These chemically diverse secondary compounds are unique to lichen symbioses and help the organism to survive in extreme environmental conditions [3].
Lichen secondary compounds are reported for manifold biological activities like antiviral, antibiotic, antioxidant, antitumor, allergenic and inhibition of plant growth [4-8]. Therefore, these compounds attracted great attention of investigators due to their marked bactericidal activity as a new significant source of antimicrobial agents [9-12]. Some of the lichen compounds fumarprotocetraric acid isolated from Cladonia furcata, gyrophoric acid from Umbilicaria polyphylla, lecanoric acid from Ochrolechia androgyna, physodic acid from Hypogymnia physodes, protocetraric acid from Flavoparmelia caperata, stictic acid from Xanthoparmelia conspersa and usnic acid from Flavoparmelia caperata, Usnea ghattensis are come to light having antimicrobial activity. These secondary compounds were also found relatively strong antimicrobial agent against many human, animal and plant pathogens, mycotoxin producers and food-spoilage organisms [13-14, 7]. Till date 1050 lichen secondary compounds are reported so far from different lichen species [15] but the number of known/ explored compounds for the bactericidal activity is still very less. The reason behind the unexploitation of lichens is their slow growth rate (2 mm/yr-1) in natural habitat [16] and requirement of high quantity of natural material for extraction of valuable phytochemicals to study the biological potential in broader spectrum.
With this background information, the aim of the present study was to evaluate the bactericidal activity of some secondary compounds isolated from three different types of lichen species; Cladonia ochrochlora, Parmotrema nilgherrensis and Parmotrema sancti-angelii.
Materials and Methods
Chemicals
Nutrient agar and broth, Muller Hinton agar and broth, sodium chloride, erythromycin, gentamicin, methicillin, streptomycin, tetracycline, vancomycin, nistatin, chloramphenicol, ketoconazole, ampicillin and Silica gel G were purchased from Hi Media Chemicals, India. Methyl thiazolete tetrazolium (MTT) was purchased from Sigma Chemicals, USA. Diethyl ether, acetic acid, toluene, 1-4 Dioxane, ethyl acetate, formic acid, n-hexane, methanol, acetone and sulfuric acid were purchased from Fisher Scientific, India. All other routine chemicals used were of analytical grade.
Lichen material
Fresh thallus of natural fruticose lichen species; Cladonia ochrochlora of Cladoniaceae family and Parmotrema nilgherrensis, Parmotrema sanctiangelii of Parmeliaceae family have been collected from different localities in Karnataka State, India. Collected lichen species were authenticated by Dr. B.O. Sharma, a lichen taxonomist at Agharkar Research Institute. A part of the material of lichen species Cladonia ochrochlora (voucher no. AMH 94.52) producing fumarprotocetraric, hypoprotocetraric, protocetraric acid; Parmotrema nilgherrensis (voucher no. AMH 94.53) producing alectoronic acid, atranorin, α- collatolic acid and Parmotrema sancti-angelii (voucher no. AMH 94.54) producing atranorin, α-collatolic acid & lecanoric acid under natural condition has been preserved as specimen in the Ajrekar Mycological Herbarium (AMH) at Agharkar Research Institute, Pune, India.
Lichen secondary compounds present in the natural thallus of C. ochrochlora, P. nilgherrensis and P. sancti-angelii were identified with routinely used standardized method of TLC [17] using standard solvent system EA (Diethyl ether : Acetic acid; 200 ml : 2 ml); HEF (Hexane : Diethyl ether : Formic acid; 140 ml : 100 ml : 20 ml); TDA (Toluene : 1-4 Dioxane : Acetic acid; 180 ml : 45 ml : 5 ml) and TEF (Toluene : Ethyl acetate : Formic acid; 65.5 ml : 41.5 ml : 4 ml). Authentication of lichen compounds were made by comparison with the standard lichen substances and sample of several species containing atranorin, constictic acid, gyrophoric acid, lecanoric acid, norstictic acid, protocetraric acid, salazinic acid and stictic acid.
Fractionation of lichen thallus
The natural thallus of lichen C. ochrochlora, P. nilgherrensis and P. sancti-angelii were separated from the tree bark and washed by keeping over night under flow of tap water and further by distilled water. The cleaned thallus was cut in to small pieces and then placed in the Soxhlet apparatus for the extraction. Different organic solvents; n-hexane, ethyl acetate, acetone and methanol were used for successive fractionation extraction and resulted fractions were filtered by Whatman filter paper (No.1) and then dried in vacuo. The dry fractions obtained from different solvents n-hexane, ethyl acetate, acetone and methanol were as follows; C. ochrochlora (11.8 mg, 35 mg, 19.1 mg, 42.8 mg) P. nilgherrensis (53.6 mg, 56.3 mg, 4.8 mg, 33.8 mg), P. sancti-angelii (335.2 mg, 201 mg, 12.2 mg, 88 mg). The dry fractions were stored at 4°C for further study.
Determination of bactericidal activity of lichen species
The screening of sensitivity of microorganisms to different organic fractions of C. ochrochlora, P. nilgherrensis and P. sancti-angelii were ascertained by standard disk diffusion method approved by the National Committee for Clinical Laboratory Standard (NCCLS) [18]. Briefly, loop full of the bacterial culture was transferred into 5 ml of Muller Hinton broth and incubated at 37°C for 18-20 hrs till light to moderate turbidity developed. Aliquot of 100 μl of this suspension containing 105-106 cells/ml was spreaded on to Muller Hinton agar medium. The lichen extract (10 μg/ml) was impregnated on small sterile disks of Whatman filter paper No. 1 of 6 mm diameter in size and placed on top of the seeded medium. Individual disks soaked with 10 μl of solvents; n-hexane, ethyl acetate, acetone and methanol were used as negative control. All the petri plates were incubated at 37°C for 24 hrs. After completion of incubation period, the size of zone of inhibition was measured in millimetre and expressed in mean values. Commercially available disk of standard antibiotics erythromycin, gentamicin, methicillin, streptomycin, tetracycline and vancomycin were used as positive control for bacteria and ampicillin, chloramphenicol, ketoconazole, nistatin for yeast strains. The bacterial strains Bacillus cereus (NCIM 2106), Bacillus subtilis (NCIM 2063), Escherichia coli (NCIM 2345), Klebsiella pneumoniae (NCIM 2957), Micrococcus luteus (NCIM 2169), Proteus vulgaris (NCIM 2027), Staphylococcus aureus (NCIM 5021), Streptococcus faecalis (NCIM 5024), Sarcina lutea (NCIM 2103) and yeast strains Candida albicans (NCIM 3471), Cryptococcus var. diffluens (NCIM 3371) were used for determination of bactericidal activity of organic fractions of lichen species and isolated compounds. All the microorganisms were obtained from National Collection of Industrial Microorganisms (NCIM) at National Chemical Laboratory, Pune, India and were maintained on recommended nutrient agar medium.
Isolation and purification of lichen secondary compounds
Crude fraction of lichens contains many lichen substances along with other compounds. However, ethyl acetate fraction of C. ochrochlora, P. nilgherrensis and P. sancti-angelii showed higher bactericidal activity in comparison to other solvent fractions. Based on the screening results we have extracted only lichen secondary compounds alectoronic acid, atranorin, α-collatolic acid, fumarprotocetraric acid, hypoprotocetraric acid, lecanoric acid and protocetraric acid using ethyl acetate from the lichen species and then separated by preparative TLC in TEF solvent (Toluene : Ethyl acetate : Formic acid; 65.5 ml : 41.5 ml : 4 ml). Lichen compounds were purified by eluting several time in the same solvent system and used for further experiments to study their bactericidal effects.
TLC bioautography overlay assay
As the quantity of pure lichen compounds obtained were very less therefore, TLC bioautography overlay assay described by Gibbons & Gray [19] was performed with minor modifications for qualitative determination of bactericidal activity of isolated lichen compounds. Briefly, lichen compounds were developed using TEF solvent on a pre-coated silica gel plates (60F254, Merck Co, USA). The developed TLC plates were sterilized by UV lamp for 30 min before encasing in nutrient agar and then covered with another layer of molted nutrient agar (45°C) containing test microorganisms. After 4 h of diffusion at 4°C, the plates were incubated for 24 h at 37°C and then sprayed with methyl thiazolyl tetrazolium bromide (MTT) (2.5 mg/ml) which converts in to a formazan dye by the test microorganisms. The zone of inhibition observed as a clear spot against purple background.
Minimal inhibitory concentration of lichen compounds
The minimal inhibitory concentrations (MIC) of lichen compounds against test microorganisms were determined by broth dilution method as described by Rankovic et al. [14] with slight modifications. The Muller Hinton broth (2 ml) was inoculated with suspension of test microorganism to give a final concentration of 106cfu/ml. A series of dilution with concentration ranging from 5 μg to 20 μg of lichen compounds were used in MIC experiment. As a positive control of growth inhibition, erythromycin and nistatin were used and for negative control broth media inoculated with each test microorganisms were used. All the test tubes were subsequently incubated at 37°C for 24 h and the turbidity was measured at 600 nm by UV visible spectrophotometer (UV-Vis 1601 Shimadzu, Japan). The MIC values were determined as the lowest concentration of tested compound which prevented the visible growth of microorganisms at 37oC after 24 h.
Kinetic time-kill assay
In order to determine the effective time of lichen compounds to inhibit the microbial growth in vitro, kinetic time-kill assay was performed [20]. Briefly, test tubes containing 2 ml of Muller Hinton broth supplemented with lichen metabolites were inoculated with microorganisms (106cfu/ml) and then incubated at 37°C. A 100 μl of aliquots were removed after post-incubation at 0, 3, 6, 24 h and plated on Muller Hinton agar plates. Inoculated plates were again incubated at 37°C for 24 h and microbial colonies were counted.
Bactericidal potential of lichen compounds under temperature and incubation time period
In order to know the stability of bactericidal activity of lichen compounds at higher temperature and incubation time period, different sets of lichen compounds alectoronic acid, atranorin, α-collatolic acid, fumarprotocetraric acid, hypoprotocetraric acid, lecanoric acid and protocetraric acid were incubated at 4°C, 40°C, 60°C for 1 h, for 30 days at 20°C and then their bactericidal activities were measured. Experiments were performed in duplicate and the average activity of each lichen compound was calculated against all the tested microorganisms.
Statistical analysis: Experimental results were expressed as a mean ± SEM of three parallel measurements.
Results
In the present study various organic fractions and the purified secondary compounds isolated from the lichen species Cladonia ochrochlora, Parmotrema nilgherrensis, Parmotrema sancti-angelii have been screened for the bactericidal potential and the results are presented.
Bactericidal activity of various solvent fractions of lichen species
The bactericidal activity of various solvent fractions n-hexane, ethyl acetate, acetone and methanol of lichen species were investigated using in vitro agar disc diffusion method against B. cereus, B. subtilis, E. coli, K. pneumoniae, M. luteus, P. vulgaris, S. aureus, S. faecalis, S. lutea, C. albicans and C. diffluens. The results in terms of zone of inhibition are presented in Table 1. All the tested fractions showed moderate to strong bactericidal activity against most of the tested microorganisms and the zone of inhibition was found ranging from 7 to 25 mm. The ethyl acetate fraction of P. nilgherrensis showed highest 8 mm to 20 mm zone of inhibition which was found to be higher than the activity shown by other lichen species P. sancti-angelii (8 to 13 mm), C. ochrochlora (8 to 12 mm) and the standard antibiotics erythromycin, gentamicin, methicillin, streptomycin, tetracycline, vancomycin (7 mm to 29 mm). Against all the tested microorganism ethyl acetate fraction of lichen species were found effective. The order of bactericidal activity of lichen species were P. nilgherrensis > P. sancti-angelii > C. ochrochlora.
| Lichen species &   Solvent extracts |
Bacterial strains |
Yeast strains |
| B. cereus |
B. subtilis |
E. coli |
K.
pneumoniae |
M. luteus |
P. vulgaris |
S. aureus |
S. faecalis |
S. lutea |
C. albicans |
C. diffluens |
| Cladonia ochrochlora |
| n-Hexane |
-- |
-- |
-- |
-- |
-- |
-- |
7 |
7 |
-- |
-- |
-- |
| Ethyl acetate |
8 |
10 |
8 |
12 |
10 |
10 |
10 |
8 |
9 |
8 |
9 |
| Acetone |
-- |
-- |
-- |
-- |
-- |
25 |
-- |
-- |
-- |
-- |
-- |
| Methanol |
-- |
7 |
7 |
-- |
7 |
20 |
-- |
-- |
-- |
-- |
-- |
| Parmotrema nilgherrensis |
| n-Hexane |
-- |
-- |
-- |
8 |
-- |
10 |
-- |
7 |
-- |
-- |
-- |
| Ethyl acetate |
11 |
10 |
8 |
11 |
10 |
20 |
8 |
12 |
10 |
12 |
13 |
| Acetone |
7 |
8 |
10 |
-- |
10 |
13 |
10 |
10 |
-- |
7 |
8 |
| Methanol |
11 |
-- |
7 |
-- |
10 |
-- |
10 |
7 |
-- |
7 |
7 |
| Parmoterema sancti-angelii |
| n-Hexane |
-- |
7 |
11 |
-- |
-- |
10 |
7 |
-- |
-- |
7 |
-- |
| Ethyl acetate |
8 |
8 |
-- |
9 |
10 |
13 |
10 |
10 |
10 |
8 |
9 |
| Acetone |
-- |
-- |
-- |
-- |
-- |
7 |
-- |
-- |
-- |
-- |
-- |
| Methanol |
-- |
-- |
-- |
7 |
-- |
-- |
-- |
-- |
-- |
-- |
7 |
| Standard antibiotics |
| Erythromycin |
10 |
25 |
25 |
29 |
22 |
15 |
23 |
17 |
Complete inhibition |
13 |
16 |
| Gentamicin |
7 |
15 |
15 |
10 |
25 |
22 |
20 |
17 |
15 |
15 |
| Methicillin |
-- |
12 |
10 |
20 |
15 |
-- |
20 |
-- |
|
| Streptomycin |
15 |
20 |
10 |
22 |
15 |
-- |
15 |
15 |
| Tetracycline |
15 |
25 |
25 |
25 |
23 |
22 |
23 |
20 |
| Vancomycin |
10 |
20 |
14 |
22 |
14 |
15 |
18 |
15 |
The value of each constituents consisted of ± S.E. of three parallel readings. (--): No inhibition
Bactericidal activity of lichen secondary compounds
Lichen secondary compound alectoronic acid, atranorin, α-collatolic acid, fumarprotocetraric acid, hypoprotocetraric acid, lecanoric acid and protocetraric acid showed bactericidal activity against all the tested microorganisms in TLC bioautography plates with clear zone of inhibition in purple background (Fig. 1). The MIC value for the lichen compounds against the tested microorganisms was found within the range of 5 μg to 740 μg/ml (Table 2). Lichen compounds atranorin, α-collatolic acid and hypoprotocetraric acid were found to be most effective bactericidal agents against ten microorganisms; B. cereus, B. subtilis, E. coli, K. pneumoniae, M. luteus, P. vulgaris, S. aureus, S. faecalis, S. lutea, C. albicans, C. diffluens with MIC value 5 μg to 70.7 μg/ml, 8.6 μg to 245 μg/ml and 12.2 μg to 278.5 μg/ml respectively. Where as, the alectoronic acid was found effective against nine microorganisms B. cereus, B. subtilis, E. coli, K. pneumoniae, M. luteus, P. vulgaris, S. aureus, S. faecalis and C. albicans with MIC value 21.9 μg to 162.1 μg/ml. Fumarprotocetraric acid was found effective against eight microorganisms B. cereus, E. coli, K. pneumoniae, P. vulgaris, S. faecalis, S. lutea, C. albicans, C. diffluens with MIC 24 μg to 227.3 μg/ml and protocetraric acid against seven microorganisms B. cereus, B. subtilis, P. vulgaris, S. aureus, S. lutea, C. albicans, C. diffluens with MIC 18.7 μg to 740.7 μg/ml and lecanoric acid against only six microorganisms B. cereus, B. subtilis, E. coli, S. faecalis, S. lutea, C. diffluens with MIC value 24.6 to 591.7 μg/ml. The order of antimicrobial activity of lichen secondary compounds were found atranorin > α-collatolic acid > hypoprotocetraric acid > alectoronic acid > fumarprotocetraric acid > protocetraric acid > lecanoric acid. The standard antibiotics erythromycin and gentamycin have shown activity with 4 μg to 7.3 μg/ml MIC.
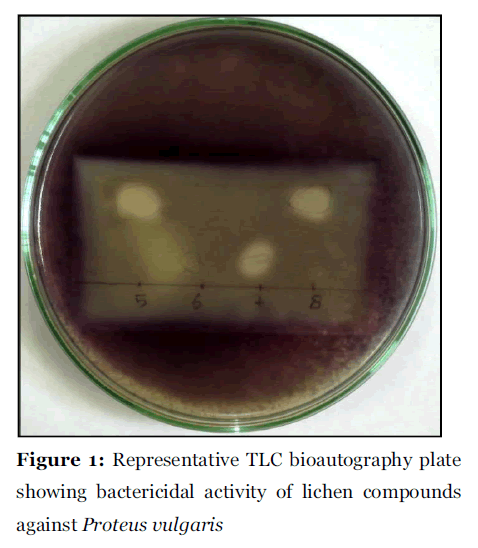
Figure 1:Representative TLC bioautography plate showing bactericidal activity of lichen compounds against Proteus vulgaris.
| Microorganism |
Alectoronic acid |
Atranorin |
a- Collatolic acid |
Fumar- protocetraric acid |
Hypo- protocetraric acid |
Lecanoric acid |
Protocetraric acid |
Erythromycin |
Gentamycin |
| Bacterial strains |
| B. cereus |
162.1 |
- |
245.0 |
59.6 |
255.4 |
26.9 |
85.5 |
4.8 |
4.3 |
| B. subtilis |
98.1 |
70.7 |
46.2 |
- |
53.1 |
24.6 |
740.7 |
4.2 |
5.0 |
| E. coli |
40.0 |
8.3 |
28.1 |
24.7 |
76.7 |
68.0 |
- |
4.7 |
4.6 |
| K. pneumoniae |
54.9 |
13.0 |
34.4 |
142.2 |
90.7 |
- |
- |
4.3 |
5.1 |
| M. luteus |
75.0 |
17.8 |
- |
- |
178.0 |
- |
- |
4.8 |
4.9 |
| P. vulgaris |
59.7 |
5.0 |
21.6 |
24.0 |
12.2 |
- |
23.4 |
5.1 |
4.6 |
| S. aureus |
62.2 |
20.5 |
83.9 |
- |
118.6 |
- |
60.7 |
4.8 |
4.4 |
| S. faecalis |
21.9 |
33.0 |
44.2 |
39.00 |
278.5 |
296.3 |
- |
4.0 |
5.0 |
| S. lutea |
- |
21.5 |
32.1 |
103.6 |
93.8 |
591.7 |
196.0 |
4.6 |
4.5 |
| Yeast strains |
| C. albicans |
22.7 |
17.0 |
8.6 |
227.3 |
- |
- |
18.7 |
5.0 |
4.9 |
| C. diffluens |
- |
15.7 |
51.5 |
24.4 |
110.3 |
50.1 |
53.1 |
5.8 |
5.5 |
*MIC: Minimal inhibitory concentration values given as µg/ml for lichen compounds and standard antibiotics; (-): Not sensitive.
Table 2: Minimal inhibitory concentration (MIC) values of isolated lichen compounds for the bactericidal activity.
Effective time to kill microbes by lichen compounds
Lichen secondary compounds showed maximum inhibition of microbial growth in terms of decrease in the count of colony forming unit (cfu) starting form 0 hrs to 24 hrs incubation period. Lichen compounds were found to be most effective in period of 0 hrs to 6 hrs. Further 6 hrs to 24 hrs no increase in the cfu of microorganisms was observed. From 0 hrs to 24 hrs the cfu count of microorganisms decreased in the presence of lichen compound alectoronic acid 24 - 280 cfu to 7 - 230 cfu/ml (Fig. 2); atranorin 6 - 110 cfu to 2 - 70 cfu/ml (Fig. 3), α-collatolic acid 5 - 200 cfu to 1 - 50 cfu/ml (Fig. 4), fumarprotocetraric acid 5 - 200 cfu to 1 - 80 cfu/ml (Fig. 5), hypoprotocetraric acid 1 - 70 cfu to 1 - 28 cfu/ml (Fig. 6), lecanoric acid 2 - 120 cfu to 1 - 60 cfu/ml (Fig. 7) and protocetraric acid the colony count decreased from 2 - 100 cfu to 1 - 50 cfu/ml (Fig. 8). These kinetic time kill results indicates the effective bactericidal activity of lichen compounds with in 0 hrs to 6 hrs and their effectiveness up to 24 hrs of incubation period against the tested microorganism.
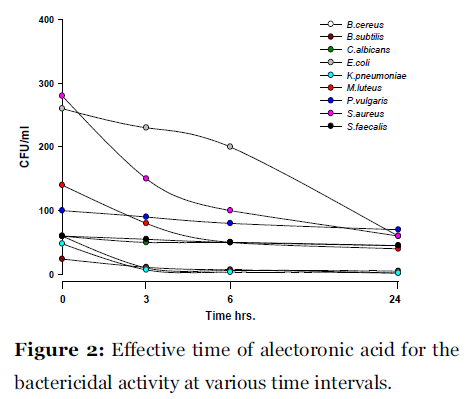
Figure 2:Effective time of alectoronic acid for the bactericidal activity at various time intervals.
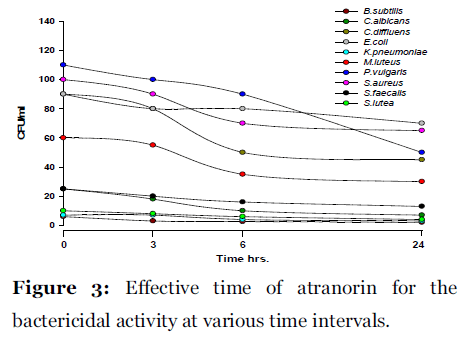
Figure 3:Effective time of atranorin for the bactericidal activity at various time intervals.
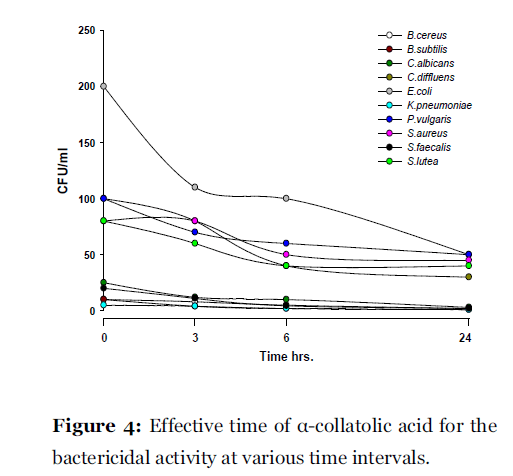
Figure 4:Effective time of a-collatolic acid for the bactericidal activity at various time intervals.
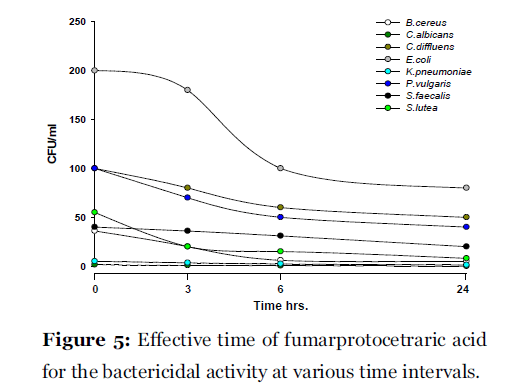
Figure 5:Effective time of fumarprotocetraric acid for the bactericidal activity at various time intervals.
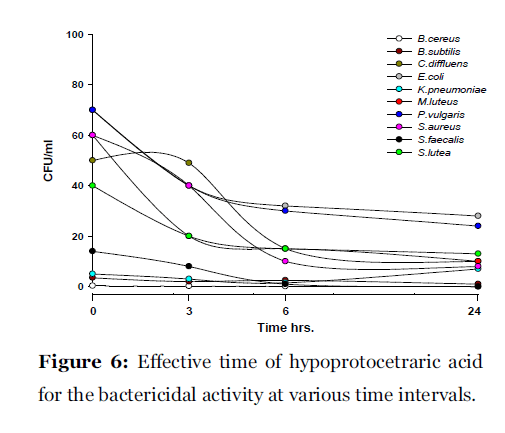
Figure 6:Effective time of hypoprotocetraric acid for the bactericidal activity at various time intervals.
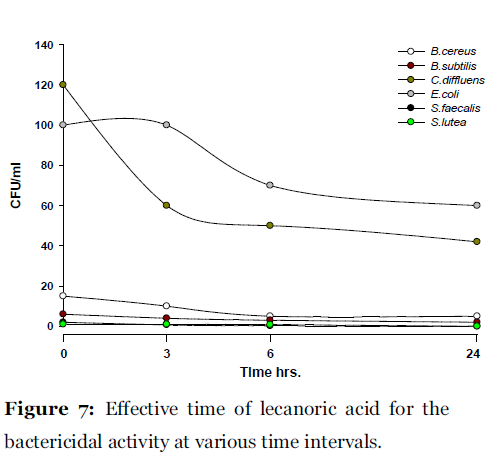
Figure 7:Effective time of lecanoric acid for the bactericidal activity at various time intervals
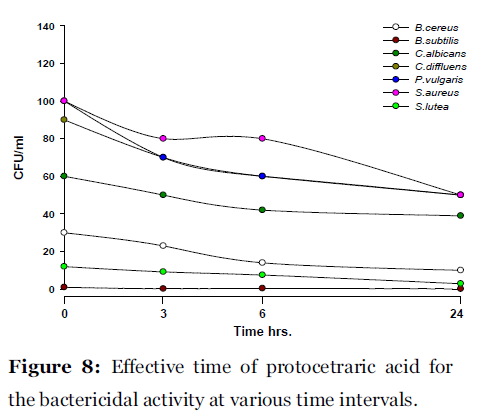
Figure 8: Effective time of protocetraric acid for the bactericidal activity at various time intervals.
| Lichen Compounds |
Average activity at 40C/1hrs/0 day |
Average activity at 400C/1hrs |
Activity decrease (%) |
Average activity after 30 day at 200C |
Activity decrease (%) |
| |
| Alectoronic acid |
51 – 99% |
34 – 83% |
17 – 25% |
12 – 50% |
15 – 87% |
| Atranorin |
37 – 84% |
13 – 65% |
14 – 64% |
23 – 59% |
14 – 47% |
| a-Collatolic acid |
39 – 93% |
28 – 62% |
20 – 69% |
10 – 58% |
20 – 84% |
| Fumarprotocetraric acid |
40 – 99% |
30 – 87% |
16 – 39% |
14 – 83% |
17 – 86% |
| Hypoprotocetraric acid |
35 – 98% |
28 – 88% |
11 – 27% |
10 – 81% |
18 – 84% |
| Lecanoric acid |
37 – 95% |
14 – 84% |
11 – 47% |
18 – 40% |
17 – 92% |
| Protocetraric acid |
29 – 97% |
17 – 86% |
11 – 54% |
3 – 53% |
86 – 94% |
* Average activity: Bactericidal activity of lichen compounds for all tested microorganism.
Table: 3. Bactericidal activity shown by the lichen compounds incubated under various temperature and finally after 30 days incubation at 20°C.
Stability of lichen compounds for bactericidal activity
As far as the stability of the lichen compounds towards the bactericidal activity is concerned, the activity was found to be varied greatly among the compounds depending on temperature and incubation time period (Table 3). A set of lichen compounds incubated at lower temperature £ 4°C showed < 2% loss in the activity. However, when these compounds were kept at ³ 40°C for 1 h showed 11% to 69% losses in the activity. The data of same activities observed at 60°C for 1 h are not shown. Furthermore, another set of compounds kept at 20°C for 30 days showed 14% to 94% decrease in the activity. The results indicated that these lichen compounds can only be preserved at < 4°C for longer period to retain their bactericidal potential.
Discussion
Lichens are valuable plant resources and since ancient time they are used as medicine, food, fodder, perfume, spice, dyes and for miscellaneous purposes throughout the world. Lichens are well known for the diversity of secondary compounds that they produce. Compounds isolated from various lichen species have been reported to display diverse biological activities [21]. In the presented work three different types of lichen species Cladonia ochrochlora, Parmotrema nilgherrensis and Parmotrema sancti-angelii and their secondary compounds were studied for the exploration of possible source of potential bactericidal agents.
Among various solvent fractions of lichen species, ethyl acetate fraction showed only potent bactericidal activity against some gram positive, gram negative bacterial strains and yeast strains of low pathogenicity and clinical importance. The ethyl acetate fraction of P. nilgherrensis gave 8 mm to 20 mm zone of inhibition, P. sancti-angelii (8 mm to 13 mm), C. ochrochlora (8 mm to 12 mm) against the tested microorganisms and the activity was found less or equivalent to the activity shown by standard antibiotics (7 mm to 29 mm). The ethyl acetate fraction of studied lichen species was also found active against B. cereus, P. vulgaris and S. faecalis, which were not found sensitive to methicillin and streptomycin antibiotics. Among the studied lichen species P. nilgherrensis showed strong bactericidal activity whereas P. sancti-angelii, C. ochrochlora showed moderate activity.
Crude extract always contains lichen substances along with the accessory pigments etc. Probably the observed biological activity may be the synergistic action of those compounds. In order to know, which phytochemical component or active principle playing the vital role of the observed bactericidal activity therefore after screening we have only isolated and purified the lichen compounds from ethyl acetate fraction and evaluated the bactericidal potential. The lichen secondary compound atranorin showed strong bactericidal activity with lowest MIC values 5 μg to 70.7 μg/ml and found to be effective against all the tested microorganisms including Bacillus subtilis and Candida albicans, which were not found sensitive in previous report by Perry et al. [22]. Here the MIC value of atranorin reported was also found very less against the studied microorganisms in comparison to the activity of atranorin isolated from Cladonia foliacea (15.6 μg to 500 μg/ml) by Yilmaz et al. [23] and from Physcia aipolia (31 mg to 250 μg/ml) by Rankovi? et al. [14]. Lichen secondary compounds hypoprotocetraric acid, α- collatolic acid and alectoronic acid inhibited the growth of ten microorganisms with MIC value 8.6 μg to 245 μg/ml, 12.2 μg to 278.5 μg/ml and 21.9 μg to 162.1 μg/ml respectively. Fumarprotocetraric acid showed activity by inhibiting the growth of eight microorganisms with MIC value 24 μg to 227.3 μg/ml and the MIC values were found higher then the value of fumarprotocetraric acid isolated from Cladonia foliacea (4.6 μg to 150 μg/ml) by Yilmaz et al. [23].
Protocetraric acid and lecanoric acid were showed weak bactericidal activity by inhibiting six to seven tested microorganisms with MIC 18.7 μg to 740.7 μg/ml. and 24.6 μg to 591.7 μg/ml respectively. The studied lichen secondary compounds isolated from the lichen species P. nilgherrensis, P. sancti-angelii and C. ochrochlora were found potential bactericidal agents. Marin et al. [24] reported the levels of phytochemicals are influenced by genetics and growth conditions here we can only conclude that the observed biological activities reported here are species specific. The results of stability of the lichen compounds towards the bactericidal activity under various physiological conditions indicates the activity of all lichen compounds is very stable at ≤4oC and are most effective from 0 hr to 6 hrs in in vitro condition.
Biological activity of material whether known in folk medicine or observed in planned screening program has been the starting point in the drug discovery. The general pattern is the isolation of active principles, elucidation their structures, followed by attempts for modulation of its activity potential by chemical modification [21]. In the presented work, the bactericidal activity of lichen secondary compounds atranorin, α-collatolic acid, hypoprotocetraric acid, alectoronic acid, fumarprotocetraric acid, protocetraric acid, lecanoric acid isolated from the P. nilgherrensis, P. sancti-angelii and C. ochrochlora has been presented for the first time. The studied lichen compounds are needed to explore medicinal/ pharmaceutical point of view for the development of their chemical derivative for pharmaceutical implementation.
Acknowledgement
We are very grateful to Council of Scientific Industrial Research (CSIR), New Delhi, India for financial support to Dr. Neeraj Verma in the form of Research Associateship (Grant no. 09/670 (0046) 2010/EMR-I) and Department of Biotechnology (DBT) (Grant no. BT/PR8551/NDB/52/15/2006) Government of India, New Delhi. We are thankful to Director, Agharkar Research Institute, Pune for providing research facilities.
5649
References
- Paudel B, Bhattarai HD, Lee JS, Hong SG,nShin HW, Yim JH. Antibacterial potential ofnAntarctic lichens against human pathogenicngram-positive bacteria. Phytother Res 2008;n22: 1269–1271.
- Türk AO, Yılmaz M, Kívanc M, Türk H. Thenantimicrobial activity of extracts of the lichennCetraria aculeata and its protolichesterinicnacid constituent. Z Naturforsch 2003; 58c:n850-854.
- Oksanen I. Ecological and biotechnologicalnaspects of lichens. Appl Microbial Biotechnoln2006; 73: 723-734.
- Crittenden PD, Porter N. Lichen-formingnfungi: potential sources of novel metabolites.nTrends Biotechnol 1991; 9: 409-414.
- Boustie J, Grube M. Lichens-a promisingnsource of bioactive secondary metabolites.nPlant Genetic Resource 2005; 3: 273-287.
- Müller K. Pharmaceutically relevantnmetabolites from lichens. Appl MicrobiolnBiotechnol 2001; 56: 9-16.
- Behera BC, Verma M, Sonone A, Makhija U.nAntioxidant and antimicrobial activities ofnlichen Usnea ghattensis in vitro. BiotechnolnLett 2005; 27: 991-995.
- Verma N, Behera BC, Makhija U. Antioxidantnand hepatoprotective activity of a lichennUsnea ghattensis in vitro. App BiochemnBiotechnol 2008; 151: 167-181.
- Mitscher L, ADrake S, Gollipudi SR, OkwutenSK. A modern look at folkloric use of antiinfectivenagents. J Nat Prod 1987; 50: 1025–n1040.
- Ingólfsdóttir K, Chung GAC, Skúlason VG,nGissurarson SR, Vilhelmsdóttir M.nAntimycobacterial activity of lichennmetabolites in vitro. Eur J Pharm Sci 1998;n6: 141–144.
- Hostettmann K, Wolfender JL. The searchnfor biologically active secondarynmetabolites. Pestic Sci 1997; 51: 471-482.
- Karaman I, Sahin F, Güllüce M, Ogütcü H,nSengül M, Adigüzel A. Antimicrobial activitynof aqueous and methanol extractsnof Juniperus oxycedrus L. JnEthnopharmacol 2003; 85: 231–235.
- Ranković B, Mišić M. Antifungal activity ofnextract of the lichens Alectoria sarmentosanand Cladonia rangiferina. Mikol Fitopatoln2007; 41: 276-281.
- Ranković B, Mišić M, Sukdlak S. Thenantimicrobial activity of substances derivednfrom the lichens Physcia aipolia,nUmbilicaria polyphylla, Parmelia caperatanand Hypogymnia physodes. World JnMicrobiol Biotechnol 2008; 24: 1239-1242.
- Stocker-Wörgötter E. Metabolic diversity ofnlichen-forming ascomycetous fungi:nCulturing, ployketide and shikimatenmetabolite production, and PKS genes, NatnProd Rep 2008; 25: 188-200.
- Hale ME. Growth. In: Ahmadjian V, Hale MEn(eds). The lichens, London, Academic Press,n1973, pp 473-492.
- Culberson CF, Kristinsson HD. Anstandardized method for the identification of lichen products. J Chromatogr 1972; 45: 85-n93.
- National Committee for Clinical LaboratorynStandards (1993) Performance standards fornantimicrobial disk susceptibility test.nApproved Standard NCCLS Publication. M2-nA5, Vilionova, PA, USA.
- Gibbons S, Gray AI. Isolation by planarnchromatography. In: Cannell RJP (ed).nNatural products isolation. Humana press,nTotowa, New Jersey, 1998, pp 209-245.
- Principe L, D’Arezzo S, Capone A, PetrosillonN, Visca P. In vitro activity of tigecycline inncombination with various antimicrobialsnagainst multidrug resistant Acinetobacternbaumannii, Ann Clin Microbiol Antimicrobn2009; 8: 18.
- Shukla V, Joshi GP, Rawat MSM. Lichens asna potential natural source of bioactivencompounds: a review. Phytochem Rev 2010;n9: 303-314.
- Perry NB, Benn MH, Bernnan NJ, BurgessnEJ, Ellis G, Galloway DJ, Lorimer SD,nTangney RS Antimicrobial, antiviral andncytotoxic activity of New Zealand lichens.nLichenologist 1999; 31: 627-636.
- Yilmaz M, Türk AO, Tay T, Kivanç M. Thenantimicrobial activity of extracts of the lichennCladonia foliacea and its (-)-usnic acid,natranorin and fumarprotocetraric acidnconstituents. Z Naturforsch 2004; 59c: 249-n254.
- Marín A, Ferreres F, Thomás-Barbeŕan FA,nGil MI. Characterization and quantitation ofnantioxidant constituents of sweet peppern(Capsicum annuum L.). J Agric Food Chemn2004; 52: 3861-3869.














