Keywords
Cyclic AMP; Immune activity; Adoptive lymphocyte immunotherapy; Cyclic AMP elevation after fasting; Lower cyclic AMP in cancer patient’s serum
Introduction
It is well known that cancer patients exhibit a significant decrease in cellular immune activity which is related to the aggravation of the cancer [1]. This phenomenon of defective immune-surveillance in cancer patients has not been elucidated. I have reported in 1985 why lowering cyclic AMP in his serum is induced defective immune-surveillance from adoptive lymphocyte immunotherapy among cancer patients [2]. Generally speaking, seral cyclic AMP concentration is low in cancer patients. So, immune activity was elevated by the application of adoptive lymphocytes immune therapy after fasting therapy on cancer patients because fasting therapy elevate seral cyclic AMP concentration. Recently, Honjo Tasuku et al., reported why T cell don’t attack cancer cells by the covering of its PD-L1 on T cell receptor, PD-1 [3]. This is also one of defective immune-surveillance from cellular side. I have recently surveyed the concentration between seral cyclic AMP concentration and T cell number and Natural Killer cell. So I have confirmed that low seral cyclic AMP is one of biochemical reason of defective immune-surveillance in cancer patient’s serum.
Materials and Methods
Fasting therapy
Food intake was gradually decreased for three days. On the day prior to commencement of the fasting therapy, a mixture of Aloe and lactulose (Lacdel, Suehiro Enzyme Institute, Osaka) was orally administered to purge the colon canal. Fasting therapy was carried out for three to seven days. After termination of the fasting Therapy, food intake was gradually increased for one to two weeks. When fasting therapy and adoptive immune therapy followed the termination of fasting therapy by two or three days.
Assay of cyclic nucleotides
Serum cyclic AMP and cyclic GMP were assayed by radio immunoassay using a kit (Yamasa Shoyu Co. Ltd, Japan) [3].
Measurement of immune activity
Peripheral blood lymphocytes were obtained from heparinized blood by centrifugation on a lymphocyte separation medium, Ficoll (Pharmazia Fine Chemicals AB, Sweden), and Conray (Daiichi Seiyaku, Tokyo), respectively.
a) T cell number: T cell number was determined by rosette formation with sheep erythrocytes.
b) Stimulation index (SI): To determine the rate of blastogenesis of lymphocytes by phytohemagglutinin (PHA), after adding PHA to isolated lymphocytes, the value of the ratio of 3H-thymidine incorporation into PHAtreated cells to the control without PHA was calculated.
c) Natural Killer (NK) activity: Lymphocytes at a concentration of 5 × 106/ml were prepared in RPMI 1640 supplemented with 10% fetal calf serum. As target cells, K562 were used. A lymphocyte suspension of 0.1 ml was poured into a 96-well microtire plate, 51Cr labelled target cells were added, and the amount of 51Cr released after incubation of effector cells for 4 hours at 37°C was measured.
Adoptive immune (sensitized lymphocyte) therapy
a) Selection of healthy donors, white blood cells of candidates should be 6000~8000 cells/mm3 and CRP within normal range and sialic acid also within normal range.
b) Lymphocyte preparation, peripheral blood (100 ml) was drawn from the selected healthy donors. According to the method of Tsuji, lymphocytes were prepared by centrifugation on lymphocyte separation medium (Lymphoprep, Nyegaard & Co, A.S. Oslo, and Norway). An approximately 1-2 × 108 lymphocyte suspension in 100 ml of physiological saline solution was aseptically infused into the recipient via intravenous drip.
c) Treatment, as subjects, 17 advanced and progressive cancer patients with decreased cellular immune activity were treated (Table 1). According to the method of tumor marker release induction into the blood previously described, retinol palmitate (vitamin A, 100,000 IU, Eisai Co., Tokyo) was administered by intramuscular injection. Hyperthermia treatment was carried out 1 to 3 hours later in a far infrared sauna bating (56™, 20min) inducing effective release of cancer antigens into the blood [4]. After hyperthermia treatment, suspension prepared from healthy donors was infused by intravenous drip (Table 1).
| Number of Cases |
| Name |
Age |
Sex |
Site |
Stage |
Fasting (Day) |
| F.S. |
36 |
F |
Breast |
IV |
7,3,3,4 |
| M.T. |
45 |
M |
Kidney |
IV |
3 |
| M.K. |
35 |
M |
Rectum |
IV |
5,3,3 |
| K.H. |
54 |
M |
Lung |
IV |
3 |
| H.N. |
28 |
F |
Breast |
IV |
3 |
| Y.N. |
32 |
F |
Thyroid |
IV |
3,3 |
| R.T. |
40 |
F |
Breast |
IV |
3 |
| R.K. |
27 |
F |
Uterus |
IV |
3 |
| S.N. |
35 |
F |
Ovary |
IV |
3,3 |
| M.T. |
39 |
F |
Rectum |
IV |
3,3 |
| E.I. |
34 |
F |
Breast |
IV |
3,3,3 |
| E.Y. |
40 |
F |
Breast |
IV |
3 |
| A.K. |
30 |
F |
Breast |
III |
3,3 |
| K.O. |
67 |
F |
Lung |
IV |
3 |
| N.Y. |
37 |
F |
Breast |
III |
3,7 |
| N.Y. |
30 |
F |
Breast |
IV |
3,3 |
| E.K. |
31 |
F |
Breast |
IV |
3,3,3 |
Table 1: Patients with progressive and advanced cancer were treated with only adoptive immune (sensitized lymphocyte) therapy.
Results
Improvement in the serum level of cyclic nucleotides by fasting therapy
Normal levels of serum cyclic AMP and cyclic GMP were taken as 10~30 pmoles/ml and 2~5 pmoles/ml, respectively. The serum levels of cyclic AMP and cyclic GMP of breast cancer patient quickly normalized as compared to the serum levels prior to commencement of the fasting therapy (Figure 1).
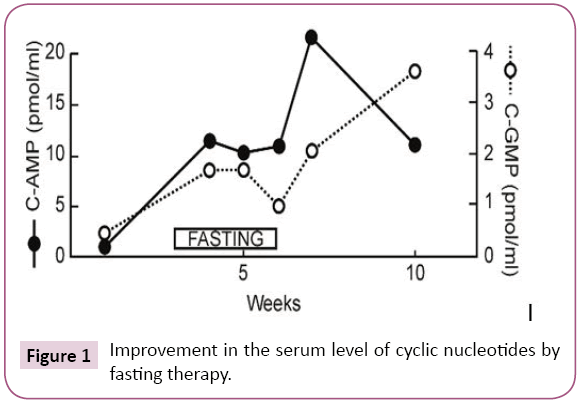
Figure 1: Improvement in the serum level of cyclic nucleotides by fasting therapy.
Possibility of cancer antigen releasing being caused by fasting therapy
Possibility of cancer antigen releasing being caused by fasting therapy. The same level of elastase-1, the tumor marker characteristic to this breast cancer patient, increased gradually after commencement of fasting therapy, and 2 weeks after termination of fasting therapy had returned to its previous level [4]. Similarly, the ferritin divided by serum iron ratio (FT/ Fe) increased after commencement of fasting therapy and decreased after its termination. Probably these cancer antigens were released from cancer tissues because of the fasting therapy (Figure 2).
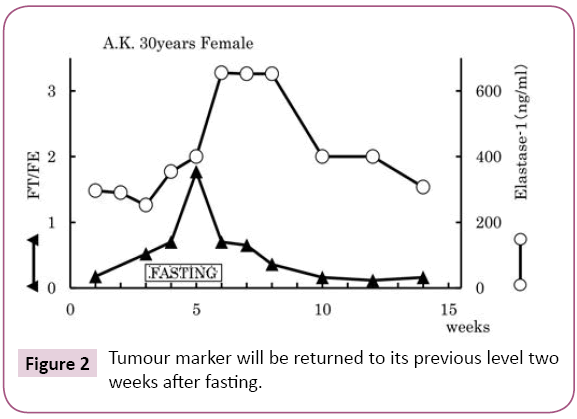
Figure 2: Tumour marker will be returned to its previous level two weeks after fasting.
Effect of fasting therapy in early cancer patient
Tumor markers will be returned to its previous level two weeks after fasting therapy (Figure 3). Figure 3 shows the measurements of the immune parameters in the blood of a 50-year-old male patient suffered from supra-mandibular carcinoma who carried out one week of fasting therapy. In such case of early cancer, fasting therapy alone produces a comparatively good improvement in immune activity, and can be maintained for several months (Figure 4).
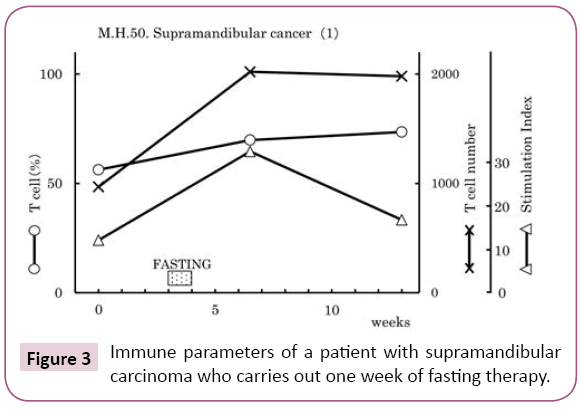
Figure 3: Immune parameters of a patient with supramandibular carcinoma who carries out one week of fasting therapy.
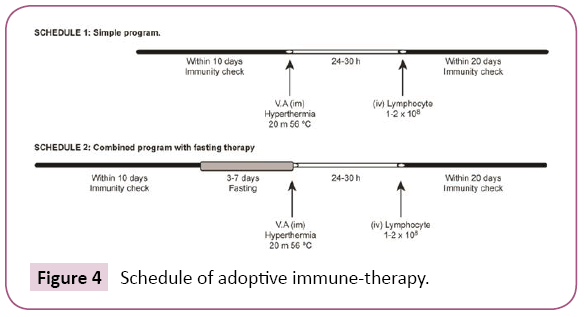
Figure 4: Schedule of adoptive immune-therapy.
Adoptive immune (sensitized lymphocyte) therapy schedule
Adoptive immune (sensitized lymphocyte) therapy schedule. Patients with progressive and advanced cancer (Table 1) were treated with only adoptive immune (sensitized lymphocyte) therapy, according to schedule 1 (Figure 5). We observed immune activity after an intravenous infusion of 1~2 × 108/ ml lymphocytes/ml in advanced cancer patients according to schedule 1 as follows. The stimulation index (SI), and NK cytotoxicity were 59% (10/17), 33% (5/14), and 53% (9/17), respectively (Figure 5).
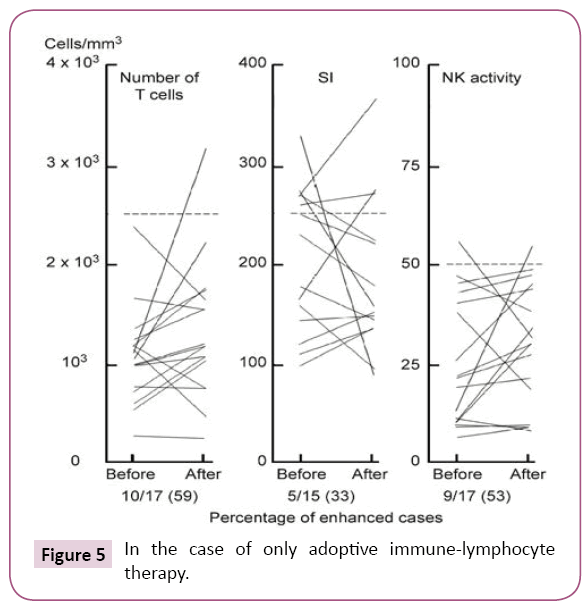
Figure 5: In the case of only adoptive immune-lymphocyte therapy.
Effects of combined fasting therapy and adoptive immune (sensitized lymphocyte) therapy
Combined fasting therapy and adoptive immune (sensitized lymphocyte) therapy were carried out according to schedule 2 (Figure 4). These therapies were carried out on the subjects, 17 patients in Table 1, for a total time of 32 times. The rates of improvement in the immune parameters, T-cell number, SI and NK cell activity increased 70% (22/32), 56% (18/32), and 70%(14/20), respectively. The improvement in immune activity by the combined therapies was significantly greater than that of the adoptive immune (sensitized lymphocyte) therapy alone (Figure 6). Immune activity [T cell number (59>70), stimulation index(SI) (33>56) and NK activity (53>70)] increased with schedule 2 compared with schedule 1 in patients with advanced cancer because of the increased serum levels of cyclic AMP after fasting therapy. Therefore, I believe that decreased serum concentrations of cyclic AMP are the biochemical because underlying defective immune-surveillance in patients with cancer [2].
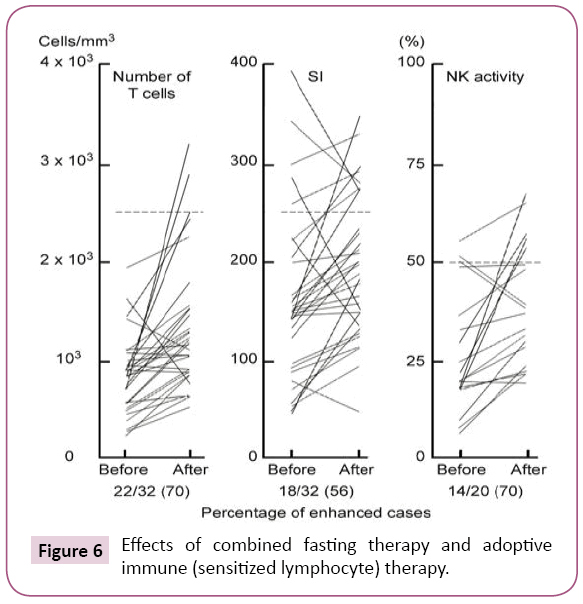
Figure 6: Effects of combined fasting therapy and adoptive immune (sensitized lymphocyte) therapy.
Survey of seral cyclic AMP in cancer patients
We have confirmed in cancer patients and preclinical cancer patients (40) who were classified into TSIV and TSV in Cancer [5], that lower immunity of T cell (Figure 7) and natural killer activity (Figure 8) showed lower cyclic AMP in their serum. The averaged cyclic AMP concentration in the serum is 16 pmol/ml in the lower T cell less than 1000/μL, conversely, the averaged cyclic AMP is 18.2 pmol/ml in the T cell number 1000/μL or above. This phenomenon’s are fundamental biological background of defective immune-surveillance in cancer patients (Figure 7). The averaged cyclic AMP is 14.5 pmol/ml in the natural killer cell less than 30%, conversely, the averaged cyclic AMP is 19 pmol/ml in the natural killer cell activity 30% or above. This phenomenon’s also show the fundamental biochemical background of defective immune-surveillance in cancer patients (Figure 8). From these data, there is the correlative tendency between the lower immune activity and lower cyclic AMP in the serum and when cancer is developing beforehand.
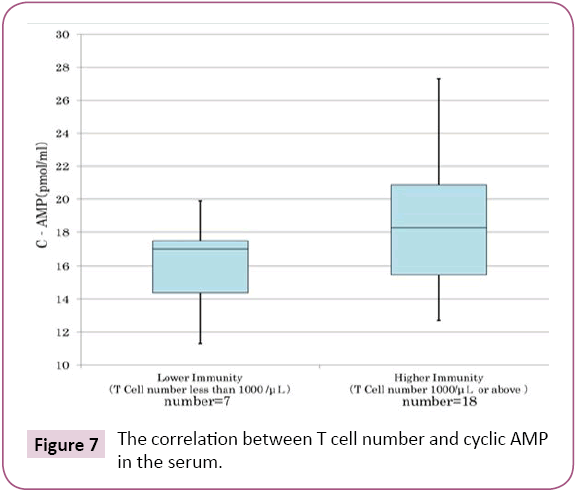
Figure 7: The correlation between T cell number and cyclic AMP in the serum.
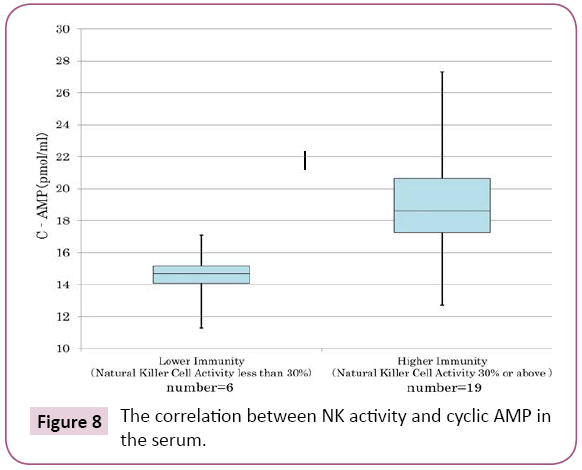
Figure 8: The correlation between NK activity and cyclic AMP in the serum.
Report of the direct effect of cyclic AMP on cancer cells
A group at Colorado University led by A.W. Hsie demonstrated that 1 mM of cyclic AMP caused the re-differentiation of ovarian cancer cells into normal fibroblasts within 5 hrs [6]. This cellular morphological change could be induced by direct and rapid influence of a small amount of cyclic AMP on damaged mitochondria or microtubules (Figure 9) [7]. This differentiation inducing phenomenon may have no correlation with genomic abnormalities in cancer cells (Figure 9). Oboshi et al., reported that glioma neuroblasts (NB-1 cells) could be re-differentiated into normal nerve cells 1 month after the addition of 1mM cyclic AMP. Thus, cyclic AMP has effective differentiation-promoting abilities in cancer cells, but also plays a key role correcting the defective immune-surveillance in cancer patients [2].
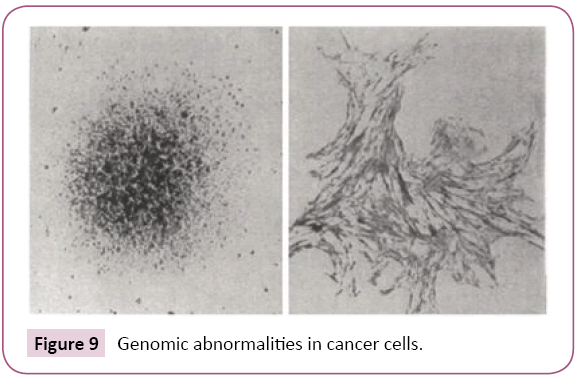
Figure 9: Genomic abnormalities in cancer cells.
Discussion
We have elucidated that defective immune-surveillance was lowering in cyclic AMP of serum concentration from biochemical side. Honjo T et al., have elucidated that defective immunesurveillance in cancer patients were inhibition of immune checkpoint disturbance by the PD-1 receptor of T cell and PD-L1 of cancer cell from cellular side. We must further investigate this correlation between seral cyclic AMP concentration and immune check point inhibition mechanism in the next step.
Conclusion
Biochemical meaning of defective immune-surveillance in cancer patients was lowering cyclic AMP concentration in the serum. So, from biochemical standpoint, cancer cells are difficult to reverse to normal cells.
References
- Ludwig G (1965) Immunological defect in aged population and its relationship to cancer. Cancer 18: 201-204.
- KobayashiT,Jo T, Hayashida S (1987) Enhancements of immune-surveillance in cancer patients. American J Acupuncuture 15: 25-33.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH(2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunology 26: 677-704.
- Kobayashi T (2018) A method to induce tumor marker release. MOJ Curr Res & Rev 1: 102-108.
- Kobayashi T, Kawakubo T (1994) Prospective investigation of tumor markers and risk assessment in early cancer screening. Cancer 73: 1946-1953.
- Hsie AW, Puck TT (1971) Morphological transformation of Chinese hamster cells by dibutyryladenosine cyclic 3’:5’-monophosphate and testosterone. Proceedings of the National Academy of Sciences of the USA. 68: 358-361.
- Kobayashi T (2018) Given the continuing dispute over the role of genetic abnormalities and protracted mitochondrial respiratory dysfunction in carcinogenesis, what is the core underlying entity? MOJ Curr Res & Rev 1:86-100.
22642
References
- Ludwig G (1965) Immunological defect in aged population and its relationship to cancer. Cancer 18: 201-204.
- KobayashiT,Jo T, Hayashida S (1987) Enhancements of immune-surveillance in cancer patients. American J Acupuncuture 15: 25-33.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH(2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunology 26: 677-704.
- Kobayashi T (2018) A method to induce tumor marker release. MOJ Curr Res & Rev 1: 102-108.
- Kobayashi T, Kawakubo T (1994) Prospective investigation of tumor markers and risk assessment in early cancer screening. Cancer 73: 1946-1953.
- Hsie AW, Puck TT (1971) Morphological transformation of Chinese hamster cells by dibutyryladenosine cyclic 3’:5’-monophosphate and testosterone. Proceedings of the National Academy of Sciences of the USA. 68: 358-361.
- Kobayashi T (2018) Given the continuing dispute over the role of genetic abnormalities and protracted mitochondrial respiratory dysfunction in carcinogenesis, what is the core underlying entity? MOJ Curr Res & Rev 1:86-100.
- Ludwig G (1965) Immunological defect in aged population and its relationship to cancer. Cancer 18: 201-204.
- KobayashiT,Jo T, Hayashida S (1987) Enhancements of immune-surveillance in cancer patients. American J Acupuncuture 15: 25-33.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH(2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunology 26: 677-704.
- Kobayashi T (2018) A method to induce tumor marker release. MOJ Curr Res & Rev 1: 102-108.
- Kobayashi T, Kawakubo T (1994) Prospective investigation of tumor markers and risk assessment in early cancer screening. Cancer 73: 1946-1953.
- Hsie AW, Puck TT (1971) Morphological transformation of Chinese hamster cells by dibutyryladenosine cyclic 3’:5’-monophosphate and testosterone. Proceedings of the National Academy of Sciences of the USA. 68: 358-361.
- Kobayashi T (2018) Given the continuing dispute over the role of genetic abnormalities and protracted mitochondrial respiratory dysfunction in carcinogenesis, what is the core underlying entity? MOJ Curr Res & Rev 1:86-100.














