Keywords
Hysteroscopy; Intrauterine adhesion; Infertility; Body mass index; Uterine surgery
Abbreviations
IUA-Intrauterine Adhesion; EBH-Examination By Hysteroscopy; IW-Infertile Women; IVF-In-Vitro Fertilization; NFC-Nordica Fertility Center; CIConfidence Interval; BMI-Body Mass Index; PI-Primary Infertility; SI-Secondary Infertility; HSG Hysterosalpingogram
Introduction
Hysteroscopy is a relatively new procedure in the investigation of congenital structural abnormalities or acquired lesions that might pose fertility challenges to women in reproductive age. In the field of gynecology, unique procedural and technological approaches have resulted in more economical, efficient, safe and useful investigative hysteroscopy. About two decades ago hystero-salpingography (HSG) was the available procedure for assessing the uterine cavity but of recent, the use of hysteroscopy for this purpose is considered superior to HSG, being increasingly employed in straight and uninterrupted visualization of the uterine cavity [1]. Many authorities on assisted fertility are of the opinion that hysteroscopy is a more accurate tool because of the high false-positive and false negative rates of intra uterine abnormality with HSG [2]. While some authors argue that abnormal uterine bleeding (AUB) is the most common indication for hysteroscopy [3,4], others regard hysteroscopy in the management of infertile women without other diagnosed or doubtful intrauterine pathologies as still a matter of debate [5].
Hysteroscopy is useful in ruling out indications of any intrauterine pathology prior to subjecting patient to any procedure for assisted reproduction [6]. Hysteroscopy is also valuable in assessing cervical, uterine, tubal and ovulatory factors when investigating female infertility and published observational studies propose increasing pregnancy rates as benefits for resection of submucosal leiomyomas, adhesions, and endometrial polyps discovered during examination of the uterine cavity with the use of hysteroscopy [7]. Thus hysteroscopy is considered a gold standard for diagnosis of intrauterine lesions [3-4,8]. Gynecological examination using hysteroscopy can indicate a normal uterine cavity with no abnormality detected or can indicate one form of abnormality or another. The prevalence of abnormal uterine finding is estimated to be 34% to 62% in infertile women [9].
Intrauterine adhesion is one of the abnormalities that can be detected during examination by hysteroscopy
Development of intrauterine adhesion (IUA) is a major risk after a gynecologic surgery [10]. Because of the potential impact on reproductive function, IUA has a precise significance, in addition to the known consequences it presents, such as abdominal/ pelvic pain or bowel obstruction [11]. Therapeutic procedures on the uterus has its complications and IUAs are one of such which, although often silent, can inhibit fertility and often hide the possibility of becoming symptomatic, as is the case with Asherman’s syndrome [11]. Surgical trauma to the endometrium, such as myomectomy, curettage, Caesarian section are known to be risk factors for the development of IUA [12].
Age is a definitive factor to be considered in the reproductive life of females, especially. Many years before the onset of menopause, decline in female fertility is initiated, though there could still be continued regular ovulatory cycles. A school of thought claims that there is no strict definition of advanced reproductive age in women but infertility becomes more pronounced after 35 years of age [13]. Possibly, the age-associated decline in female fecundity and increased risk of spontaneous abortion are largely attributable to abnormalities in the oocyte [13]. In a report on the effect of female age on fertility, one study found that the percentage of women not using contraception who remained childless rose steadily according to their age at marriage: 6% at age 20-24, 9% at 25-29, 15% at age 30-34, 30% at age 35-39 and 64% at age 40-45 [14]. Nagele and co-workers [14] claimed that the prevalence of uterine pathology, such as fibroids and endometrial polyps, increases with age. It is also possible that some other uterine pathologies equally increase with age.
Body Mass Index has recently become a central issue in male and female infertility investigations. A study suggested that elevated body mass index at age 18, even at levels lower than those considered to be obese is a risk factor for subsequent ovulatory infertility [15]. Another study [16] observed a U-shaped association between body mass index (BMI) and relative risk of ovulatory infertility, with increased risk for BMI below 20.0 or above 24.0 kg/m2.
There is little evidence in literature to highlight demographic differences in normal and abnormal findings on examination by hysteroscopy among infertile women. There is also meager proof of any association between previous uterine surgery, age, intrauterine adhesions, body mass index and type of infertility, especially in sub-Saharan Africa. The aim of this study therefore was to conduct a comparative analysis of demographic indices and history of previous uterine surgery among 765 infertile women with or without intrauterine adhesions detected at hysteroscopy. Data obtained from diagnostic examinations by hysteroscopy, performed for infertility investigation were analyzed.
Materials and Methods
This study took place in one of the southern islands of Lagos State. In all, 1115 infertile women were recruited into the study of whom 350 had other abnormal findings on examination by hysteroscopy (EBH) apart from intrauterine adhesions (IUA). These women were excluded from further statistical analysis in this study, leaving 765 infertile women for analysis among who 427 examinations by hysteroscopy was normal while examination and 338 with confirmed IUA.
Study site
The study was conducted at Nordica Fertility Center, a private invitro fertility (IVF) facility in Lagos State, Nigeria. The facility also serves as center for counselling, acupuncture and examination of sperm count, sperm morphology and sperm motility in accessing male infertility, and multifactorial dynamics relating couples’ inability to have a child. Nordica Fertility Center, where this study took place is located in one of the southern islands of metropolitan Lagos with good access by road, acceptable infrastructures and clean water supply. The center has no in-patient facility apart from admitting patients who undergo laparoscopy for overnight stay or transferring them to a sister clinic for any extra days required. Data for this study were retrieved from the medical records of patients who consulted the IVF facility over a 9-year period.
Study design
This was a retrospective descriptive appraisal of medical records of infertile women that patronized the Nordica Fertility Center in the ten years spanning June 2005 to November 2014. The medical record of each infertile woman in the study period was retrieved and data contained therein transcribed into a spreadsheet, cleaned and coded and were imported into a statistical package for analysis.
Inclusion and exclusion criteria
The main inclusion criterion for this particular study was either diagnostic or therapeutic hysteroscopy. Other criteria were history of previous uterine surgeries and history suggestive of Asherman’s syndrome. The main exclusion criteria were: (i) history of pelvic inflammatory disease and (ii) pelvic cancer. Hysteroscopy was performed to appraise and manage the existence of intrauterine abnormalities. The operating surgeon provided a comprehensive description of the process and operative technique and all eligible women signed an informed consent before submitting themselves to the procedure. Stepwise discoveries at hysteroscopy were recorded manually on the medical case note of each patient. All sequential infertile women who met the inclusion criteria submitted to short general anesthesia followed by hysteroscopy. The procedure has been described in another paper (7).
Training and data collection
To avoid transcriptional and other forms of error, one-day training was given to three data-entry clerks (two females and one male) to familiarize them with medical terminologies and gynecological nuances used by the attending gynecologists. The training included retrieving data from medical records of the study patients, coding the data, entering the data into a laptop and cleaning the data. They were assisted and supervised by a seasoned obstetrician/gynecologist in the team (OB). Actual data collection started in October 2014 and lasted till February 2015.
Ethics committee approval and Informed consent
All patients were counseled on the processes of hysteroscopy. Informed consent for the process and for using results for training and research was obtained from subjects of the study. Anonymity of individual patients was maintained by coding. The study was approved by the local Ethics Committee.
Definitions
For the purpose of this paper, age (years) was categorized into < 35 and ≥ 35 and Body Mass Index (BMI kg/m2) was stratified into underweight (< 18.5), normal (18.5 - 24.9), overweight (25.0 - 29.9) and obese (≥ 30.0). Variables were accordingly coded for ease of statistical analysis.
Statistical analysis
Data of each patient was coded for anonymity, ease of reference, avoidance of bias and fed into a lap top computer, cleaned and cross-checked for errors. Analysis of the cleaned data was done using STATA 13 statistical software. The data were analyzed descriptively obtaining frequencies and percentages, and inferentially using chi-square test to determine associations, where appropriate. Student’s t-test was used to compare means of two categorical variables and Chi-square was used to test association and a p-value < 0.05 was regarded as significant. Confidence Interval (CI) in this study refers to a range of values for specific variable constructed so that this range has a specified probability of including the true value of that variable.
Results
A description of the outcome of examination by hysteroscopy (EBH) among the initial 1115 infertile women (IW) is as shown in Table 1. In all, EBH was normal in 427 (38.3%) out of the 1115 IW and abnormal in another 688 (61.7%) IW, among who were 338 (49.1%) IW with intrauterine adhesion (IUA) while the rest had other types of intrauterine lesions apart from IUA. The latter were not considered again in further analysis. The overall prevalence of IUA among IW was 30.3%. A comparative analysis of Age, Body Mass Index, Type of Infertility and history of previous uterine surgery was conducted between the IW who had normal finding on EBH (i.e. no IUA, n = 427; control) and those who had abnormal findings on EBH, specifically IUA (n = 338; cases), totaling 765 IW. The 765 IW were subjects who were further analyzed from this point on.
| Findings on hysteroscopy |
Intrauterine adhesions (IUA) |
| Normal |
Frequency (%) |
All |
Absent |
Present |
| 427 (38.3) |
427 (55.0) |
0 (0.0) |
| Abnormal |
Frequency (%) |
688 (61.7) |
350 (45.0) |
338 (100.0) |
| Total |
Frequency (%) |
1115 (100.0) |
777 (69.7) |
338 (30.3) |
Table 1: Finding of intrauterine adhesions on examination by hysteroscopy among 1115 infertile women.
Demographic characteristics of 765 IW with (case) or without (control) IUA is as illustrated in Table 2. The mean (± SD) age of IW without IUA [38.1 (6.37) years] was significantly lower (t = -5.67, df = 748.2; P-value = 0.000) than that of IW with IUA [40.6 (5.80) years]. When age was categorized into two groups of < 35 and ≥ 35 years, IW ≥ 35 years were three times more likely to have IUA compared to those aged < 35 (χ2 = 38.53; P-value = 0.000; OR = 3.06, 95% CI = 2.13, 4.41), and the proportion of older IW with IUA (289, 85.5%) was significantly higher than that of younger IW with IUA (49, 14.5%). The Table 2 also shows that there was no significant difference in the overall mean Body Mass Index (Kg/m2) of IW with IUA [27.7 (4.78)] when matched with the BMI of those without IUA [28.1 (4.64)]. In addition, the proportion of older IW with IUA increased with increase in BMI from undernourished IW (5, 1.5%) up to overweight (168, 49.7%), then decreased (99, 29.3%) among obese IW. The proportion of IW without IUA followed the same pattern.
| Variable |
Item |
Result of examination by hysteroscopy |
t |
df |
P-value |
| IUA absent |
IUA present |
| Age (All) in years |
Freq. (%) |
427 |
338 |
|
|
|
| Mean |
38.1 |
40.6 |
-5.67 |
748.2 |
0 |
| ± SD |
6.37 |
5.8 |
|
|
|
| Range |
24-57 |
27-58 |
|
|
|
| Age<35 |
Freq. (%) |
146 (34.2) |
49 (14.5) |
|
|
|
| Mean |
31.2 |
31.3 |
-0.27 |
89.6 |
0.4 |
| ± SD |
2.43 |
2.22 |
|
|
|
| Range |
24-34 |
27-34 |
|
|
|
| Age ≥35 years |
Freq. (%) |
281 (65.8) |
289 (85.5) |
|
|
|
| Mean |
41.6 |
42.2 |
-1.54 |
567.1 |
0.06 |
| ± SD |
4.68 |
4.63 |
|
|
|
| Range |
35-57 |
35-58 |
|
|
|
| Comparing ages <35 and ≥35 years |
t |
-30.2 |
-26.1 |
|
|
|
| df |
425 |
132.9 |
|
|
|
| P-value |
0 |
0 |
|
|
|
| χ2 = 38.53; P-value = 0.000; OR = 3.06, 95%, CI = 2.13,4.41 |
| Body Mass Index (All) (Kg/m2) |
Mean |
27.7 |
28.1 |
-0.17 |
732.2 |
0.12 |
| ± SD |
4.78 |
4.64 |
|
|
|
| Range |
17.0-45.0 |
16.3-44.6 |
|
|
|
| <18.5 |
Freq. (%) |
2 (0.5) |
5 (1.5) |
|
|
|
| |
Mean |
17.3 |
17.3 |
0 |
4.45 |
1 |
| ± SD |
0.4 |
0.88 |
| χ2 = 1.16*; P-value = 0.28; OR = 0.31; 95% CI = 0.06,1.63 |
| 18.5-24.9 |
Freq. (%) |
129 (30.2) |
66 (19.5) |
|
|
|
| |
Mean |
22.85 |
22.57 |
1.2 |
128.86 |
0.88 |
| ± SD |
1.52 |
1.55 |
|
|
|
| χ2 = 11.34; P-value = 0.0008; OR = 1.78; 95% CI = 1.27, 2.50 |
| 25.0-29.9 |
Freq. (%) |
172 (40.3) |
168 (49.7) |
|
|
|
| |
Mean |
27.17 |
27.26 |
-0.6 |
336.65 |
0.27 |
| ± SD |
1.44 |
1.32 |
|
|
|
| χ2 = 6.78; P-value = 0.009; OR = 0.68; 95% CI = 0.51, 0.91 |
| ≥30.0 |
Freq. (%) |
124 (29.0) |
99 (29.3) |
|
|
|
| |
Mean |
33.6 |
33.6 |
0 |
214.08 |
1 |
| ± SD |
3.49 |
3.33 |
| χ2 = 0.006 P-value = 0.94; OR = 0.99; 95% CI = 0.72, 1.35 |
| *Fishers exact test; 95% CI = 95% Confidence Interval; OR = Odds ratio |
Table 2: Demographic characteristics of patients with normal (IUA-ve) and abnormal (IUA+) findings on examination by hysteroscopy.
The study attempted to relate BMI, type of infertility and previous uterine surgery with presence or absence of IUA in Table 3. The first observation was that obese IW aged ≥ 35 years were three time more likely to develop IUA than obese <35 years old (χ2 = 7.15; p = 0.008; OR = 3.01, 95% CI = 1.31, 6.94). The second observation was that older overweight IW were about twice more likely to develop IUA compared to younger overweight infertile women (χ2 = 3.91; p = 0.04; OR = 1.76, 95% CI = 1.00, 3.08). Overall, older IW were about twice more likely to develop IUA than younger IW (χ2 = 12.81; p = 0.0003; OR = 2.01, 95% CI = 1.37, 2.96) regardless of their BMI status. The Table 3 also shows that there was no substantial alteration in the proportion of women with primary infertility who developed IUA regardless of their age whereas older women with secondary infertility were twice likely to develop IUA when matched with younger women (χ2 = 9.98; p = 0.002; OR = 2.08, 95% CI = 1.31, 3.28).
| Variable |
Item |
IUA Present |
IUA Absent |
χ2 |
P-value |
OR |
95% CI |
| Age <35 |
Age≥35 |
Age <35 |
Age ≥35 |
| Freq. (%) |
Freq. (%) |
Freq. (%) |
Freq. (%) |
| Body Mass Index |
| <18.5 |
|
0 (0) |
5 (1.7) |
1 (1.1) |
3 (1.1) |
0.01 |
0.91 |
0 |
Un
defined |
| χ2 |
0.1 |
0.04 |
0.1 |
0.04 |
- |
- |
- |
- |
| P-value |
0.75 |
0.83 |
0.75 |
0.83 |
- |
- |
- |
- |
| |
OR |
0 |
1.51 |
0 |
0.66 |
- |
- |
- |
- |
| 95% CI |
undefined |
0.36, 6.40 |
undefined |
0.16, 2.79 |
- |
- |
- |
- |
| 18.5-24.5 |
|
14 (28.6) |
52 (18.0) |
30 (33.7) |
73 (28.0) |
1.31 |
0.25 |
1.53 |
0.74, 3.16 |
| χ2 |
0.38 |
7.77 |
0.38 |
7.77 |
- |
- |
- |
- |
| P-value |
0.54 |
0.005 |
0.54 |
0.005 |
- |
- |
- |
- |
| OR |
0.78 |
0.57 |
1.27 |
1.77 |
- |
- |
- |
- |
| 95% CI |
0.36, 1.68 |
0.38, 0.85 |
0.59, 2.72 |
1.18, 2.65 |
- |
- |
- |
- |
| 25.0-29.9 |
|
26 (53.0) |
142 (49.1) |
36 (40.4) |
112 (42.9) |
3.91 |
0.04 |
1.76 |
1.00, 3.08 |
| χ2 |
2.03 |
2.14 |
2.03 |
2.14 |
- |
- |
- |
- |
| P-value |
0.15 |
0.14 |
0.15 |
0.14 |
- |
- |
- |
- |
| OR |
1.66 |
1.29 |
0.6 |
0.78 |
- |
- |
- |
- |
| 95% CI |
0.82, 3.36 |
0.92, 1.80 |
0.30, 1.21 |
0.56, 1.09 |
- |
- |
- |
- |
| ≥30 |
|
9 (18.4) |
90 (31.2) |
22 (24.7) |
73 (28.0) |
7.15 |
0.008 |
3.01 |
1.31, 6.94 |
| χ2 |
0.73 |
0.66 |
0.73 |
0.66 |
- |
- |
- |
- |
| P-value |
0.39 |
0.42 |
0.39 |
0.42 |
- |
- |
- |
- |
| OR |
0.69 |
1.16 |
1.46 |
0.86 |
- |
- |
- |
- |
| 95% CI |
0.29, 1.63 |
0.81, 1.68 |
0.61, 3.48 |
0.59, 1.24 |
- |
- |
- |
- |
| Total |
49 (14.5) |
289(85.5) |
89 (22.4) |
261 (74.6) |
12.81 |
0.0003 |
2.01 |
1.37, 2.96 |
| Type of infertility |
| Primary |
|
12 (24.5) |
49 (17.0) |
33 (37.1) |
86 (33.0) |
1.4 |
0.24 |
1.57 |
0.74, 3.31 |
| Secondary |
|
37 (75.5) |
240 (83.0) |
56 (62.9) |
175 (67.0) |
9.98 |
0.002 |
2.08 |
1.31, 3.28 |
| Total |
|
49 (14.5) |
289 (85.5) |
89 (22.4) |
261 (74.6) |
12.81 |
0.0003 |
2.01 |
0.34, 0.73 |
| χ2 |
2.28 |
18.95 |
2.28 |
18.95 |
- |
- |
- |
- |
| P-value |
0.13 |
0.00001 |
0.13 |
0.00001 |
- |
- |
- |
- |
| |
OR |
0.55 |
0.42 |
1.82 |
2.41 |
- |
- |
- |
- |
| |
95% CI |
0.25, 1.20 |
0.28, 0.62 |
0.83, 3.97 |
1.61, 3.60 |
- |
- |
- |
- |
| Previous uterine surgery |
| Yes |
|
39 (79.6) |
277 (95.8) |
73 (82.0) |
229 (87.7) |
14.57 |
0.0001 |
2.26 |
1.48, 3.47 |
| No |
|
10 (20.4) |
12 (4.2) |
16 (18.0) |
32 (12.3) |
0.95 |
0.33 |
0.6 |
0.21, 1.68 |
| Total |
|
49 (14.5) |
289 (85.5) |
89 (22.4) |
261 (74.6) |
12.81 |
0.0003 |
2.01 |
1.37, 2.96 |
| χ2 |
0.12 |
12.25 |
0.12 |
12.25 |
- |
- |
- |
- |
| P-value |
0.73 |
0.0004 |
0.73 |
0.0004 |
- |
- |
- |
- |
| OR |
0.85 |
3.23 |
1.17 |
0.31 |
- |
- |
- |
- |
| 95% CI |
0.35, 2.06 |
1.62, 6.41 |
0.48, 2.82 |
0.16, 0.62 |
- |
- |
- |
- |
| IUA is more prevalent among overweight and obese IW regardless of age. Older (≥35 y) IW with previous uterine surgery (PUS) are more than three times more likely to develop IUA than those without PUS. Previous uterine surgery influence IUA especially among those aged 35 years and above; OR = Odds Ratio; 95% CI = 95% Confidence Interval. |
Table 3: Presence or absence of IUA among infertile women indifferent age groups relative to Body Mass Index (Kg/m2), type of infertility and previous uterine surgery.
The Table 3 and Figure 1 illustrate that older IW with IUA were over two times more likely to have had previous uterine surgery (χ2 = 14.57; p = 0.0001; OR = 2.26, 95% CI = 1.48, 3.47). Thus, a higher proportion of IW aged ≥ 35 years and with IUA (277/316, 87.7%) have had previous uterine surgery performed on them in comparison to IW aged <35 years (39, 12.3%).
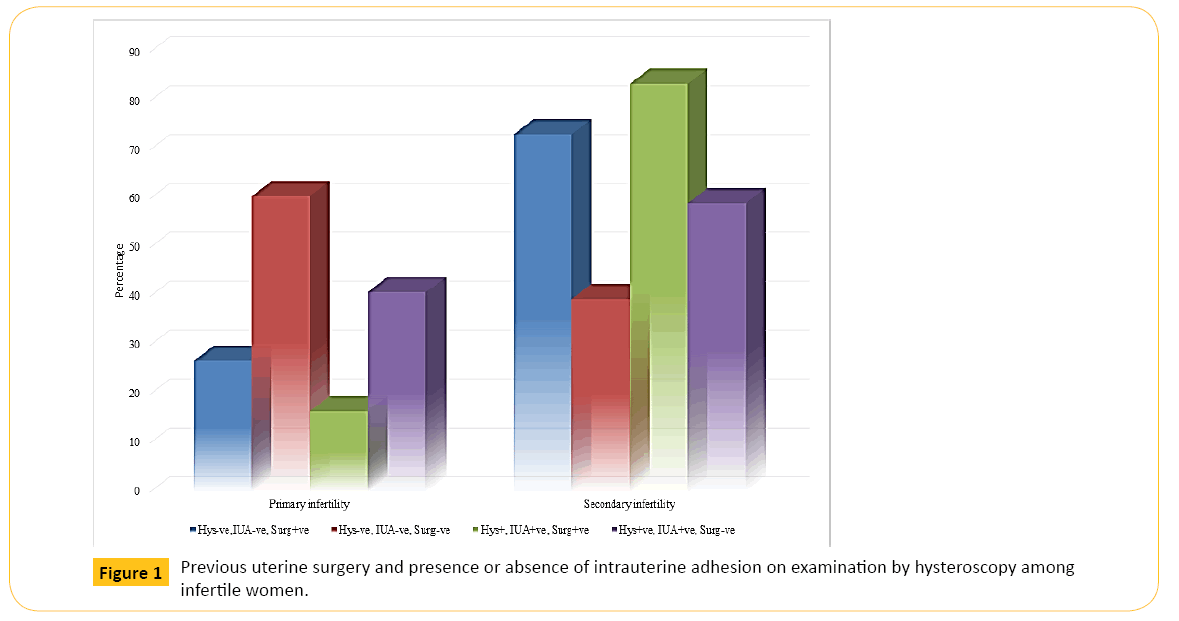
Figure 1: Previous uterine surgery and presence or absence of intrauterine adhesion on examination by hysteroscopy among infertile women.
Table 4 probes further the association between presence or absence of IUA and previous history of uterine surgery on one hand and age, BMI and type of infertility on the other hand. A higher proportion of older IW with IUA (277, 87.7%) gave a history of previous uterine surgery than their counterpart without IUA (239, 68.1%) with a statistically significant difference (χ2 = 19.36; p = 0.000001; OR = 4.06, 95% CI = 2.09, 7.88). Considering IW aged ≥ 35 years, those with IUA were four times more likely to have had previous uterine surgery compared those without IUA. Considering IW aged < 35 years with IUA, only 39 (12.3%) gave a history of previous uterine surgery compared 112 (31.9%) without IUA. The difference in these proportions did not approach any level of significance. Older IW with IUA were about 6 times more likely to have had previous uterine surgery compared to younger IW with IUA (χ2 = 18.19; p = 0.00002; OR = 5.92, 95% CI = 2.40, 14.61). Older IW without IUA were about 2 times more likely to have had previous uterine surgery compared to younger IW without IUA (χ2 = 4.57; p = 0.03; OR = 1.73, 95% CI = 1.04, 2.86). In addition, the Table 4 also illustrates that a significantly higher proportion of overweight IW with IUA had had previous uterine surgery compared with overweight IW without IUA (χ2 = 12.22; p = 0.0004; OR = 0.27, 95% CI = 0.123, 0.58). Furthermore, Table 4 indicates that older women with secondary infertility who presented with IUA were 3½ times more likely to have had previous uterine surgery than younger IW with primary infertility who presented with IUA (χ2 = 8.31; p = 0.004; OR = 3.51, 95% CI = 1.43, 8.65).
| Variable |
Item |
Previous uterine surgery |
χ2 |
P |
OR |
95% CI |
| IUA present |
IUA absent |
| Freq. (%) |
Freq. (%) |
Freq. (%) |
Freq. (%) |
| Age group (years) |
| |
<35 |
112 (31.9) |
34 (44.7) |
39 (12.3) |
10 (45.5) |
0.17 |
0.68 |
1.18 |
0.53, 2.62 |
| |
≥35 |
239 (68.1) |
42 (55.3) |
277 (87.7) |
12 (54.5) |
19.36 |
0.00001 |
4.06 |
2.09, 7.88 |
| |
χ2 |
4.57 |
18.19 |
- |
- |
- |
- |
| |
P-value |
0.03 |
0.00002 |
- |
- |
- |
- |
| |
OR |
1.73 |
5.92 |
- |
- |
- |
- |
| |
95% CI |
1.04, 2.86 |
2.40, 14.61 |
- |
- |
- |
- |
| BMI |
|
|
<18.5 |
2 (0.6) |
0 (0.0) |
5 (1.6) |
0 (0.0) |
2.75* |
0.1 |
undefined |
undefined |
| |
18.5-24.9 |
105 (29.9) |
24 (31.6) |
62 (19.6) |
4 (18.2) |
4.61* |
0.03 |
3.54 |
1.17, 10.69 |
| |
25.0-29.9 |
142 (40.5) |
30(39.5) |
159 (50.3) |
9 (40.9) |
12.22 |
0.0004 |
3.73 |
0.71, 8.13 |
| |
≥30.0 |
102 (29.0) |
22(28.9) |
90 (28.5) |
9 (40.9) |
3.44 |
0.06 |
0.46 |
0.20, 1.06 |
| Type of infertility |
| |
Primary |
94 (26.8) |
46 (60.5) |
52 (16.5) |
9 (40.9) |
7.01 |
0.008 |
0.35 |
0.16, 0.78 |
| |
Secondary |
257 (73.2) |
30 (39.5) |
264 (83.5) |
13 (59.1) |
6.63 |
0.01 |
0.42 |
0.22, 0.83 |
| |
χ2 |
32.28 |
8.31 |
- |
- |
- |
- |
| |
P-value |
0 |
0.004 |
- |
- |
- |
- |
| |
OR |
4.12 |
3.51 |
- |
- |
- |
- |
| |
95% CI |
2.50, 7.03 |
1.43, 8.65 |
- |
- |
- |
- |
| *Fisher’s Exact Test; 95% CI = 95% Confidence Interval |
Table 4: Previous uterine surgery among infertile women with or without intrauterine adhesions discovered on examination by hysteroscopy relative to age (years), BMI (Kg/m2) and type of infertility.
Multivariate logistic regression analysis, as shown in Table 5, demonstrates significant correlation between age (t = -4.61, p = 0.000, 95% CI: -0.015, -0.006), type of infertility (t = -3.55, p = 0.000, 95% CI: -0.172, -0.049), and history of previous uterine surgery (t = 2.61, p = 0.009, 95% CI: 0.027, 0.190) with intrauterine adhesion. Table 6 depicts that, at age below 35 years, intrauterine adhesion is negatively associated with the type of infertility (t = -2.51, p = 0.0130) but at age 35 years and above (Table 7), it is negatively associated with type of infertility (t = -2.98, p = 0.003) and positively with history of previous uterine surgery (t = 3.68; p = 0.000).
| Independent variables |
Coefficient |
Std. Err |
t |
P-value |
95% CI |
| Age |
-0.01 |
0.002 |
-4.61 |
0 |
-0.015, -0.006 |
| BMI Kg/m2 |
-0.022 |
0.018 |
-1.23 |
0.218 |
-0.056, 0.013 |
| Type of infertility |
-0.111 |
0.031 |
-3.55 |
0 |
-0.172, -0.049 |
| History of previous uterine surgery |
0.109 |
0.042 |
2.61 |
0.009 |
0.027, 0.190 |
Table 5: Multiple regression analysis with intrauterine adhesions as dependent variable and BMI (Kg/m2), type of infertility, findings onhysteroscopy and history of previous surgery as independent variables among all infertile women.
| Independent variables |
Coefficient |
Std. Err |
t |
P-value |
95% CI |
| BMI Kg/m2 |
-0.006 |
0.029 |
-0.21 |
0.836 |
-0.064, 0.052 |
| Type of infertility |
-0.122 |
0.048 |
-2.51 |
0.013 |
-0.217, -0.026 |
| History of previous uterine surgery |
-0.042 |
0.058 |
-0.73 |
0.469 |
-0.157, 0.072 |
Table 6: Multiple regression analysis with intrauterine adhesions as dependent variable and Age (years), BMI (Kg/m2), type of infertility, findings on hysteroscopy and history of previous surgery as independent variables among infertile women <35 years’ old.
| Independent variables |
Coefficient |
Std. Err |
t |
P-value |
95% CI |
| BMI Kg/m2 |
-0.034 |
0.021 |
-1.59 |
0.111 |
-0.076, 0.008 |
| Type of infertility |
-0.115 |
0.039 |
-2.98 |
0.003 |
-0.190, -0.039 |
| History of previous uterine surgery |
0.202 |
0.055 |
3.68 |
0 |
0.094, 0.309 |
Table 7: Multiple regression analysis with intrauterine adhesions as dependent variable and BMI (Kg/m2), type of infertility, findings on hysteroscopy and history of previous surgery as independent variables among infertile women ≥ 35 years’ old.
Figure 2 shows that older women in each category of BMI had higher percentage of IUAs than younger women. Figure 3 illustrates that older women, whether with primary or secondary infertility, experienced more IUA than younger women while Figure 4 indicates that age is also a predisposing factor to previous history of uterine surgery and to IUA.
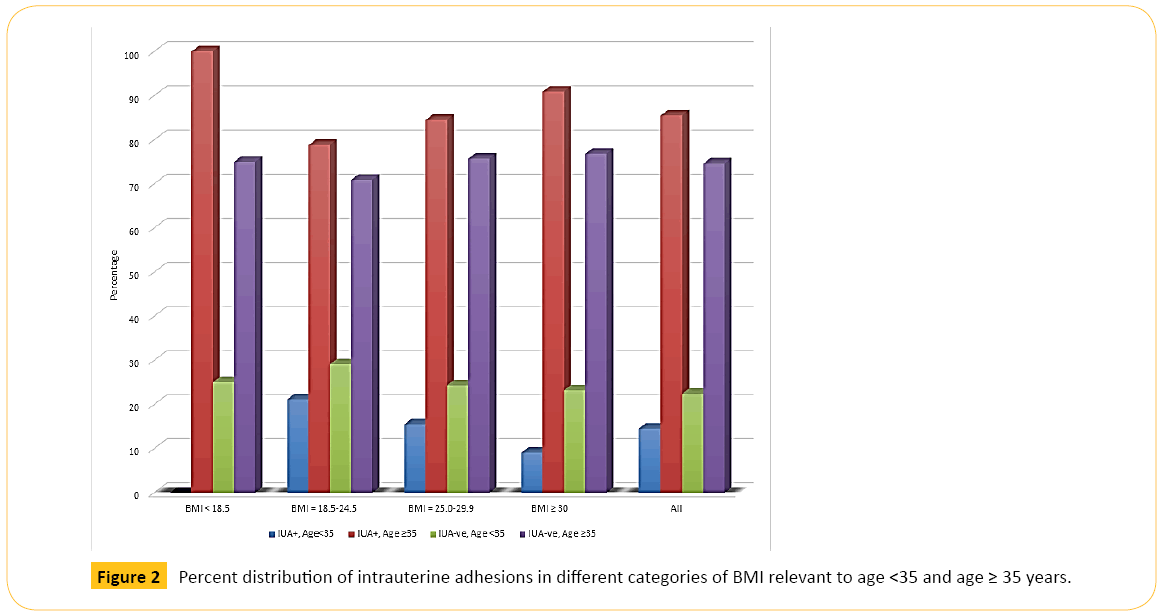
Figure 2: Percent distribution of intrauterine adhesions in different categories of BMI relevant to age <35 and age ≥ 35 years.
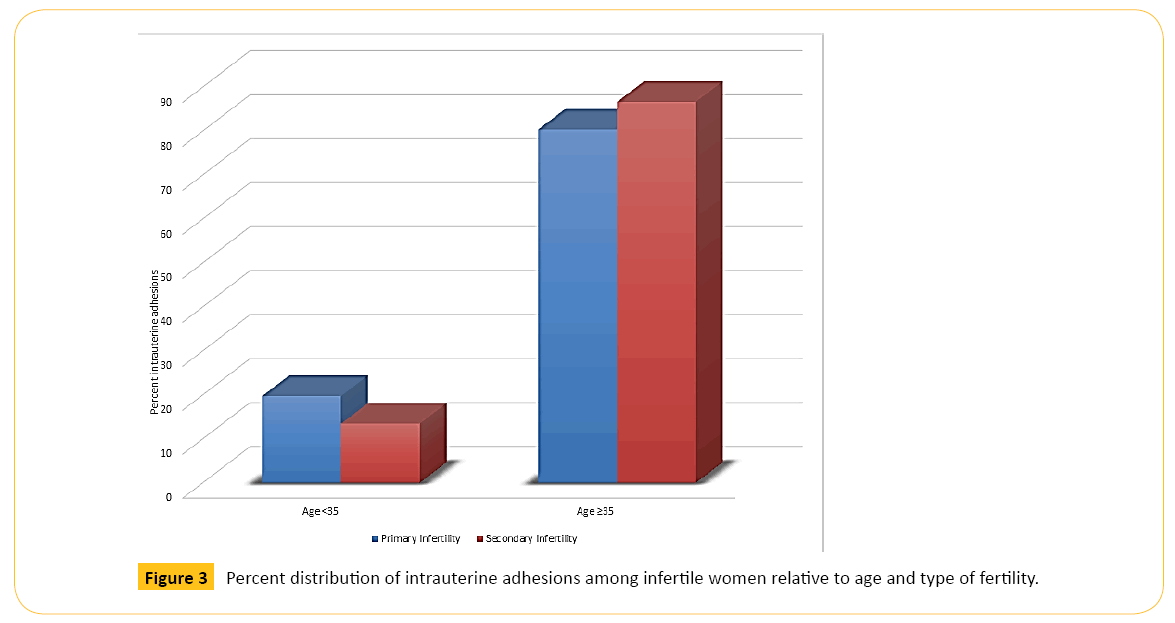
Figure 3: Percent distribution of intrauterine adhesions among infertile women relative to age and type of fertility.
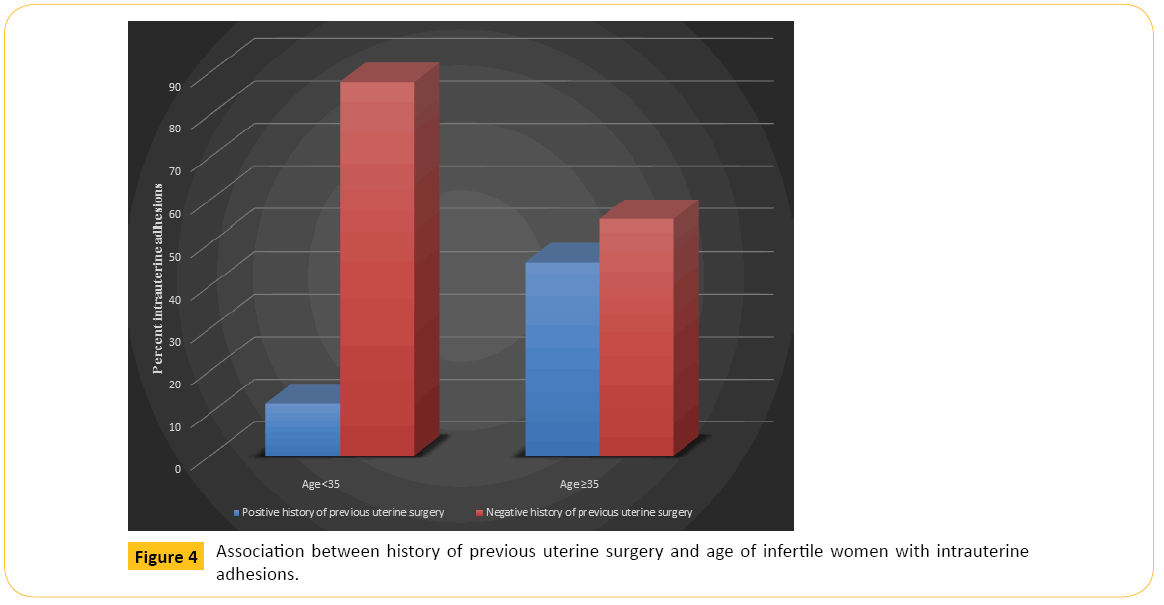
Figure 4: Association between history of previous uterine surgery and age of infertile women with intrauterine adhesions.
Discussion
The evaluation of infertility in women is a complex procedure as disruption of the physiological process of reproduction is associated with many factors, some of which are congenital, acquired or following chronological sequence of natural degeneration. Among the 1115 infertile women examined by hysteroscopy, 688 (61.7%) had abnormal findings, a figure much higher than the 6.3% described by Siam [17], the 33.1% reported by El Mazny [18] and the 40% stated by Kroska et al. [19]. Our result of higher rate of abnormal outcome of examination by hysteroscopy among older infertile women, compared to younger infertile women (χ2= 38.53; P-value = 0.000; OR = 3.06, 95% CI = 2.13, 4.41), was not in consonance with the result of Magos et al. [20] that showed no significant difference between the two but tallies with the report of Dicker et al. [21]. The overall prevalence of IUA in our study was 34.8% (388/1115), though this figure became 49.1% among all abnormal findings on hysteroscopy. These figures were similar to another study [17] but much higher than report from Koskas et al. [19]. The uterus could be the most physically assaulted internal organ of the female body. Trauma to the endometrium, especially the basal layer, is usually taken as the most important factor in the genesis of uterine scar resulting in synechae, though the mechanism of this process is still under debate and largely unknown [11]. Various studies have suggested that tissue hypoxia potentiates the initial tissue injury and triggers the cascade of responses that leads to the creation of adhesions [22,23]. Initial multivariate logistic regression analysis to assess the independency of variables such age, body mass index, type of infertility and history of previous uterine surgery as predictors of the presence of intrauterine abnormalities, demonstrated a significant correlation between age and IUA, (Coeff. = -0.010, SE = 0.002, t = -4.61, P = 0.000, 95% CI: -0.014 - 0.006), type of infertility (Coeff. = -0.111, SE = 0.031, t = -3.55, P = 0.000, 95% CI: -0.172 - 0.049) and history of previous uterine surgery (Coeff. = 0.109, SE = 0.042, t = 2.61, P = 0.009, 95% CI: 0.027, 0.190) in the studied infertile women. Interestingly, BMI was not a determinant of IUA among infertile women in our study which departs from what Fatemi et al. [24] described. Some studies [14,24] have reported a higher proportion of older women than younger women suffer from IUA and this is confirmed by our study where (85.5%) of older infertile women presented with IUA compared to only 14.5% of younger women. Various authors have documented adhesion rates following surgical interventions but very few categorized their patients by BMI, and type of infertility. For example, Taskin et al. [25] described adhesion rates of 35%- 46.15%, Guida et al. [26] reported a range of 16%-33.3% while Touboul [27] gave a lower rate of 7.5%. No study in sub-Saharan Africa has put forward a relationship between IUA and type of infertility as well as previous history of uterine surgery relative to age of the patient. Be that as it may, our study reports a higher prevalence of IUA among older infertile women who presented with primary (49/61, 80.3%) or secondary infertility (240/277; 86.6%) than younger infertile women with primary (12/61; 19.7%) or secondary infertility (37/277; 13.4%). Older women with secondary infertility may have been trying to get pregnant and therefore subjected themselves to many interventions than younger women with primary infertility. The picture is clearer as our study also shows that a much higher proportion of older infertile women with IUA (277/316; 87.7%) gave history of previous uterine surgeries than younger infertile women with IUA (39/316; 12.3%). Most uterine pathologies that prevent the possibility of a woman getting pregnant are corrected by surgical interventions. Previously published data [14,19,24] show that intrauterine pathologies increase with increasing age, supporting the findings in our study that IUA occurred more among older than younger women. This is probably because older women, more than those aged <35 years, may have been more exposed to dilatation and curettage, caesarian section, infection and other conditions that predispose to IUA. Our study is the first in Africa to report that a higher proportion of overweight and obese IW presented with IUA while lesser proportion were without IUA. Increased weight can be a risk factor for conception as it contributes to ovulatory disorders. As a woman’s weight increases, insulin resistance may increase as well, leading to elevated production of male hormones, such as testosterone, which affect ovulation. However, we are not sure why IUA is more prominent among overweight and obese IW. The reason might probably be multi-factorial as inability to get pregnant might have led some women to psychogenic eating disorder that increased their weight while in the interim period these women attempted various surgical interventions to correct any anomaly. This might have resulted in IUA and unhealthy BMI.
To conclude, the present study demonstrated that infertile women with intrauterine adhesions were older than those without intrauterine adhesions and that the proportion of infertile women with intrauterine adhesions also increased with increase in body mass index till overweight when this proportion reduced. We also showed that overall, age, type of infertility and previous uterine surgery were risk factors for intrauterine adhesions; that at age <35 years only type of infertility was a risk factor to intrauterine adhesions but at the age of 35 years and over, type of infertility and history of previous uterine surgery were risk factors to intrauterine adhesions.
Study limitations and strength
There were certain limitations to this study which need to be mentioned. First, the study was retrospective and we had to rely on the correctness of the data as recorded in the medical records of the patients. Secondly, the control group was inherent in the overall sample size, extracted from those who had abnormal outcome of examination by hysteroscopy, specifically intrauterine adhesion. Another limitation of this study was that follow-up of the patients was not taken into consideration. The strength of the study was mainly in the relatively large sample size and use of modern gynecological equipment.
Conflict of Interests
The authors declares that there is no conflict of interest regarding the publication of this paper.
8830
References
- Golan A,Eilat E,Ron-El R,Herman A, Soffer Y, et al. (1996) Hysteroscopy is superior to hysterosalpingography in infertility investigation. Acta Obstetricia et GynecologicaScandinavica 75: 654-656.
- Sahu L,Tempe A,Gupta S (2012) Hysteroscopic evaluation in infertile patients: a prospective study. Int J Reprod Contracept Obstet Gynecol 1: 37-44.
- Bosteels J, Kasius J, Weyers S, Broekmans FJ, Mol BWJ (2013) Hysteroscopy for treating subfertility associated with suspected major uterine cavity abnormalities. Cochrane Database of Systematic Reviews.
- Taylor E, Gomel V (2008) The uterus and fertility. Fertility and Sterility 89: 1-16.
- Schild RL, Knobloch C, Dorn C, Fimmers R, van der Ven H, et al. (2001) Endometrial receptivity in an in vitro fertilization program as assessed by spiral artery blood flow, endometrial thickness, endometrial volume and uterine artery blood flow. Fertility and Sterility 75: 361-6.
- Palshetkar N, Pai H, Pisat S (2015) Role of Hysteroscopy Prior to Assisted Reproductive Techniques. Journal of Gynecological Endoscopy and Surgery 1: 27-30.
- Carneiro MM (2014) What Is the Role of Hysteroscopic Surgery in the Management of Female Infertility? A Review of the Literature. Surgery Research and Practice p: 6.
- Bakour SH, Jones SE, O’Donovan P (2006) Ambulatory hysteroscopy: evidence-based guide to diagnosis and therapy. Best Practice & Research Clinical Obstetrics & Gynecology 20: 953-975.
- Brown SE, Coddington CC, Schnorr J, Toner JP, Gibbons W, et al. (2000) Evaluation of outpatient hysteroscopy, saline infusion hysterosonography and hysterosalpingography in infertile women: a prospective, randomized study. Fertility and Sterility 74: 1029-34.
- Davey AK, Maher PJ (2007) Surgical adhesions: a timely update, a great challenge for the future,” Journal of Minimally Invasive Gynecology 14: 15-22.
- Gambadauro P, Gudmundsson J, Torrejon R (2012) Intrauterine Adhesions following Conservative Treatment of Uterine Fibroids. Obstetrics and Gynecology International 2012: 6
- Deans R, Abbott J (2010) Review of intrauterine adhesions. Journal of Minimally Invasive Gynecology 17: 555-569.
- Practice Committee (2006) American Society for Reproductive Medicine, Birmingham, Alabama. Aging and infertility in women. Fertility and Sterility 86: S249-S251.
- Nagele F, O’Connor H, Davies A, Badawy A, Mohamed H, et al. (1996) 2500 outpatient diagnostic hysteroscopies. Obstet Gynecol 88: 900-901
- Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, et al. (1994) Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol 171: 171-177.
- Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ (2002) Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology13: 184-90.
- Siam S (2015) Role of office hysteroscopy in the evaluation of infertile women after controlled ovarian stimulation/intra uterine insemination failure, Middle East Fertil Soc J.
- El-Mazny A, Abou-Salem N, El-Sherbiny W, Saber W (2011) Outpatient hysteroscopy: a routine investigation before assisted reproductive techniques? Fertility and Sterility 95: 272-276.
- Koskas M, Mergui J, Yazbeck C, Uzan S, Nizard J (2010) Office Hysteroscopy for Infertility: A Series of 557 Consecutive Cases. Obstetrics and Gynecology International p: 4.
- Magos A, Al-Khouri A, Scott P (2005) “One stop fertility clinic,” Journal of Obstetrics and Gynaecology 25: 153-159.
- Dicker D, Goldman JA, Ashkenazi J, Feldberg D, Dekel A (1990) The value of hysteroscopy in elderly women prior to in vitro fertilization-embryo transfer (IVF-ET): a comparative study. Journal of In-vitro Fertilization and Embryo Transfer 7: 267-270.
- Saed GM, Diamond MP (2004) Molecular characterization of postoperative adhesions: the adhesion phenotype. Journal of the American Association of Gynecologic Laparoscopists 11: 307-314.
- Shavell VI, Saed GM, Diamond MP (2009) Cellular metabolism: contribution to postoperative adhesion development. Reproductive Sciences 16: 627-634.
- Fatemi HM, Kasius JC, Timmermans A, van Disseldorp J, Fauser BC (2010) Prevalence of unsuspected uterine cavity abnormalities diagnosed by office hysteroscopy prior to in vitro fertilization. Human Reproduction p. 1-7.
- Taskin O, Sadik S, Onoglu A (2000) Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. Journal of the American Association of Gynecologic Laparoscopists 7: 351-354.
- Guida M,Acunzo G,Sardo ADS,Bifulco G,Piccoli R, et al.(2004) Effectiveness of auto-cross-linked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: a prospective, randomized, controlled study. Human Reproduction 19: 1461-1464.
- Touboul C, Fernandez H, Deffieux X, Berry R, Frydman R (2009) Uterine synechae after bipolar hysteroscopic resection of submucosal myomas in patients with infertility. Fertility and Sterility 92: 1690-1693.









