Research Article - (2023) Volume 17, Issue 5
Blood Arsenic contamination causing Breast Cancer Risk in Exposed Population of Bihar
Yerravarapu Vamsi Krishna1,
Chandrajeet Kumar1 and
Arun Kumar2*
1Department of Biochemistry, YBN University, Ranchi, Jharkhand, India
2Mahavir Cancer Sansthan and Research Centre, Patna, Bihar, India
*Correspondence:
Arun Kumar, Mahavir Cancer Sansthan and Research Centre, Patna, Bihar,
India,
Tel: +91-9334740800,
Email:
Received: 02-May-2023, Manuscript No. iphsj-23-13742;
Editor assigned: 04-May-2023, Pre QC No. iphsj-23-13742 (PQ);
Reviewed: 18-May-2023, QC No. iphsj-23-13742;
Revised: 23-May-2023, Manuscript No. iphsj-23-13742(R);
Published:
30-May-2023
Abstract
Groundwater arsenic poisoning in the recent times has posed serious health hazards to human beings. After the long-term exposure to arsenic, it has also caused disease of cancer in them. Breast cancer is the disease, which has become a fast-growing disease in the women in the present times. The exposed population is posed to hormonal imbalance due to arsenic poisoning which causes breast carcinogenesis.
In the present study, n=203 women subjects were voluntarily selected for breast cancer study and their blood samples were collected for arsenic estimation by Atomic Absorption Spectrophotometer against the control female subjects n=100.
The study reveals that there is significant elevation in the blood arsenic concentration in the breast cancer patients. Out of n=203, female breast cancer patients, n=148 subject’s blood had significant blood arsenic concentration (72%), while n-55 subject’s blood arsenic concentration in normal levels (28%). The maximum arsenic concentration observed in the breast cancer patient was 1620μg/L, which is very significant. Moreover, the control female subjects didn’t have any significant blood arsenic concentration, as only n=03 subjects had very mild increased blood arsenic concentration.
In the state of Bihar, the exposed women population due to prolonged arsenic poisoning develop hormonal imbalance in their body. Due to elevated estrogen hormone, there is significant stress on the other regulated hormones such as prolactin. This elevated prolactin for long duration causes formation of lumps in the breast. The untreated breast lumps causes inflammation in the breast lobules, which in long duration time of 10-15 years gets converted into to malignant, which is the major cause of the breast carcinogenesis. Hence, the elevated arsenic levels are associated with the linkage between arsenic and breast cancer carcinogenesis.
Keywords
Breast Cancer; Blood Arsenic Contamination; Gangetic Plains; Hormonal Imbalance
Introduction
In the entire world, about 300 million people are exposed to arsenic poisoning and as such in India; about 70 million people are exposed. In Bihar, about 10 million people in the present scenario are exposed to arsenic through the drinking of contaminated water. In the Gangetic plains of Bihar, arsenic has caused serious health hazards in the exposed population of Bihar. Arsenic is moreover is also called the king of poison or, the poison of kings. The trivalent form of arsenic has got a special point of consideration because of its hazardous nature. According to WHO (world health organization), exposure of 10ppb is the maximum permissible limit for inorganic arsenic in the drinking water [1] The US EPA also set a limit for total inorganic arsenic in the drinking water that is 10μg/L [2] Not only drinking water but also food crops are becoming a major route for arsenic exposure due to the usage of arsenic-contaminated groundwater for irrigation. The source of arsenic is geogenic and it is present in the alluvial sediment of the Delta [3, 4]. Arsenic contamination of groundwater in southern Asia affects tens of millions of people. In the southern Asian lowlands, high population density coincides with dangerous levels of arsenic in the groundwater [5]. According to the latest estimates in 2014 by the Council of Scientific and Industrial Research (CSIR), in India alone, more than 7 Crore people are exposed to the risk of arsenic in drinking water, most of them reside at the Ganga Basin. The most exposed countries globally are Argentina, Bangladesh, Bolivia, Brazil, Chile, China, Cambodia, Ghana, Greece, Hungary, India, Japan, Korea, Mexico, Mongolia, Nepal, New Zealand, Poland, Taiwan, Vietnam, and the USA.
Presently in Bihar, arsenic contamination in the groundwater is reported from 22 districts, threatening more than 10 million people in the state [6, 7]. The groundwater arsenic contamination in Bihar was first reported in Samaria Ojha Patti village of Shahpur, a block of Bhojpuri district in 2002 [8, 9] reported groundwater arsenic contamination in 50 blocks in 11 districts of Bihar. Recently, Singh and Ghosh (2012) estimated that there is a very high health risk in the arsenic-contaminated areas in the Maner block of Patna district. Moreover, the arsenic affected areas of Bihar, where the level of arsenic in drinking water exceeded 1000μg/L are Bhojpur, Patna, Samastipur, and Bhagalpur districts. More than 50μg/L of arsenic was detected in Vaishali, Saran, Begusarai, Khagaria, Munger, and Katihar districts [10-14].
Breast cancer disease in women in the recent times has increased many folds in the country. The common etiology of the disease is hereditary factors, reproductive factors, hormonal imbalance, women who discontinue breastfeeding etc. Moreover, in the recent times the environmental factors are also thought to be the causative agent behind the cause of breast cancer. The environmental pollutants could be pesticides, heavy metals and metalloids such as arsenic [15-20]. The breast cancer incidences in the recent times have increased many folds in the Gangetic plains of Bihar. Unfortunately, in this area arsenic poisoning in groundwater has also been reported severely. Hence, the present study tends to find out the association between the arsenic intoxication and breast cancer disease prevalence.
Materials and Methods
Ethical Approval: The study was approved from the Ethics Committee (IEC) of Mahavir Cancer Sansthan and Research Centre with the approved IEC No. MCS/Research/2019-2020/11, dated 08/01/2019. Informed consent was obtained from the studied breast cancer patients and the control subjects.
Location
The study was carried out in our institute itself in Mahavir Cancer Sansthan and Research Centre, Patna, Bihar, India. The study was carried out from January 2019 and was completed in November 2019.
Selection of the subjects for the study
A total of n= 100 control normal subjects were selected for the study, while for the cross-sectional design, n=203 subjects with pathologically confirmed breast disease were selected as the arsenic exposed group in the study.
Collection of blood samples for the study
Collection of blood samples: About 5ml of blood by volume were collected from the peripheral vein of the arm of the patients using the disposable syringes and then after were transferred to the heparinized vacationer as per the IUPAC guidelines [21]. The blood samples were stored in -80 degree centigrade for the further use.
Estimation of blood arsenic contamination
In the present study for the blood arsenic estimation, an volume of 0.5ml of whole blood sample were taken in 30ml conical flask (glass) and for overnight reaction to it, 5ml of HNO3 was added. The following day, the overnight left samples were digested on hotplate at 90oC-120oC, until the sample reached to 3ml volume. Then in the conical flask to the pre-digested solution, 5ml volume of HNO3:HClO4 (6:1) mixture was added. The samples were redigested on the hotplate at 90oC – 120oC until the volume of the solution reached to about 2ml. The final volume was adjusted to 10ml with distilled water after rinsing it with 1% HNO3 and was filtered through Whatman filter paper no.41 for the final reading on Graphite Furnace Atomic Absorption Spectrophotometer (GFAAS).
Statistical Analysis
All the data were analysed using the software Graph Pad Prism 5.0 and the values were generated as Mean ± SEM. The data variables were also analysed statistically through one-way analysis of variance (ANOVA) by using the Dennett’s test. The scattered plots were made using the statistical software SPSS- 16.0 using the linear regression analysis model as earlier carried out by [22, 23].
Results
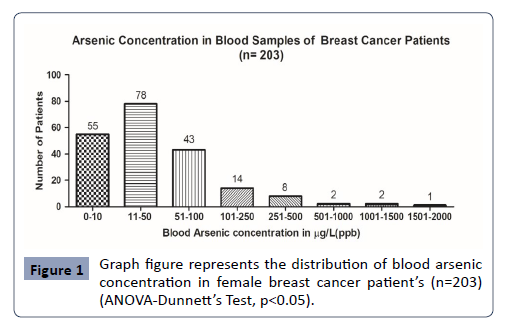
Breast Cancer Patient’s Blood arsenic concentration: In the present study, in n= 203 breast cancer patient’s blood arsenic concentration was analysed with maximum arsenic concentration of 1620μg/L. The minimum value of arsenic concentration observed was 1.34μg/L (Figure 1).
Figure 1: Graph figure represents the distribution of blood arsenic concentration in female breast cancer patientâ??s (n=203) (ANOVA-Dunnettâ??s Test, p<0.05).
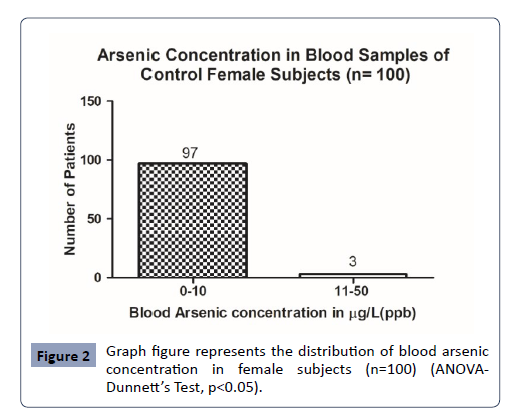
Control Subjects Blood arsenic concentration: In the present study, in n= 100 control female subjects the blood arsenic concentration was analysed with maximum arsenic concentration of 13.7 μg/L. The minimum value of arsenic concentration observed was 0.56μg/L (Figure 2).
Figure 2: Graph figure represents the distribution of blood arsenic concentration in female subjects (n=100) (ANOVADunnettâ??s Test, p<0.05).
Discussion
Arsenic in the recent times has caused serious health hazards among the exposed population. Female subjects are severely affected with arsenic poisoning as arsenic mimics the estrogen hormone, which in turn causes hormonal imbalance in the females. This leads to elevation in the levels of the hormones such as estrogen, luteinizing, prolactin and oxytocin. The elevated prolactin’s causes formation of breast lumps which in long run causes cancer of breast [24, 25]. Moreover, the AsIII is more toxic than the AsV due to instability of the compounds [26-31]. The arsenic is usually eliminated through the body via the metabolic organ’s liver and kidney.
In the present study, there is significant elevation in the blood arsenic concentration in the breast cancer patients. Out of n=203, female breast cancer patients, n=148 subject’s blood had significant blood arsenic concentration (72%), while n-55 subject’s blood arsenic concentration in normal levels (28%). The maximum arsenic concentration observed in the breast cancer patient was 1620μg/L, which is very significant. Moreover, the control female subjects didn’t have any significant blood arsenic concentration, as only n=03 subjects had very mild increased blood arsenic concentration. Recently, very high blood arsenic concentration and its association with cancer risk has been reported [32-34].
According to IARC, EPA and WHO, arsenic is considered as category- I carcinogen, causing cancer of Gallbladder, skin, lung, kidney, bladder and colorectal cancer (Kumar et al., 2023; Palma- Lara et al., 2015; Cuzick 2017. Arsenic poisoning due to prolonged exposure causes deregulated signaling pathways which causes breast carcinogenesis. This wrong signaling is involved in BCL- 2, PTEN, MLH, MMP-2 and Bax gene transformations which are significantly mutated due to arsenic toxicity [35-49].
In the state of Bihar, the exposed women population due to prolonged arsenic poisoning develop hormonal imbalance in their body. Due to elevated estrogen hormone, there is significant stress on the other regulated hormones such as prolactin. This elevated prolactin for long duration causes formation of lumps in the breast. The untreated breast lumps causes inflammation in the breast lobules, which in long duration time of 10-15 years gets converted into to malignant, which is the major cause of the breast carcinogenesis [50-57]. In the present study, there is significant arsenic contamination found in the blood samples of breast cancer, which denotes that it could be the agent which is causing the breast carcinogenesis [58-76].
Conclusion
The present study, significantly demonstrates the increase in the blood arsenic concentration in the 72% of studied breast cancer patients. Moreover, these patients were from the Gangetic plains of Bihar. The elevated arsenic levels associate the linkage between arsenic and breast cancer carcinogenesis. Furthermore, study is required to validate the association between arsenic and breast cancer.
Acknowledgement: The authors are thankful to YBN University and Mahavir Cancer Sansthan and Research Centre (MCSRC) for providing all necessary infrastructures required for this study. The financial assistance for the study was provided by the University itself.
Author contributions
Y.V.K, C.K and A.K conceptualized the entire work. Y.V.K is the principal author and had the major contributions in writing the manuscript but support was also provided by A.K. and C.K., literature search was done by Y.V.K., experimental work and data analysis were done by Y.V.K and A.K, final data interpretation was done by Y.V.K, C.K and A.K. All authors read and approved the final manuscript.
References
- Alatise OI, Schrauzer GN (2010) Lead exposure: a contributing cause of the current breast cancer epidemic in Nigerian women. Biol Trace Elem Res 136: 127-139.
Indexed at, Google Scholar, Crossref
- Benderli Cihan Y, Sozen S, Ozturk Yildirim S (2011) Trace elements and heavy metals in hair of stage III breast cancer patients. Biol Trace Elem Res 144: 360-79.
Indexed at, Google Scholar, Crossref
- Benner S (2010) Arsenic occurs naturally in the groundwater of southern Asia. Analyses of an agricultural site in Bangladesh suggest that human activities, including widespread farming practices, can dictate where elevated arsenic is found. Nature Geoscience 3:5-6.
Indexed at, Google Scholar, Crossref
- Bibi MH, Ahmed F, Ishiga H (2008) Geochemical study of arsenic concentrations in groundwater of the Meghna River Delta, Bangladesh. J Geochemical Explore 97: 43-58.
Indexed at, Google Scholar, Crossref
- Blaurock-Busch E, Busch YM, Friedle A et al. (2014) comparing the metal concentration in the hair of cancer patients and healthy people lives in the Malwa region of Punjab, India. Clin Med Insights Oncol 8: 1-13.
Indexed at, Google Scholar, Crossref
- Bundschuh J, Litter M, Bhattacharya P (2010) Targeting arsenic-safe aquifers for drinking water supplies. Env Geochem Health 32: 307-315.
Indexed at, Google Scholar
- Calaf GM (2021) Role of organ phosphorous pesticides and acetylcholine in breast carcinogenesis. Seminars in cancer biology 76: 206-217.
Indexed at, Google Scholar, Crossref
- Casentini B, Hug SJ, Nikolaidis NP (2011) Arsenic accumulation in irrigated agricultural soils in Northern Greece. The Science of the Total Environment 409: 4802-4810.
Indexed at, Google Scholar, Crossref
- Chakraborti D, Singh SK, Rahman MM, Dutta RN, Mukherjee SC et al. (2018) Groundwater Arsenic Contamination in the Ganga River Basin: A Future Health Danger. Int J Environ Res Public Health 15: 180.
Indexed at, Google Scholar, Crossref
- Chakraborti D, Mukherjee SC, Pati S, Sengupta MK, Rahman MM et al. (2003) Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: a future danger. Environ Health Persp 111: 1194-1201.
Indexed at, Google Scholar, Crossref
- Chakraborti D, Sengupta MK, Rahman MM, Ahamed S, Chowdhury UK (2004) Groundwater arsenic contamination and its health effects in the Ganga-Meghna-Brahmaputra plain. J Env monitoring JEM 6: 74N-83N.
Indexed at, Google Scholar, Crossref
- Chakraborti D, Singh SK, Rahman MM, Dutta RN, Mukherjee SC (2018) Groundwater Arsenic Contamination in the Ganga River Basin: A Future Health Danger. Int J Env Res public health 15: 180.
Google Scholar, Crossref
- Chanda S, Dasgupta UB, Guhamazumder D (2006) DNA hyper methylation of promoter of gene p53 and p16 in arsenic-exposed people with and without malignancy. Toxicol Sci 89: 431-37.
Indexed at, Google Scholar, Crossref
- Cornelis R, Heinzow B, Herber RF (1995) Sample collection guidelines for trace elements in blood and urine (technical report). Pure Appl Chem 67: 1575-1608.
Indexed at, Google Scholar, Crossref
- Cui X, Wakai T, Shirai Y, Hatakeyama K, Hirano S (2006) Chronic oral exposure to inorganic arsenate interferes with methylation status of p16INK4a and RASSF1A and induces lung cancer in A/J mice. Toxicol Sci 91: 372-381.
Google Scholar
- Danes JM, de Abreu ALP, Kerketta R (2020) Inorganic arsenic promotes luminal to basal transition and metastasis of breast cancer. FASEB J 34: 16034-16048.
Indexed at, Google Scholar, Crossref
- Dantzig PI (2009) Breast cancer, dermatofibromas and arsenic. Indian J Dermatol 54: 23-25.
Indexed at, Google Scholar, Crossref
- Davey JC, Bodwell JE, Gosse J (2007) Arsenic as an endocrine disruptor: effects of arsenic on oestrogen receptor-mediated gene expression in vivo and in cell culture. Toxicol Sci 98: 75-86.
Indexed at, Google Scholar, Crossref
- Davey JC, Nomikos AP, Wungjiranirun M, Sherman JR, Ingram L (2008) Arsenic as an endocrine disruptor: arsenic disrupts retinoic acid receptor-and thyroid hormone receptor-mediated gene regulation and thyroid hormone-mediated amphibian tail metamorphosis. Env health persp 116: 165-172.
Indexed at, Crossref
- Easton DF, Pharoah PD, Antoniou AC (2015) Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 372: 2243-2257.
Indexed at, Google Scholar, Crossref
- Foster H, Kennedy G, Maisonneuve PA (2008) Case-control study of toenail selenium, mercury, arsenic and cadmium and cancer of the breast, colon and prostate in Montreal. Trends Cancer Res 4: 15-8.
Indexed at, Google Scholar
- Gamboa-Loira B, Cebrian ME, Salinas-Rodriguez A (2017) Genetic susceptibility to breast cancer risk associated with inorganic arsenic exposure. Environ Toxicol Pharmacol 56: 106-113.
Indexed at, Google Scholar, Crossref
- Gamboa-Loira B, Cebrian ME, Salinas-Rodriguez A (2017) Genetic susceptibility to breast cancer risk associated with inorganic arsenic exposure. Environ Toxicol Pharmacol 56: 106-113.
Indexed at, Google Scholar, Crossref
- Garland M, Morris JS, Colditz GA (1996) Toenail trace element levels and breast cancer: a prospective study. Am J Epidemiol 144: 653-660.
Indexed at, Google Scholar, Crossref
- Girard L, Reix N, Mathelin C (2020) Impact des pesticides perturbations endocriniens sur le cancer du sein Gynecologie, obstetrique fertilite senologie 48: 187-195.
Indexed at, Google Scholar, Crossref
- Goyal A, Sahu RK, Kumar M (2019) Promoter methylation, expression, and its association with estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 subtype of breast carcinoma. J Can Res Ther 15: 1147-1154.
Google Scholar
- Joo NS, Kim SM, Jung YS (2009) Hair iron and other minerals’ level in breast cancer patients. Biol Trace Elem Res 129: 28-35.
Indexed at, Google Scholar, Crossref
- Kortenkamp A (2011) Are cadmium and other heavy metal compounds acting as endocrine disrupters. Met Ions Life Sci 8: 305-317.Â
Indexed at, Google Scholar, Crossref
- Kumar A, Rahman MS, Kumar R, Ali M, Niraj PK (2019) Arsenic contamination in groundwater causing impaired memory and intelligence in school children of Simri village of Buxar district of Bihar. J Mental Health Hum Behav 24:132-138.
Google Scholar, Crossref
- Kumar A, Ghosh AK (2019) Arsenic and Cancer. In Environmental Exposures and Human Health Challenges 106-132.
Google Scholar
- Kumar A, Ali M, Kumar R, Kumar M, Sagar P et al. (2021) Arsenic exposure in Indo Gangetic plains of Bihar causing increased cancer risk. Scientific reports 11: 2376.
Indexed at, Google Scholar, Crossref
- Kumar A, Ali M, Kumar R, Kumar M, Sagar P (2021) Arsenic exposure in Indo Gangetic plains of Bihar causing increased cancer risk. Scientific reports 11:2376.
Indexed at, Google Scholar, Crossref
- Kumar A, Ali M, Rahman SM, Iqubal AM, Anand G et al. (2015) Ground water arsenic poisoning in Tilak Rai Ka Hatta village of Buxar district, Bihar, India causing severe health hazards and hormonal imbalance. J Environ Anal Toxicol 5: 1-7.
Google Scholar
- Kumar A, Ali M, Raj V, Kumari A, Rachamalla M (2023) Arsenic causing gallbladder cancer disease in Bihar. Scientific reports 13: 4259.
Indexed at, Google Scholar
- Kumar A, Ghosh AK (2021) Assessment of arsenic contamination in groundwater and affected population of Bihar. N. Kumar (ed.), Arsenic Toxicity: Challenges and Solutions.
Google Scholar
- Kumar A, Kumar R, Rahman MS, Ali M, Kumar R (2021) Assessment of arsenic exposure in the population of Sabalpur village of Saran District of Bihar with mitigation approach. Environmental science and pollution research international
Google Scholar, Crossref
- Kumar A, Rahman MS, Ali M, Salaun P, Gourain A (2022) Assessment of disease burden in the arsenic exposed population of Chapar village of Samastipur district, Bihar India and related mitigation initiative. Env Sci pollution Res Int 29: 27443-27459.
Google Scholar, Crossref
- Kumar A, Rahman MS, Iqubal MA, Ali M, Niraj PK (2016) Ground Water Arsenic Contamination: A Local Survey in India. Int J prev med 7: 100.
Indexed at, Google Scholar, Crossref
- Kumar A, Rahman MS, Ali M, Kumar R, Niraj PK et al. (2021) Assessment of arsenic exposure and its mitigation intervention in severely exposed population of Buxar district of Bihar, India. Toxicol Environ Health Sci.
Crossref
- Kumar A, Ravi C, Dhingra S, Krishna Murti MA, Ghosh AK (2022) Arsenic Causing Gallbladder Cancer Disease near the Himalayan bound Rivers in Bihar: A Case study of Gallbladder Cancer. J Cancer Sci Clin Therap 6: 388-391.
Indexed at, Google Scholar, Crossref
- Kumar A, Ravi C, Dhingra S, Krishna Murti MA, Ghosh AK (2022) Arsenic Causing Gallbladder Cancer Disease near the Himalayan bound Rivers in Bihar: A Case study of Gallbladder Cancer. Journal of Cancer Science and Clinical Therapeutics 6: 388-391.
Indexed at, Google Scholar
- Kuo CC, Moon KA, Wang SL, Silbergeld E, Navas-Acien A (2017) the Association of Arsenic Metabolism with Cancer, Cardiovascular Disease, and Diabetes: A Systematic Review of the Epidemiological Evidence. Environ Health Perspect 125: 087001.
Indexed at, Google Scholar, Crossref
- Ledda C, Bracci M, Lovreglio P, Senia P, Larrosa M (2021) Pesticide exposure and gender discrepancy in breast cancer. European review for medical and pharmacological sciences, 25: 2898-2915.
Crossref
- Lopez-Carrillo L, Hernandez-Ramirez RU, Gandolfi AJ (2014) Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol Appl Pharmacol 280: 53-59.
Google Scholar, Crossref
- Lopez-Carrillo L, Hernandez-Ramirez RU, Gandolfi AJ, Ornelas-Aguirre JM, Torres-Sanchez L et al. (2014) Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol Appl Pharmacol 280: 53-59.
Indexed at, Google Scholar
- Marciniak W, Derkacz R, Muszynska M (2020) Blood arsenic levels and the risk of familial breast cancer in Poland. Int J Cancer 146: 2721-2727.
Crossref
- Marsit CJ, Karagas MR, Schned A, Kelsey KT (2006) Carcinogen exposure and epigenetic silencing in bladder cancer. Ann N Y Acad Sci 1076: 810-821.
Indexed at, Google Scholar
- Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL (2011) Arsenic exposure and the induction of human cancers. J Toxicol 2011: 431287.
Google Scholar, Crossref
- Mass MJ, Wang L (1997) Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for a mechanism of carcinogenesis. Mutate Res 386: 263-277.
Indexed at, Google Scholar, Crossref
- Michel-Ramirez G, Recio-Vega R, Lantz RC (2020) Assessment of YAP gene polymorphisms and arsenic interaction in Mexican women with breast cancer. J Appl Toxicol 40: 342-351.
Google Scholar, Crossref
- Mukherjee A, Sengupta MK, Hossain MA, Ahamed S (2006) Arsenic Contamination in Groundwater: A Global Perspective with Emphasis on the Asian Scenario. J Health Popul Nutr 24: 142-163.
Indexed at, Google Scholar
- Nickson R, Sengupta C, Mitra P, Dave S, Banerjee A (2007) Current Knowledge on the Distribution of Arsenic in Groundwater in Five States of India. J Env Sci Health Part A 42:1707-1718.
Indexed at, Google Scholar
- Nielsen FC, van Overeem Hansen T, Sorensen CS (2016) hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer 16: 599-612.
Indexed at, Google Scholar, Crossref
- Palma-Lara P I, Martinez-Castillo M, Quintana-Perez JC (2020) Arsenic exposure: A public health problem leading to several cancers. Regul Toxicol Pharmacol 110: 104539.
Indexed at, Google Scholar, Crossref
- Paydar P, Asadikaram G, Fallah H, Zeynali Nejad H, Akbari H (2021) Serum levels of Organochlorine Pesticides and Breast Cancer Risk in Iranian Women.Environ Contam Toxicol 77: 480-489.
Indexed at, Google Scholar
- Pineda-Belmontes CP, Hernandez-RamIrez RU, Hernández-Alcaraz C Mex (2016) Genetic polymorphisms of PPAR gamma, arsenic methylation capacity and breast cancer risk in Mexican women. Salud Publica 58: 220-700.
Indexed at, Google Scholar, Crossref
- Pullella J, Kotsopoulos KJ (2020) Arsenic Exposure and Breast Cancer Risk: A Re-Evaluation of the Literature. Nutrients 12: 3305.
Indexed at, Google Scholar, Crossref
- Ravenscroft P, Brammer H, Richards KS (2009) Arsenic pollution: A global synthesis. UK: Wiley-Blackwell.
Indexed at, Google Scholar, Crossref
- Reichard JF, Puga A (2010) Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics 2: 87-104.
Indexed at, Google Scholar, Crossref
- Ren X, McHale CM, Skibola CF, Smith AH, Smith MT et al. (2011) An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ Health Perspect 119: 11-19.
Indexed at, Google Scholar, Crossref
- Rocha PRS, Oliveira VD, Vasques CI, Dos Reis AA (2021) Exposure to endocrine disruptors and risk of breast cancer: A systematic review. Critical reviews in oncology/ hematology, 161: 103330.
Indexed at, Google Scholar, Crossref
- Saha D (2009) Arsenic groundwater contamination in parts of middle Ganga plain, Bihar. Current Science 97: 753-755.
Indexed at, Google Scholar, Crossref
- Sahoo PK, Kim K (2013) A review of the arsenic concentration in paddy rice from the perspective of geoscience. Geoscie J 17: 107-122.
Indexed at, Google Scholar, Crossref
- Sanz E, Munoz-Olivas R, Camara C, Sengupta MK, Ahamed S (2007) Arsenic speciation in rice, straw, soil, hair and nails samples from the arsenic-affected areas of Middle and Lower Ganga plain. J Environ Sci Health A Toxicol Hazard Subst Environ Eng 42: 1695â??1705.
Indexed at, Google Scholar, Crossref
- Selmin OI, Donovan MG, Skovan B, Paine-Murieta GD, Romagnolo DF (2019) Arsenic induced BRCA1 CpG promoter methylation is associated with the down regulation of ERa and resistance to tamoxifen in MCF7 breast cancer cells and mouse mammary tumor xenografts. Int J Oncol 54: 869-878.
Indexed at, Google Scholar, Crossref
- Singh SK, Ghosh AK (2012) Health Risk Assessment due to Groundwater Arsenic Contamination: Children are at High Risk, Human and Ecological Risk Assessment. An Int J 18: 751-766.
Indexed at, Google Scholar, Crossref
- Singh SK, Ghosh AK, Kumar A, Kislay K, Kumar et al. (2017) Â Groundwater Arsenic Contamination and Associated Health Risks in Bihar, India. Int J Env Res 8: 49-60.
Indexed at, Google Scholar, Crossref
- Smedley PL, Kinniburgh DG (2002) A review of the source, behavior and distribution of arsenic in natural waters. Applied Geochemistry. 17: 517-568.
Indexed at, Google Scholar, Crossref
- SOES (2012) School of Environmental Studies, Groundwater arsenic contamination in middle Ganga plain, Bihar, India: A Future Danger? School of Environmental Science, Jabalpur University, Kolkata, India.
Indexed at, Google Scholar, Crossref
- Soh MA, Garrett SH, Somji S (2011) Expression in breast cancer Arsenic, cadmium and neuron specific Enolase. Cancer Cell Int 11: 41.
Indexed at, Google Scholar, Crossref
- Thakur JK, Thakur RK, Ramanathan AL, Kumar M, Singh SK (2011) Arsenic contamination of groundwater in Nepal-An overview Water 3: 1-20.
Indexed at, Google Scholar, Crossref
- US EPA. (2007) Guidance for evaluating the oral bioavailability of metals in soils for use in human health risk assessment. OSWER. 9285: 7-80.
Indexed at, Google Scholar, Crossref
- Wadhwa SK, Kazi TG, Afridi HI, Talpur FN, Naeemullah (2015) Interaction between carcinogenic and anti-carcinogenic trace elements in the scalp hair samples of different types of Pakistani female cancer patients. Clin Chim Acta 439: 178-184.
Indexed at, Google Scholar, Crossref
- WHO (2010) Exposure to arsenic: A major public health concern, In Preventing disease through healthy environments. 1-5.
Indexed at, Google Scholar, Crossref
- Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP (1997) Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc National Academy Sci USA 94: 10907-1012.
Indexed at, Google Scholar, Crossref
- Zimta AA, Schitcu V, Gurzau E (2019) Biological and molecular modifications induced by cadmium and arsenic during breast and prostate cancer development. Environ Res 178: 108700.
Indexed at, Google Scholar, Crossref
Citation: Kumar A, Kumar C, Krishna YV (2023) Blood Arsenic Contamination Causing Breast Cancer Risk in Exposed Population of Bihar. Health Sci J. Vol. 17 No. 5: 1017