Keywords
NIRS, blood transfusion, tissue oxygenation, beta-thalassemia
Introduction
Beta-thalassemia is a hereditary disease that results from mutations causing decreased synthesis of structurally normal β-globin chains. Deficiency of β-chain synthesis results in an accumulation of α-chains which are unstable and aggregate, forming insoluble inclusions in bone-marrow erythroid precursors [1]. These are harmful and affect red cell survival. Severe anemia compromises the oxygen-carrying capacity of blood and results in an accelerated decrease of capillary oxygen pressure [2]. Regular transfusions, to maintain pre-transfusion hemoglobin (Hb) levels of about 9-10 g/dl, constitute the basis of treatment, permit normal growth and development and suppress endogenous erythropoiesis and Hb synthesis [3]. A primary goal of red blood cell (RBC) transfusion is to increase the availability of oxygen-saturated Hb in microcirculation and the tissue oxygen content.
Near Infrared Spectroscopy (NIRS), with the vascular occlusion technique, is a simple, non-invasive method which allows estimation of tissue oxygenation, yielding also information on oxygen consumption and function of microcirculation [4].
In anemic states, fractional oxygen extraction increases to provide the oxygen required for aerobic metabolism, and an increased Hb desaturation ensues.
This could affect peripheral oxygenation signals obtained by NIRS. However, there are no studies, so far, evaluating tissue oxygenation and related microcirculatory alterations in patients suffering from beta-thalassemia major and/or possible differences after blood transfusions.
With the present study we aimed to investigate the effect of RBC transfusion on peripheral microcirculation in beta-thalassemia major patients.
Methodology
Patients
The study was approved by the Scientific Council and the Ethics committee of our hospital. The population study consisted of patients of the Thalassaemia Unit of the 1st Department of Paediatrics, of Athens University, attending a regular transfusion program. All patients were receiving chelation therapy with either desferrioxamine (40 mg/kg/day, 10-12 h sc, 5 days/week), or deferiprone (75 mg/kg/day, per os) or both. At the time of the study, all patients were clinically stable, under appropriate medical treatment. Patients with symptoms or signs of respiratory or cardiac disease were excluded from the study.
Informed consent was obtained from all patients before their evaluation.
NIRS measurements
The principles of NIRS and its application in vivo have been described elsewhere [5-7]. In short, NIRS uses the principles of light transmission and chromophore absorption and measures, non-invasively, the percentage saturation of Hb with O2 in microcirculation.
A probe is placed on the surface of the skin. Light in the infrared region (680-800 nm) is transmitted from the probe and is absorbed, mainly, by the heme containing Hb at the level of small arterioles, capillaries and venules in tissues. Its penetration depth enables the measurement of tissue oxygen saturation (StO2) in the corresponding muscle.
In the present study, thenar-muscle StO2 was measured non-invasively with NIRS (InSpectra; Hutchinson Technology; Hutchinson; MN). The measurement was carried out with the patient in a supine position after a resting period of 15 minutes. StO2 was monitored continuously during upper limb ischemia with a 25mm probe. Vascular occlusion was obtained with a pneumatic cuff that was placed above the elbow and inflated to 50 mmHg above the patients’ systolic blood pressure. The occlusion was retained for three minutes and variations in StO2 were recorded. No muscular contractions in the upper limbs were allowed during measurements [6,8-10].
The measurements were carried out before and 30 min after the completion of the transfusion.
StO2 curves were analyzed by InSpectra Analysis Program version 2.0 (Hutchinson Technology, USA) running in Mat Lab 7.0 (Math Works, USA). Linear regression was used to extrapolate the O2 consumption rate (%/min) of the thenar muscle during stagnant limb ischemia and the consequent oxygen reperfusion rate (%/min) after the release of the vascular occlusion, which reflects endothelial function.
Statistical analysis
Data were analyzed with Wilcoxon’s test for paired data and Repeated Measures Analysis of Covariance (ANCOVA) using SPSS 13.0 (Chicago IL, 2003). Normality of distribution of continuous variables was assessed with Kolmogorov-Smirnov test. Linear regression was performed as appropriate. In all cases, statistical significance level was set at p<0.05.
Results
Seventeen consecutive patients (7 males, 10 females) were included in the study. Six patients had previously undergone splenectomy. Mean age (±SD) was 25.7±9.3 years, body mass index (BMI) 24.1±3.3 kg/m2, body surface area (BSA) 1.68±0.18 m2, serum ferritin [median (interquartile range)] 2511 (870 – 5355) ng/ml and cardiac ejection fraction (EF) 63.7±7.0%. The amount of packed leukocyte-depleted RBC transfused was 7.85±1.98 ml/kg.
Measured variables before and after RBC transfusion are shown in table 1. Data are presented with median values and the corresponding interquartile ranges. RBC transfusion produced a significant increase of the hematocrit and the Hb concentration.
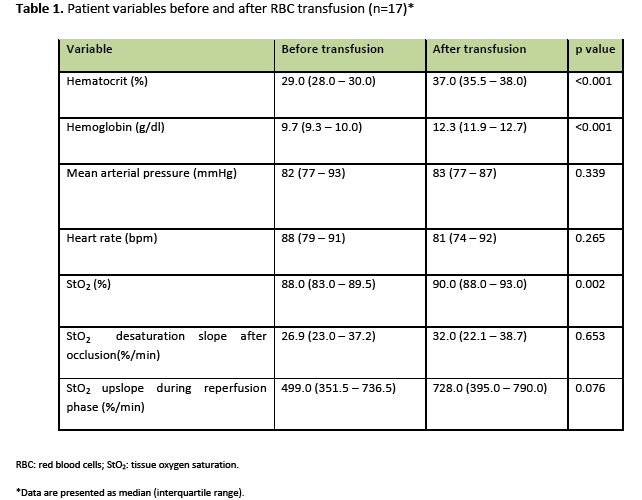
StO2 increased significantly after RBC transfusion compared to baseline (90% vs. 88% respectively, p=0.002). Delta StO2 (StO2 change after RBC transfusion) showed a significant negative correlation with baseline StO2), which means that RBC transfusion conferred a greater StO2 increase in patients with lower baseline StO2 (figure 1). The O2 consumption rate showed no significant change after RBC transfusion, while the reperfusion rate displayed an increasing trend of borderline significance (p=0.076).
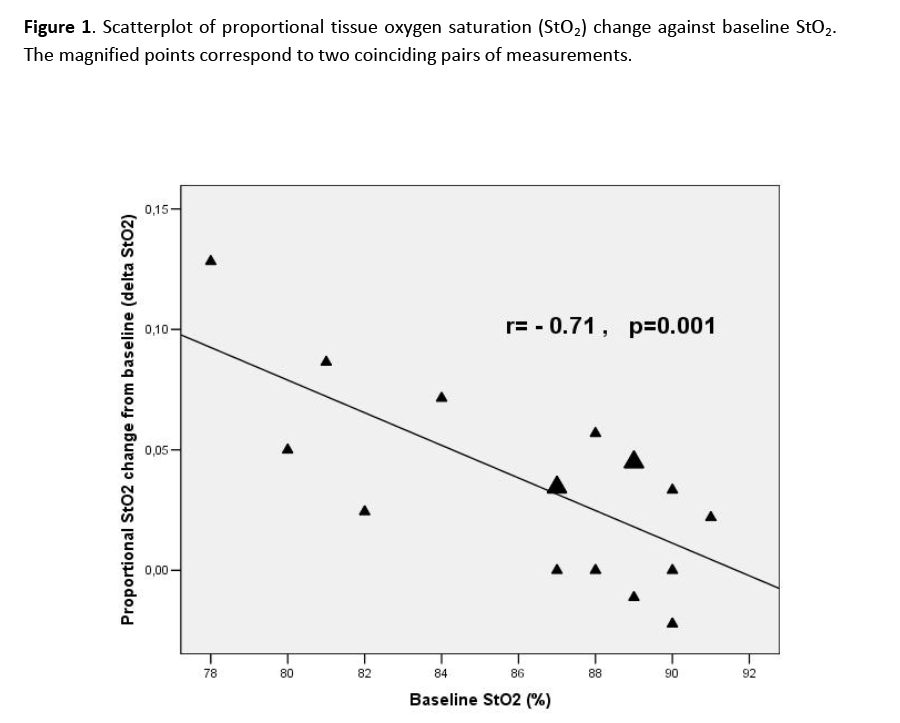
Figure 1: Scatterplot of proportional tissue oxygen saturation (StO2) change against baseline StO2. The magnified points correspond to two coinciding pairs of measurements.
In a repeated measures ANCOVA model assessing a potential association between the changes of StO2 and Hb after RBC transfusion, there was a StO2-Hb interaction of borderline significance (partial eta squared=0.231, p=0.051), which indicates that the increase of Hb with RBC transfusion contributes to the increase of StO2.
No significant differences of the measured variables were recorded in patients with regard to previous splenectomy.
Discussion
The findings of our study indicate that RBC transfusion increases StO2 in patients with beta-thalassemia major. To our knowledge, this is the first study utilizing NIRS to investigate the impact of RBC transfusion on microcirculation in this special patient population, with chronic anemia.
Previous studies have addressed the effect of blood transfusions on the microcirculation in other patient populations with conflicting findings. In trauma patients, regional tissue oxygen saturation measured by NIRS was a reliable indicator of the need for blood transfusion [11] and could identify poor perfusion, and predict the development of organ dysfunction and death similarly, as the maximum base deficit [12]. Transfusions in chronically anemic hematology outpatients resulted in an increase in StO2 (from 81% to 86%) and in the tissue hemoglobin index, which reflects the amount of Hb in the NIRS measurement volume [13]. Cerebral and peripheral oxygenation estimated by NIRS diminished after compensated blood loss in humans [14]. In contrast, StO2 measured at the thenar eminence was not affected significantly after 500 ml blood loss in healthy volunteers [15]. Additionally, RBC transfusions in hemodynamically stable intensive care unit (ICU) patients did not affect StO2; however oxygen consumption and vascular reactivity improved after transfusion in patients with altered values at baseline [16].
Different patient populations can account for the variable effect of blood transfusion on tissue oxygenation indices obtained by NIRS.
The lower pre-transfusion StO2, which is partially restored after RBC transfusion in thalassemics, reveals that oxygen supply might have been relatively compromised.
Although the StO2 increase is not quantitatively large, it could be considered clinically significant if it corresponds to such a difference at the level of tissue oxygenation, which would be easily perceivable by the oxygen sensing mechanism in tissues that regulates, accordingly, the response to hypoxia.
The strong correlation between StO2 at baseline and the StO2 change after transfusion suggests that this is indeed the case.
Hypoxia sensing mechanism functions through the hypoxia inducible factors (HIFs). These are transcription factors for multiple protein products elaborated in response to hypoxic stimulus e.g. erythropoietin [17].
It has been demonstrated that prolyl hydroxylases (PHDs), which are the enzymes that regulate the levels of HIF-1α, exhibit a strikingly low oxygen affinity [18,19]. This low affinity allows the PHDs to be very sensitive even to very small changes in oxygen concentration, resulting in pronounced changes in the velocity of their reaction (hydroxylation reaction in the HIF-1α molecule) and in the rate of HIF-1α turnover.
Therefore, the recorded differences in tissue oxygenation, although not apparently large, could potentially indicate a clinically significant hypoxic stimulus in beta-thalassemics at the pre-transfusion period, which may influence the patients’ erythropoietic response.
RBC transfusions on a regular basis in beta-thalassemics aim at suppressing the erythropoietic response which is induced by tissue hypoxia. If the oxygenation index obtained by NIRS can be used for an optimal transfusion regimen remains to be established.
In our study, StO2 increase was associated with the increase of Hb concentration, yet not very strongly. However, a similar association was not a constant finding in previous studies [13]. Although RBC transfusion was the only intervention implemented, other factors possibly involved (i.e. distribution of blood, matching of flow to demands with a chronically impaired endothelium, differences in Hb dissociation curve of the stored blood) may have compromised the strength of association.
In thalassemics the oxygen consumption rate did not change significantly with RBC transfusion. Apparently, oxygen delivery at the pre-transfusion period was not diminished to such an extent that could limit the oxygen consumption rate required for unaltered metabolic demands. An adequate oxygen flow can be achieved, as mentioned, by increased fractional oxygen extraction. This is indicated by the lower pre-transfusion StO2.
A similar observation, of unaltered systemic oxygen consumption rate after RBC transfusion, was reported in hemodynamically stable ICU patients [16].
The reperfusion rate displayed a trend to increase after RBC transfusion. The reperfusion rate is a surrogate marker of the endothelial response to ischemia which involves vasodilatation and capillary recruitment [20-22]. Beta-thalassemia major is associated with endothelial dysfunction, [23] with iron overload being a major contributor in its pathogenesis. The improvement of the reperfusion rate could be attributed to a mitigated hypoxic stimulus, assuming more oxygen was available in microcirculatory vessels during ischemia. The accelerated StO2 increment, however, can be accounted for, as well, by the increased oxygen content of blood, which affects the adjustment of fractional oxygen extraction that is required to cover stable metabolic demands. Although this finding did not reach statistical significance, it should be further pursued with more powered studies.
Splenectomy may affect microcirculation in beta-thalassemics. It leads to persistence of beta-thalassemic erythrocytes (nucleated, immature forms, containing precipitated globin chain inclusions) which would be removed from the circulation in case of an intact spleen. It has been shown that in thalassemic syndromes, specifically in beta-thalassemia intermedia, there are different deformability profiles of the beta-thalassemic erythrocytes in patients with and without splenectomy [24]. Erythrocyte membranes from splenectomized individuals were mechanically unstable. Increased mechanical instability may compromise rheology in microcirculation. However in our study no significant difference in the measured variables was established between splenectomized and non-splenectomized individuals.
Limitations of the study
Our study has certain limitations. First, the small sample size does not provide adequate power to detect all potential associations (e.g. the reperfusion rate did not reach statistical significance). However, the use of conservative nonparametric methods in primary data analysis ensures the detection of robust associations (e.g. the StO2 increase and its negative correlation with baseline StO2, which were consistent findings in all analyses performed). As a consequence, multivariable analysis was also limited in rather simple models.
Secondly, the RBC storage time was not available for all RBC packs transfused and it was not taken into account in the analysis. Finally the small sample size, probably, did not allow the demonstration of potential differences between splenectomized and non-splenectomized thalassemics.
Conclusion
The present study indicates that RBC transfusions in patients with beta-thalassemia major, increase oxygenation at the microcirculatory level. The StO2 increment after RBC transfusion correlated negatively with baseline StO2. Potential clinical implications of these findings remain to be further investigated.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgements
This study was funded by a grant from the special for research grants of the National and Kapodistrian University of Athens.
2801
References
- Rund D, Rachmilewitz E. ?-Thalassemia. New Engl J Med 2005; 353(11): 1135-1146.
- Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Physiology of exercise. In: Weiberg R, editor. Principles of exercise testing and interpretation. Baltimore-Maryland: Lippincott Williams & Wilkins; 1999.
- Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood 2011; 118(13): 3479-3488.
- Lipcsey M, Woinarski NC, Bellomo R. Near infrared spectroscopy of the thenar eminence in anesthesia and intensive care. Ann Intensive Care 2012; 2(1): 11.
- Lima A, Bakker J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med 2005; 31(10): 1316-1326.
- Gerovasili V, Dimopoulos S, Tzanis G, Anastasiou?Nana M, Nanas S. Utilizing vascular occlusion technique with NIRS technology. Review. Int J Ind Ergonom 2010; 40(2): 218-222.
- Myers DE, Anderson LD, Seifert RT, Ortner JP, Cooper CE, Beilman GJ, et al. Noninvasive method for measuring local hemoglobin oxygen saturation in tissue using wide gap second derivative near-infrared spectroscopy. J Biomed Opt 2005; 10(3): 034017.
- Nanas S, Gerovasili V, Dimopoulos S, Pierrakos C, Kourtidou S, Kaldara E, et al. Inotropic agents improve the peripheral microcirculation of patients with end-stage chronic heart failure. J Card Fail 2008; 14(5):400-406.
- Gerovasili V, Tripodaki E, Karatzanos E, Pitsolis T, Markaki V, Zervakis D, et al. Short-term systemic effect of electrical muscle stimulation in critically ill patients. Chest 2009; 136(5):1249-1256.
- Siafaka A, Angelopoulos E, Kritikos K, Poriazi M, Basios N, Gerovasili V, et al. Acute effects of smoking on skeletal muscle microcirculation monitored by near-infrared spectroscopy. Chest 2007; 131(5):1479-1485.
- Smith J, Bricker S, Putnam B. Tissue oxygen saturation predicts the need for early blood transfusion in trauma patients. American Surg 2008; 74(10): 1006-1011.
- Cohn SM, Nathens AB, Moore FA, Rhee P, Puyana JC, Moore EE, et al. StO2 in Trauma Patients Trial Investigators. Tissue oxygen saturation predicts the development of organ dysfunction during traumatic shock resuscitation. J Trauma 2007; 62(1): 44-54.
- Yuruk K, Bartels SA, Milstein DMJ, Bezemer R, Biemond BJ, Ince C. Red blood cell transfusions and tissue oxygenation in anemic hematology outpatients. Transfusion 2012; 52(3): 641-646.
- Torella F, Haynes SL, McCollum CN. Cerebral and peripheral near-infrared spectroscopy: an alternative transfusion trigger? vox Sang 2002; 83(3): 254-257.
- Jeger V, Jacob SM, Fontana S, Wolf M, Zimmermann H, Exadaktylos AK. 500 ml of blood loss does not decrease non invasive tissue oxygen saturation (StO2) as measured by near infrared spectroscopy-A hypothesis generating pilot study in healthy adult women. J Trauma Manag Outcomes 2010; 4: 5.
- Creteur J, Neves AP, Vincent JL. Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care 2009; 13(Suppl 5): S11.
- Yoon D, Ponka P, Prchal JT. Hypoxia.5.Hypoxia and hematopoiesis. Am J Physiol-Cell Physiol 2011; 300(6): C1215-1222.
- Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood 2009; 114(10): 2015-2019.
- Siddiq A, Aminova LR, Ratan RR. Hypoxia inducible factor prolyl 4-hydroxylase enzymes: center stage in the battle against hypoxia, metabolic compromise and oxidative stress. Neurochem Res 2007; 32(4-5): 931-946.
- Vallet B. Vascular reactivity and tissue oxygenation. Intensive Care Med 1998; 24(1): 3-11.
- Murrant CL, Sarelius IH. Coupling of muscle metabolism and muscle blood flow in capillary units during contraction. Acta Physiol Scand 2000; 168(4): 531-541.
- Creteur J. Muscle StO2 in critically ill patients. Current Opin Crit Care 2008; 14(3): 361-366.
- Aggeli C, Antoniades C, Cosma C, Chrysohoou C, Tousoulis D, Ladis V, et al. Endothelial dysfunction and inflammatory process in transfusion-dependent patients with beta-thalassemia major. Intern J Cardiol 2005; 105(1): 80-84.
- Schrier SL, Rachmilewitz E, Mohandas N. Cellular and membrane properties of alpha and beta thalassemic erythrocytes are different: implication for differences in clinical manifestations. Blood 1989; 74(6): 2194-2202.







