Keywords
Cardiovascular disease; Coronavirus infections; Clinical medicine; Systematic review
Introduction
In December 2019, a cluster of pneumonia cases, caused by a novel identified as β-coronavirus, initially named as 2019-novel coronavirus (2019-nCoV), SARS-CoV-2, occurred in Wuhan, China [1,2] and it was called COVID-19. It spread to other countries and, in a short time, a new pandemic was declared on 12 January, 2020 by World Health Organization (WHO) [3]. Accelerated by human migration, exported cases have been reported in several regions of the world, including Europe, Asia, North America, and Oceania [4]. Globally, as of 2:39pm CEST, 27 May 2021, there have been 168.040.871 confirmed cases of COVID-19, including 3.494.758 deaths, reported to WHO. As of 26 May 2021, a total of 1.545.967.545 vaccine doses have been administered [5,6].
According to the WHO, there has been a recent increase in the burden of cardiovascular disease (CVD), especially in low and middle income countries [7]. It is estimated that 17.7 million people died from CVD in 2015, representing 31% of all deaths globally. Of these deaths, it is estimated that 7.4 million are due to CVD (WHO, 2017). In 2020, CVDs are the number 1 cause of death globally, taking an estimated 17.9 million lives each year [8,9].
COVID-19 brought back to discussion a topic already highlighted during the SARS-CoV-1 and Coronavirus-related SARS known as the MiddleEast Respiratory Distress Syndrome (MERS) of 2002 and 2013. During those outbreaks it was observed a particularlyelevated incidence of cardiovascular disease among patients [10]. Studies has shown that patients with comorbidities such as hypertension, heart failure, diabetes [11] and elderly people [12] are, among others causes, risk factor for severe illness by SARS-CoV-2. Also, COVID-19 caused by binding of the viral surface spike protein to thehuman angiotensin-converting enzyme 2 (ACE2) receptor following activation of the spikeprotein by transmembrane protease serine 2 (TMPRSS2) [13]. Thus, the cardiovascular impacts by COVID-19 pandemic are not yet well established although there are constant publications about its potential deleterious effects. we believe it is important to discuss the challenges faced, prognostic risk factors, and outcomes of COVID-19 in post-hematopoietic stem cell transplantation patients based on the available real-world data.
We aimed to carry a systematic review of the literature with meta-analysis based on thefollowing guiding question: what practical contributionsto the scientific literature produced in theperiod of 2019-2020 does the impact of the COVID-19 on cardiovascular system have to offer? This review highlights in a pandemic period, cardiovascular pathologies are risk factor from a worsening results and the pandemic prevention and control measures can also be made as a way to prevent cardiovascular diseases for the population, because fewer people exposed to the virus means less cardiovascular risk [14].
Method
Literature review
A qualitative systematic review with meta-analysis of the literature using the Virtual Health Library (VHL), which hosts recognized databases – LILACS (Literatura Latino-americana e do Caribe emCiências da Saúde), MEDLINE, SciELO (Scientific Electronic Library Online), and PubMed was performed. Initially, the following descriptors were used: #1 "cardiovascular disease" [MeSH] AND #2 "COVID-19" [keyword], as well as their equivalents in the Portuguese and Spanish language.
Eligibility criteria
The period reported in the literature ranged from December 2019 to March 2020, as it is the period when the pandemic started. Compilation of the data was performed in April 2020. Manuscript selection occurred primarily through the analysis of titles and abstracts. Article analysis followed the eligibility criteria: (1) At least a combination of the terms described in the search strategy were present in the title or words that refer to the theme; (2) Articles were written in English, Portuguese or Spanish; (3) Articles address cardiovascular impact of COVID-19 pandemic; (4) Original articles with the full text available through the CAPES (Coordination of Personal Improvement of Higher Level) Periodicals Portal, a virtual library created by the Brazilian Ministry of Health where content is restricted to authorized users. Monographs, dissertations and thesis were excluded. Manuscripts that were repeated in more than one of the databases were counted only once. Some articles were excluded because they generally approached others viruses/pandemics or the sample was children.
To ensure trustworthiness of the findings, data collection was performed, individually, by two researchers with divergences being solved by a third senior researcher.
Each sample article was thoroughly read and the information was inserted in a spreadsheet (Table 1), including the author, publishing year and main study findings. According to the PRISMA protocol (http://www.prisma-statement.org/).
| Author (Year) |
Journal |
Sample (Study type) |
Main Findings |
| Guo et al [1] |
JAMA Cardiol |
187 patients with confirmed COVID-19 at the Seventh Hospital of Wuhan City, China (cross sectional retrospective observational study). |
During hospitalization, 66 (35.3%) patients had underlying CVD including hypertension, coronary heart disease, and cardiomyopathy, and 52 (27.8%) exhibited myocardial injury as indicated by elevated TnT levels. Patients with elevated TnT levels had more frequent malignant arrhythmias, and the use of glucocorticoid therapy (37 [71.2%] vs 69 [51.1%]) and mechanical ventilation (41 [59.6%] vs 14 [10.4%]) were higher compared with patients with normal TnT levels. |
| Clerkin et al [13] |
Circulation |
(Integrative Review) |
COVID-19 is caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), which invades cells through the angiotensin converting enzyme 2 (ACE2) receptor. Among those with COVID-19, there is a higher prevalence of cardiovascular disease and more than 7% of patients suffer myocardial injury from the infection (22% of the critically ill). |
| Bansal [16] |
Diabetes Metab Syndr |
(Narrative Review) |
Acute cardiac injury, defined as significant elevation of cardiac troponins, is the most commonly reported cardiac abnormality in COVID-19. |
| Cheng et al [15] |
Curr Cardiol Rep |
(Integrative Review) |
Emerging epidemiological evidence suggest cardiovascular risk factors are associated with increased disease severity and mortality in COVID-19 patients. Patients with a more severe form of COVID-19 are also more likely to develop cardiac complications such as myocardial injury and arrhythmia. |
| Li et al [28] |
Infection Dis Poverty |
31 normal human tissues (Experimental study) |
ACE2 expression levels were the highest in the small intestine, testis, kidneys, heart, thyroid, and adipose tissue, and were the lowest in the blood, spleen, bone marrow, brain, blood vessels, and muscle. ACE2 showed medium expression levels in the lungs, colon, liver, bladder, and adrenal gland |
| Han et al [46] |
J Cardiovasc Magn Reson |
(Integrative Review) |
First, continued urgent and semi-urgent care for the patients who have no known active COVID-19 should be provided in a safe manner for both patients and staff. Second, when necessary, CMR on patients with confirmed or suspected active COVID-19 should focus on the specific clinical question with an emphasis on myocardial function and tissue characterization while optimizing patient and staff safety. |
| S?awi?ski and Lewicka [38] |
Kardiol Pol |
(Integrative Review) |
Among comorbidities in patients with COVID?19, cardiovascular disease is most commonly found. And in the most common symptoms of COVID?19 dyspnea is responsible by18.6%-59%. |
| Berre et al [44] |
Diagn Interv Imaging. |
71-year-old man with COVID-19 pneumonia (Case Report) |
A case report about concomitant acute aortic thrombosis and pulmonary embolism complicating COVID-19 pneumonia |
| Vignera et al [29] |
Int J Mol Sci. |
(Short Communication) |
Data on the experimental animal have shown that 17ß-estradiol increases the expression and activity of ACE2 in both adipose tissue and kidney. Spontaneously hypertensive male mice have a higher myocardial ACE2 expression than females and its levels decrease after orchiectomy |
| Zhu et al [34] |
Curr Cardiol Rep |
(Integrative Review) |
The literature reports association between history of cardiac disease and worsened outcome during COVID infection. Development of new onset myocardial injury during COVID-19 also increases mortality. |
| Celina and Oliva [43] |
Diagn Interv Imaging. |
60-year-old man with COVID-19 pneumonia (Case Report) |
A case report about acute pulmonary embolism complicating COVID-19 pneumonia |
| Gonzallez-Jamarillo, Low and Franco [32] |
Eur J Epidemiol. |
(Short Communication) |
SARS-CoV-2 infection produces enzymatic shedding that inactivates ACE2 and prevents conversion of Ang-II.This effect could in part explain the cardiovascular and respiratory manifestations of COVID-19. |
| Gao et al [45] |
Respir Res |
102 patients with severe COVID-19 (cross sectional observational study) |
N terminal pro B type natriuretic peptide (NT-proBNP) might be an independent risk factor for in-hospital death in patients with severe COVID-19. |
| Rico-Mesa, White and Anderson [47] |
Curr Cardiol Rep |
(Integrative Review) |
Worse outcomes appear to be more prevalent in patients with hypertension and diabetes mellitus (DM), possibly due to overexpression of angiotensin-converting enzyme 2 (ACE2) receptor in airway alveolar epithelial cells. |
| Wang and Xu [26] |
Cells |
17,520 testicular cells (Experimental Study) |
ACE2 is predominantly enriched in spermatogonia and Leydig and Sertoli cells. Gene Set Enrichment Analysis (GSEA) indicates that Gene Ontology (GO) categories associated with viral reproduction and transmission are highly enriched in ACE2-positive spermatogonia, while male gamete generation related terms are downregulated. |
| Rizzo et al [33] |
Basic Res Cardiol |
(Short Communication) |
We might be able to target Notch also to fight heart and lung disease caused directly by SARS-CoV-2 infection and by the cytokine storm in response to the virus. |
| Laccarino et al [10] |
High Blood Press Cardiovasc Prev |
(Short Communication) |
In vitro studies are available to support the eventual role of ACE inhibitors and ARBs in both the promotion and antagonism of the disease. The available literature, indeed, presents contrasting results. |
| Schiffrin et al [39] |
Am J Hypertens |
(Short Communication) |
There is as yet no evidence that hypertension is related to outcomes of COVID-19, or that ACE inhibitor or ARB use is harmful, or for that matter beneficial, during the COVID-19 pandemic. |
| Tan and Aboulhousn [35] |
Int J Cardiol |
(Integrative Review) |
COVID-19 results in mild symptoms in the majority of infected patients, but can cause severe lung injury, cardiac injury, and death. |
| Gupta and Misra [24] |
Diabetes Metab Syndr |
(Integrative Review) |
Patients with COVID-19 infection have elevated natriuretic peptides, significance of which is uncertain and Cardiac troponin I levels are significantly increased in patients with severe SARS-CoV-2 infection. |
| Gackowski et al [17] |
Kardiol Pol |
(Integrative Review) |
Transesophageal echocardiography is considered an aerosol?generating procedure and should be performed only as a lifesaving procedure. Personnel should use appropriate personal protection equipment in the immediate vicinity of the patients in accordance with the relevant guidelines. |
| Guo et al [23] |
J Am Heart Associat |
(Integrative Review) |
ACE2 plays a protective role in both cardiovascular diseases and acute lung injury. For uninfected patients, we tend to believe it is unnecessary to discontinue ACEIs/ARBs given the lack of evidence to support the hypothesis that ACEIs/ARBs might lead to an increased risk of SARS-CoV-2 infection. For infected patients, although higher ACE2 expression might be associated with higher viral loads, ACEIs/ARBs should not be discontinued assertively because they can block the RAS and protect patients from the potential heart injuries in COVID-19 and might also reduce the severity of lung damage caused by the infection. |
| Sommerstein et al [37] |
J Am Heart Associat |
(Integrative Review) |
Cardiovascular diseases and/or their therapy, by affecting ACE2 levels, may play a pivotal role with regard to infectivity and outcome of COVID-19. Whether treatment or disease induced upregulation of ACE2 influences the course of COVID-19 urgently needs to be determined. |
| Meng et al [12] |
Emerg Microbes Infect. |
51 patients with hypertension and COVID-19 (cross sectional retrospective study) |
Patients receiving ACEI or ARB therapy had a lower rate of severe diseases and a trend toward a lower level of IL-6 in peripheral blood. In addition, ACEI or ARB therapy increased CD3 and CD8 T cell counts in peripheral blood and decreased the peak viral load compared to other antihypertensive drugs. |
| Li et al [3] |
N Engl J Med |
(Integrative Review) |
Insufficient data are available to determine whether these observations readily translate to humans, and no studies have evaluated the effects of RAAS inhibitors in COVID-19 |
| Chen et al [41] |
Cardiovasc Res |
Human heart tissues were obtained from abandon donors in Center of Cardiovascular Treatment in China (Experimental Study) |
The pericytes injury due to virus infection may result in capillary endothelial cells dysfunction, inducing microvascular dysfunction. And patients with basic heart failure disease showed increased ACE2 expression at both mRNA and protein levels, meaning that if infected by the virus these patients may have higher risk of heart attack and critically ill condition. |
| Fang et al [11] |
Lancet Respir Med |
(Short Communication) |
patients with cardiac diseases, hypertension, or diabetes, who are treated with ACE2-increasing drugs, are at higher risk for severe COVID-19 infection |
| Chen, Zhou and Wang [41] |
Herz |
(Short Communication) |
The condition of some patients with severe SARS-CoV-2 infection patients might deteriorate rapidly with acute respiratory distress syndrome and septic shock, which is eventually followed bymultiple organ failure and fulminant myocarditis |
| Hulot et al [27] |
Arch Cardiovasc Dis |
(Short Communication) |
COVID-19 can be caused palpitations and chest tightness, myocardial damage with an increase in high-sensitivity cardiac troponin I. |
| South, Diz and Chappel [25] |
Am J Physiol Heart Circ Physiol |
(Short Communication) |
In lieu of the fact that many older patients with hypertension or other CVDs are routinely treated with RAAS blockers and statins, new clinical concerns have developed regarding whether these patients are at greater risk for SARS-CoV-2 infection, whether RAAS and statin therapy should be discontinued, and the potential consequences of RAAS blockade to COVID-19-related pathologies such as acute and chronic respiratory disease. |
| Abassi et al [31] |
Am J Physiol Heart Circ Physiol |
(Short Communication) |
In patients infected with SARS-CoV-2, ACE2 may transform to a Trojan horse. Its binding with ACE2 neutralizes the advantageous cardiac effects of this enzyme, especially in patients with heart failure. |
CVD: Cardiovascular Disease; TnT: Troponin T; ACE2: Angiotensin Converting Enzyme 2; CMR: Magnetic Ressonance; NT-proBNP: N Terminal Pro B Type Natriuretic Peptide; Ang-II: Angiotensin II; ARB: Angiotensin-Receptor Blockers; RAAS: Renin-Angiotensin-Aldosterone System; CD: Cluster of Differentiation.
Table 1 Main findings.
Ethical issues
Since this is a systematic review, Resolution 510/16 of the Brazilian National Health Council (CNS) ensures the dispensation of submission to a Human Beings Research EthicsCommittee.
Results
According to the search strategy, 101 articles were found in Pubmed and 27 were selected. In VHL there were 59 articles and four were selected. After the eligibility criteria was applied (Figure 1), these were then input in Table 1.
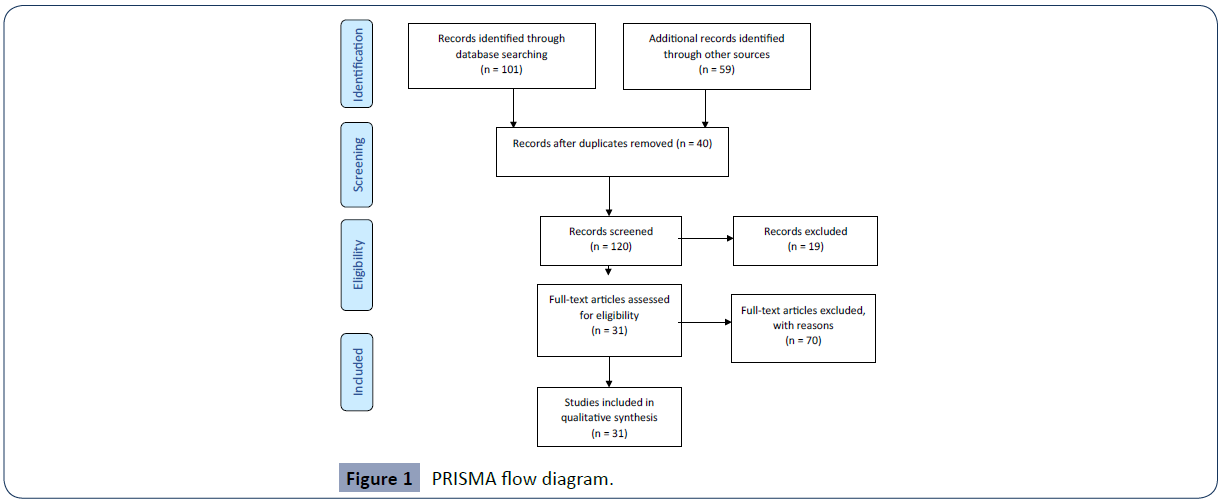
Figure 1 PRISMA flow diagram.
The findings were then divided into three subcategories: Etiology, Physiopathology and Risk factors of SARS-CoV-2 in Cardiovascular System; Clinical presentation, laboratory markers and imagenological aspects of SARS-CoV-2 in cardiovascular system; and Anti-Hypertensive Drugs, Cardiovascular System and SARS-CoV-2.
Discussion
Etiology, physiopathology and risk factors of SARS-CoV-2 in cardiovascular system
SARS-CoV-2 is caused by a novel enveloped beta coronavirus that belongs to Coronaviridae family, a group of positive strand RNA viruses causinghuman respiratory infections, it was named after the crown shaped outer coat seen on the electron-microscopy. First discoveredin the 1960s, it received great attention during the 2003 SARS coronavirus (SARS-CoV) outbreak [15]. Seven species of these beta-coronaviruses are known to cause human infections, with four causing mainly mild flulike symptoms and the remaining three resulting in potentially fatal illnesses (SARS, MERS and the ongoing COVID-19) [16].
The transmission of SARS-CoV-2 occurs mainly through the droplet route, but the possibility of airborne transmission and transmission through the fecal?oral route is also postulated, though the latter seems less relevant so far. The estimated median incubation time for COVID-19 is 5.1 days, and only 2.5% of patients develop symptoms within 2.2 days after infection, whereas 97.5% of patients develop symptoms within 11.5 days after infection [17]. The widely agreed upon routes of transmission of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) are droplet infection, aerosols, and close contact. However, the rate of spread, disease load, and the symptom pattern of the coronavirus disease 2019 (COVID-19) have raised the probability of other routes of transmission, such as feco-oral. A significant proportion of persons infected with SARS CoV-2 had diarrhea. Similar to earlier coronaviruses, SARS CoV-2 has also been reported to be found in fecal samples. Plausible explanation for its presence in stool is that the ACE-2 receptors to which SARS CoV-2 binds are present in gastrointestinal mucosa as well. Literature search revealed the presence of infectious SARS CoV-2 in feces of COVID-19 patient [18].
Stool samples tested positive for SARS CoV-2 RNA even up to 5 weeks after the respiratory samples tested negative for COVID-19 [19]. These findings establish the presence of SARS CoV-2 in stools. Feco-oral transmission, if it is happening, can be very significant in developing and underdeveloped countries, where open defecation is common, and poor water sanitation and hygiene (WASH) practices are followed. In addition, reports have attributed SARS transmission from aerosol plumes of SARS CoV-1 patient with diarrhea, in 2003 [20]. Hence, as a precaution, it should be ensured that COVID-19 patients are provided with separate toilets, cleaned at least twice daily. The lid should be down while flushing, to avoid bio-aerosolization or water splashes. If the feco-oral route contributes to transmission of COVID-19 cases, then it is an issue of serious consideration with regard to modifying or adding the public health recommendation for COVID-19 prevention. However, further research is warranted to confirm the feco-oral transmission [18,21,22].
Patients with severe COVID-19 were reported to have a higher proportion of pulmonary hypertension as compared to mild COVID-19 disease [22% vs 2%]. Elevated pulmonary artery systolic pressure was significant in predicting mortality. COVID-19 patients with chronic obstructive pulmonary disease, congestive heart failure, myocardial injury, pulmonary embolism, and prior pulmonary hypertension were at a higher risk of worsening pulmonary hypertension. Multiple mechanisms for developing pulmonary hypertension that have been postulated are i) concomitant worsening myocardial injury, ii) cytokine storm, endothelial injury, hypercoagulability attributing to development of venous thromboembolism, iii) and the presence of thrombotic microangiopathy. Among patients with severe COVID-19 disease and pulmonary hypertension, complications including acute respiratory distress syndrome, acute myocardial injury, the requirement of intensive care unit admission, the requirement of mechanical ventilation, and mortality are higher [22,23].
SARS?CoV?2 is particularly dangerous to cardiac patients. It invades the lungs and causes interstitial pneumonia with the dynamic destruction of the alveoli and development of ARDS. Severely depressed gas exchange, accompanied by insufficiently active or hyper regulated immune system, add a risk of mortality, mostly in patients with coexisting illnesses, particularly cardiovascular disease. There are also reports about direct acute and chronic damage caused by SARS?CoV?2 to the cardiovascular system [24]. The infection has been associated with the development of myocarditis, arrhythmias, heart failure, myocardial infarction, and thromboembolism [25]. In Wuhan, it has been observed that patients treated in intensive care units had significantly higher cardiac troponin levels comparing with other patients [26]. Among those who died from COVID?19, 11.8% of patients without a history of previous heart disease had elevated troponin concentrations. It was estimated that 7% of deaths were caused by myocarditis with heart failure, and overall myocarditis might have been implicated in 33% of deaths. Fulminant myocarditis has also been reported in some patients effectively treated with steroids and immunoglobulins. As a result, Chinese authors recommend transtho? racic echocardiography in all patients with complicated COVID?19 disease. Apart from respi? ratory failure, the typical mode of death is distributive or cardiogenic shock. Neither pericardial nor pleural effusion is a typical finding in COVID?19.
Although respiratory tract is the primary target for SARS-CoV- 2, cardiovascular system (CVS) may get involved in several different ways [16] as destabilized coronary plaque [23], hypoxemia, systemic inflammation and enhanced myocardial oxygen demand, a direct cardiovascular injury, likely develops, initiated by binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE2). This receptor is widely expressed in lungs, kidney [10]- renal tubules [24], brain, gut [25], gastrointestinal epithelium, Leydig cells in testis [24,26], but also in the heart, where it is localized to macrophages, vascular endothelium, smooth muscle and myocytes [27].
Experimental study shows that there were correlations between ACE2 expression levels and immune signature enrichment levels (CD8+ T cells, interferon response, B cells, and NK cells) in various male and female human tissues. In the skin, digestive system (esophagus, stomach, colon, and pancreas), brain, and blood vessels, significant positive correlations between ACE2 expression levels and CD8+ T cell enrichment levels were observed in both males and females (Pearson’s correlation test, adjusted P < 0.05, 0.27 ≤ r ≤ 0.78) [28]. On the other hand, data on the experimental animal have shown that 17ß-estradiol increases the expression and activity of ACE2 in both adipose tissue and kidney of mice´s. Spontaneously hypertensive male mice have a higher myocardial ACE2 expression than females and its levels decrease after orchiectomy [29].
The fact is that the virus shares the ACE2 as the host cellular receptor for virus spike (S) protein according to structural analysis [30,31] following activation by transmembrane protease serine 2 (TMPRSS2) [25]. The virus produces enzymatic shedding that inactivates ACE2 and prevents conversion of Ang-II [32]. Besides that, the pericytes injury due to virus infection may result in capillary endothelial cells dysfunction, inducing microvascular dysfunction. Patients with basic heart failure disease showed increased ACE2 expression at both mRNA and protein levels, meaning that if infected by the virus these patients may have higher risk of heart attack and critically ill condition [30].
Laboratory studies have suggested that other intracellular signaling pathways such as Notch could also serve to explain the cytokine storm that ultimately induces heart and lung disease caused by SARS-CoV-2 direct damage to tissues [33]. Besides that, other theory is that the systemic release of cytokines, characterized byincreased IL-2, IL-6, IL-10, GCSF, IFN-γ, MCP-1, MIP-1-α, and TNF-α, likely contributes to cardiac injury in a situationanalogous to cardiotoxicity in the setting of chimeric antigenreceptor (CAR)-T cell therapy. A prior study demonstratedthat cardiac injury and cardiovascular events in the form ofelevated troponin and left ventricular systolic dysfunction are common post-CAR-T [34].
Therefore, the exact mechanism of cardiac involvement in COVID-19 remains under investigation but it seems that the SARSCoV- 2 could be (a) cause cardiac injury indirectly since COVID-19 may overwhelmimmune inflammatory response and cytokine storm; (b)invade of cardiomyocytes and direct damage via this process; (c) cause Severe hypoxia from acute respiratory damage caused by the virusmay result in oxidative stress and myocardial injury from increasedmyocardial oxygen demand in the presence of severe hypoxia due to acute lung injury (ARDS) [35].
Cardiovascular disease patients are at particularly high risk of mortality from SARS-CoV-2 due to their frailty and susceptibility for a myocardial involvement [36], perhaps due to the virus's affinity for ACE2 mainly due to the interaction with the renin-angiotensin- aldosterone system (RAAS).
RAAS plays an important role in regulating electrolytebalance and blood pressure and comprises twopathways: the ACE/Ang II/AT1R pathway and theACE2/Ang (1–7)/Mas receptor pathway. Undernormal physiological conditions, the activity of theACE/ Ang II/AT1R axis and the ACE2/Ang (1–7)/Mas receptor axis are in a dynamic equilibrium state,maintaining the normal function of the correspondingsystem [12]. So, RAAS is widely implicated in Diabetes mellitus (DM), hypertension, heart failure and Coronary heartdisease [37].
Available data indicate that patients with COVID-19 are often diagnosed with hypertension (15%–30.4%), diabetes (7.3%–18.8%), coronary artery disease (2.5%–8%), or other cardiovascular disease (4%–14.6%). In addition, patients with concomitant cardiovascular diseases have a worse prognosis and more often require admission to the intensive care unit (ICU) compared with patients without such comorbidities [23,38,39].
Another fact to be considered is that COVID-19 is more aggressive in elderly patients. The literature tells us that elderly and male have more ACE2 receptors than the general population [37]. About that, Li et al. [28] refers that, when studying the expression of ACE2 receptors in various tissues of the body and its correlation with immunogenicity, in the thyroid, lungs, adrenal gland, liver, and kidneys, ACE2 expression levels showed significant positive correlations with CD8+ T cell enrichment levels solely in males [40,41].
Finally, patients with chronic kidney and those who have received renal transplant - and have a higher cardiovascular risk - are at increased risk of COVID-19 infection and severity. Moreover, there are frequent renal function abnormalities and increased incidence of acute kidney injury in patients with COVID- 19 [24].
Clinical presentation, laboratory markers and imagenological aspects of SARS-CoV-2 in cardiovascular system
There appears to be two clinical stages to the disease. The first stage is the replicative stage, when SARS-CoV-2 is replicating over the course of several days and the patient presents with relatively mild symptoms [35] fever, cough, and myalgia or fatigue; less common symptoms were sputum production, headache, hemoptysis, and diarrhea [41]. The second stage is the adaptive immunity stage, when the body develops an antibody response to the virus. This leads to falling titers of the virus and resolution of symptoms in most patients. There is a minority of patients, however, that become critically-ill and have a high risk of mortality [35]. It is important to remember that some symptoms in patients with COVID-19 pneumonia suggest cardiovascular diseases. Fatigue, dyspnea, cough is typical in COVID-19, but these symptoms may also result from exacerbation of chronic heart failure [38].
Reports from China demonstrate that a significant majority of patients (81%) had mild symptoms (no pneumonia or mild pneumonia) from COVID-19. Among those with more significant symptoms, 14% experienced severe symptoms (dyspnea, respiratory rate ≥30/min, blood oxygen saturation ≤93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300, and/or lung infiltrates >50% within 24 to 48 hours) and 5% were critical (respiratory failure, septic shock, and/or multiple organ dysfunction or failure) [42]. Other published and anecdotal reports indicate manifestations that arrhythmia [23], cardiac arrest, acute heart failure [33] and theoretically fulminant myocarditis [41].
COVID-19 virus enters cells through the angiotensin converting enzyme II (ACE2) receptor, resulting in down-regulation of ACE2 receptor function. This leads to an increase of angiotensin II activity, activation of the renin-angiotensin-aldosterone system (RAAS) following a decrease in ACE2, an increase in vasoactive, proliferative, and profibrotic Ang-II leads to cardiopulmonary damage through hemodynamic changes such as pulmonary hypertension and interstitial edema followed by respiratory failure in the most severe cases [32].
In laboratory markers, definitive diagnosis of SARS-CoV-2 Infection is based primarily on nucleic acid amplification tests, such as real time reverse transcriptase–polymerase chain reaction (rRTPCR).
The most common laboratory abnormalities found in COVID-19 include decreased lymphocyte count (35%–82.1%), thrombocytopenia (17%–36.2%), elevated serum Creactive protein (60.7%–93%), lactate dehydrogenase (41%–76%) and D’dimer concentration (36%–46.4%). Elevated concentrations of serum creatine kinase (7%–13.7%), transaminases (21%–28%), or total bilirubin (10.5%–18%) have been rarely reported [38].
Interesting to note that elevated D-dimer values are common in COVID-19 patients, even in the absence of thrombophlebitis and acute pulmonary embolism and it seems to correlate with acute pulmonary embolism [43], arterial thrombosis, acute respiratory distress syndrome and death [44]; elevated cardiac troponin I (cTnI) levels (Chen, Zhou and Wang, 2020) and N terminal pro B type natriuretic peptide (NT-proBNP), with the cut-off value of 88.64 pg/mL [45] are correlate with cardiovascular injury, hospitalization and death. Furthermore, plasma TnT levels in patients with COVID-19 correlated significantly with both plasma high-sensitivity C-reactive protein levels, NT-proBNP elevation and malignant arrhythmias [45].
According Clerkin et al. [13] the rise in elevated high sensitivity cTnI tracks with other inflammatory biomarkers (D-dimer, ferritin, interleukin-6 (IL-6), lactate dehydrogenase and elevated creatinine kinase raising the possibility that this reflects cytokine storm or secondary hemophagocytic lymphohistiocytosis more than isolated myocardial injury.
Due to the high prevalence of myocarditis and heart failure, transthoracic echocardiography is routinely recommended in patients with complicated COVID-19 in order to differentiate causes of dyspnea and monitor the sequelae of ARDS. Echocardiography may be used to monitor fluid management in shock or extracorporeal membrane oxygenation. Ultrasound evaluation of the lung may be useful and the most common changes present are: pleural line abnormalities, Bline artifacts, and consolidation. Pleural line is normally about 1 mm thick but in COVID-19 it may thicken, appear irregular, and lose its continuity. Bline artifacts are the earliest signs in the disease course. They are a sensitive marker of fluid accumulation in the interstitial space.
Cardiovascular Magnetic Resonance (CMR) appears most appropriate in patients with clinically suspected acute myocardial injury, as definedby clinical criteria (symptoms, ECG abnormalities) and serologic evidence of cardiomyocyte damage with troponin elevation. In these patients, if unable to differentiate based on other clinical findings, CMR can differentiate between ischemic and non-ischemic etiologies, and further demonstrate the extent and severity of the injury and its impact on ventricular function [46].
Anti-hypertensive drugs, cardiovascular system and SARS-CoV-2
Even at the beginning of the pandemic, a publication suggested that due to hyper expression of ACE2 receptors in DM and hypertension, they would be more likely to develop severe manifestations of COVID-19 [11] which was not confirmed with subsequent studies [47]. Concurrently, there was a theory that anti-hypertensive drugs could cause more severe cases of COVID-19, however it has been refuted. Meng et al. (2020) show that ACE inhibitors (ACEi) or angiotensin receptor-1 blockers (ARB) therapy increased CD3 and CD8 T cell counts in peripheral blood and decreased the peak viral load compared to other antihypertensive drugs and Rico-Mesa, White and Anderson [47] suggest that positive effects of these drugs, include ACE2 receptor blockade, disabling viral entry into the heart and lungs, and an overall decrease in inflammation secondary to ACEI/ARB.
Moreover, Societies of Hypertension affirms that in hypertensive patients with COVID-19 or at risk of COVID-19 infection, ACEi and ARBs treatment should be maintained according to the recommendations contained in the 2018 ESC/ESH guidelines [10], because blood pressure control remainsan important consideration inorder to reduce disease burden, evenif it has no effect on susceptibility to the SARS-CoV-2 viral infection [39].
Conclusion
Cardiovascular diseases (CVD) are one of the most important causes of morbidity and mortality in the world being a great challenge for clinicians and researchers in the context of COVID-19. The pathophysiological explanation suggests an intimate correlation between SARS-CoV-2 protein S and ACE2 receptors, which the virus takes advantage of to increase its ability to penetrate host cells. The aggression of the cardiovascular system can be divided into three hypotheses - direct damage of the cardiomyocyte by the virus; hypoxemia due to lung injury or coronary events; or exacerbated immune response. When it comes to patients with COVID-19, the coexistence of previous cardiovascular diseases or risk factors such as hypertension, diabetes, coronary heart disease and heart failure, in addition to biochemical markers such as high troponin and pro-BNP seem to increase mortality.
Thus, when it comes to the cardiovascular system, these issues are aggravated and urge as a joint commitment from researchers, medical and governmental organizations for carry out more robust studies with bold methodologies aimed at mapping prognostic factors and assertive therapeutic approaches in the management of cardiovascular complications of COVID- 19.
Limitations
Despite the interesting results found in the study, it is important to point out some limitations. Not all autonomous communities have experienced the same number of infections by the CARDIOVASCULAR SYSTEM and SARS-COV-2 nor do they have the same health resources, so the results obtained should be viewed with caution. It would be interesting to compare the results with others found previously or later, in order to observe the evolution of symptoms at different times in time. Future studies should include more basic sociodemographic data, such as marital status, number of partners or number of children, or perceived emotional/social support, which may play a role in moderating the impact of the CARDIOVASCULAR SYSTEM AND SARS-COV-2 burden.
38409
References
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, et al. (20200 The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – An update on the status. Mil Med Res 13:1-11.
- Júnior JG, De Sales JP, Moreira MM, Pinheiro WR, Lima CKT, et al. (2020) A crisis within the crisis: the mental health situation of refugees in the world during the 2019 coronavirus (2019-nCoV) outbreak. Psychiatry Res 288: 113000.
- LiQ, Guan X, Wu P, Wang X, Zhou L, et al. (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382: 1199-1207.
- Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, et al. (2020) Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med 9: 601.
- World Health Organization (2019) Coronavirus disease (COVID-19) pandemic. WHO, Geneva.
- World Health Organization (2021) WHO Coronavirus (COVID-19) Dashboard. WHO, Geneva.
- Massa KHC, Duarte YAO, Filho, AGPC (2020) Analysis of the prevalence of cardiovascular diseases and associated factors among the elderly, 2000-2010. Cienc Saude Colet 24:105-114.
- World Health Organization (2017) Doenças Cardiovasculares. WHO, Geneva.
- Laccarino G, BorghiC, Cicero AFG, Ferri C, Minuz P, et al. (2020) Renin-Angiotensin System Inhibition in Cardiovascular Patients at the Time of COVID19: Much Ado for Nothing? A Statement of Activity from the Directors of the Board and the Scientific Directors of the Italian Society of Hypertension. High Blood Press Cardiovasc Prev 27:105–108.
- Fang L, Karakiulakis G, Roth M (2020) Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet 8: E21.
- Meng J, Xiao G, Zhang J, He X, Ou M, et al. (2020) Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect 9: 757-760.
- Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, et al. (2020) Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease. Circulation 141:1648-1655.
- Kamal KS, Ahmad DS (2021) A review on recipients of hematopoietic stem cell transplantation patients with COVID-19 infection. Ther Adv Infect Dis 8:20499361211013252.
- Cheng P, Zhu H, Witteles RM, Wu JC, Quertermous T, et al. (2020) Cardiovascular Risks in Patients with COVID-19: Potential Mechanisms and Areas of Uncertainty. Current Cardiology Reports 22:34.
- Bansal M (2020) Cardiovascular disease and COVID-19. Diabetes MetabSyndr 14: 247-250.
- Gackowski A, Lipczyńska M, Lipiec P, Szymański P (2020) Echocardiography during the coronavirus disease 2019 (COVID‑19) pandemic: expert opinion of the Working Group on Echocardiography of the Polish Cardiac Society. Kardiologia Polska 78:357-363.
- Gandhi PA, Singh T (2020) Feco-Oral Transmission of SARS-CoV-2. Asia Pac J Public Health 32:370.
- Patil AM, Göthert JR, Khairnar V (2020) Emergence, Transmission, and Potential Therapeutic Targets for the COVID-19 Pandemic Associated with the SARS-CoV-2. Cell Physiol Biochem 54:767-790.
- Wu LP, Mei ZQ, Wang NC, Zhao XF, Na DY, et al. (2004) Distribution and timing of antibody to SARS-CoV in SARS cases of transmission chain or non-transmission chain. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 18:109-112.
- Deniz M, Tezer H (2020) Vertical transmission of SARS CoV-2: a systematic review. J Matern Fetal Neonatal Med 21:1-8.
- Mishra A, Lal A, Sahu KK, George AA, Martin K, et al. (2020) An Update on Pulmonary Hypertension in Coronavirus Disease-19 (COVID-19). Acta Biomed 91:e2020155.
- Guo T, Fan Y, Chen M, Wu X, Zhang L, et al. (2020) Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 5:811-818.
- Gupta R, Misra A (2020) Contentious issues and evolving concepts in the clinical presentation and management of patients with COVID-19 infection with reference to use of therapeutic and other drugs used in Co-morbid diseases (Hypertension, diabetes etc). Diabetes Metab Syndr 14: 251-254.
- South AM, Diz DI, Chappell MC (2020) COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 318: H1084–H1090.
- Wang Z, Xu X (2020) scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, A Target for SARS-CoV-2 Infection in Spermatogonia, Leydig and Sertoli Cells. Cells 9: 920.
- Hulot JS (2020) COVID-19 in patients with cardiovascular diseases. ArchCardiovasc Dis 113: 225-226.
- Li MY, Li L, Zhang Y, Wang XS (2020) Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 9: 45.
- Vignera SL, Cannarella R, Condorelli RA, Torre F, Aversa A, et al. (2020) Sex-Specific SARS-CoV-2 Mortality: Among Hormone-Modulated ACE2 Expression, Risk of Venous Thromboembolism and Hypovitaminosis D. Int J Mol Sci 21: 2948.
- Chen L, Li X, Chen M, Feng Y, Xiong C (2020) The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 116:1097-1100.
- Abassi Z, Assady AS, Khoury EE, Heyman SN (2020) Angiotensin-converting enzyme 2: an ally or a Trojan horse? Implications to SARS-CoV-2-related cardiovascular complications. Am J Physiol Heart Circ Physiol 318: H1080–H1083.
- Gonzallez-Jamarilo N, Low N, Franco OH (2020) The double burden of disease of COVID-19 in cardiovascular patients: overlapping conditions could lead to overlapping treatments. Eur J Epidemiol 35:335–333.
- Rizzo P, Sega FVD, Fortini F, Marracino L, Rapezzi C, et al. (2020) COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res Cardiol 115:31.
- Zhu H, Rhee JW, Cheng P, Waliany S, Chang A, et al. (2020) Cardiovascular Complications in Patients with COVID-19: Consequences of Viral Toxicities and Host Immune Response. Curr Cardiol Rep 22:32.
- Tan W, Aboulhousn J (2020) The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol 309: 70–77.
- Gori T, Lelieveld J, Münzel T (2020) Perspective: cardiovascular disease and the Covid‑19 pandemic. Basic Res Cardiol 115:32.
- Sommerstein R, Kochen MM, Messerli FH, Gräni C, et al. (2020) Coronavirus Disease 2019 (COVID-19): Do Angiotensin-Converting Enzyme Inhibitors/ Angiotensin Receptor Blockers Have a Biphasic Effect?J Am Heart Assoc 9:e016509.
- Sławiński G, Lewicka E (2020) What should a cardiologist know about coronavirus disease 2019?Kardiol Pol78: 278-83.
- Guo J, Huang Z, Lin L, Lv J (2020) Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Am Heart Assoc 9:e016219.
- Chen C, Zhou Y, Wen WD (2020) SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz 45:230–232.
- Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46:586-590.
- Celina M, Oliva G (2020) Acute pulmonary embolism in a patient with COVID-19 pneumonia. Diagn Interv Imaging 101:325-326.
- Berre AL, Marteau V, Emmerich J, Zins M (2020) Concomitant acute aortic thrombosis and pulmonary embolism complicating COVID-19 pneumonia. Diagn Interv Imaging 101: 321–322.
- Gao L, Jiang D, Wen XS, Cheng XC, Sun M, et al. (2020) Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res 21:83.
- Han Y, Chen T, Bryant J, Bucciarelli-Ducci C, Dyke C, et al. (2020) Society for Cardiovascular Magnetic Resonance (SCMR) guidance for the practice of cardiovascular magnetic resonance during the COVID-19 pandemic. J Cardiovasc Magn R 22:26.
- Rico-Mesa JS, White A, Anderson AS (2020) Outcomes in Patients with COVID-19 Infection Taking ACEI/ARB. Curr Cardiol Rep 22:31.






