Keywords
|
| Lung cancer; Lambert-Eaton syndrome; Paraneoplastic syndrome |
Introduction
|
| Lambert-Eaton myastenic syndrome (LEMS) is an autoimmune disease, affecting the presynaptic neuronal transmission. It is the results of an autoimmune reaction in which antibodies are formed against presynaptic voltage-gated calcium channels (VGCC) in the neuromuscular junction. Its clinical features include muscle weakness and autonomic symptoms. LEMS can occur sporadically or as a paraneoplastic syndrome, associated with different cancers–breast, colon, prostate, pancreas, lung. LEMS is a rare paraneoplastic syndrome associated in 50% with small cell lung cancer (SCLC), with prevalence 1% among patients with SCLC. |
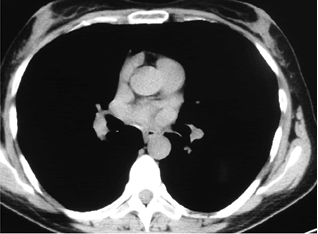
| We present a clinical case of ? 45-years old female patient with complaints of symmetrical, proximal, progressive leg weakness, unstable gait, painful cramps in the calfs and the thighs, weakness and tingle of the hands, ptosis of the lids, diplopy. The onset of the described clinical symptoms has been for four years. Transient complaints of muscle weakness, painful cramps and diploply have developed for the first time during the second pregnancy of the patient. The patient was hospitalized at Gastroenterology Clinic in the fifth lunar month because of abdominal pain, nausea, vomiting, diplopy and muscle weakness. Then it was diagnosed cholethiasis. By reason of diplopy and ptosis of the lids, it has been discussed in differential diagnosis tumor of the brain, as it was made MRI of the brain, it wasn’t determined pathological findings. Because of progressive course of the muscle weakness in the patient, it was made an electroneuromyography, which revealed axonal peripheral polyneuropathy. The planned Caesareau section was made in 38 weeks in the patient. Two months after the childbirth through persistent muscle weakness, it was done new electroneuromyography, as it was diagnosed myastenic syndrome. Because of the doubt about mediastinal tumor, computed tomography of lung and mediastinum (one year after the beginning of muscle symptoms), native and with contrast, was made. It was found a high density zone in X-th bronchopulmonary segment in the right lung, probably postpneumonic fibrosis (Figure 1). |
| Mantoux test for tuberculosis was performed and it was positive (18 mm), as well as following Quantiferon test, on the basis it was diagnosed chronic tuberculosis intoxication. It was applied tuberculostatic treatment for four months, without improvement. Following some electroneuromyography confirmed the diagnosis myastenic syndrome. It was diagnosed idiopathic myastenic syndrome as the cancer hasn’t been proved in the patient. It was used acetylcholinesterase inhibitors (Pyridostigmine, Kalimin®), with slight improvement of the muscle weakness. |
| Physical examination revealed: muscle weakness in the proximal muscle groups of the lower limbs; neurological status showed binocular diplopy, ptosis of the lids, decrease muscle tonus, hyporeflexia; pulmonary system–reduced vesicular breath in the middle and inferior lobe in the right lung. Liver, spleen and peripheral lymph node were not enlarged. |
| Laboratory findings showed increased erythrocytes sedimentation rate (46 mm), C-reactive protein 7.35 g/l (normal <6 g/l) and mild elevated creatinine phosphokinase 310 U/l (<180 U/l). |
| In differential diagnosis were discussed: |
| • Lambert-Eaton myastenic syndrome–idiopathic or paraneoplastic |
| • Polymyositis |
| • Polymyositis assocated with other connective tissue disease |
| • Dystrophia musculorum progressiva in adults |
| • Myastenia gravis |
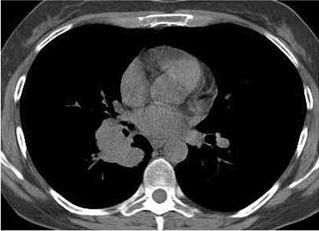
| Electromyography showed low amplitude of compound muscle action potential (CMAP), decremental responce at lowrate repetitive nerve stimulation (2-5 Hz) and marked incremental responces at high-rate nerve stimulation (50 Hz), changes characterized by the myastenic syndrom. It was made a new CT of lung and mediastinum (native), which showed a huge paracardial soft-tissue multilobulated mass (7sm in diameter) with finny spicules with central localization in the right lung, compressing middle and lower lobe bronchi (Figure 2). |
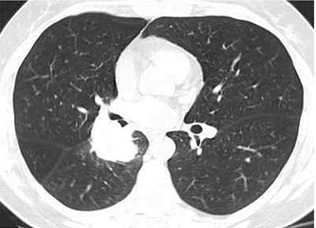
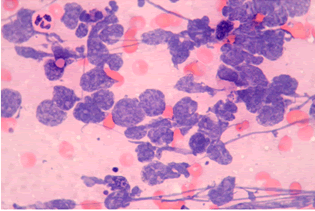
| The results of fibrobronchoscopy revealed narrowed and infiltrated right middle lobe bronchus and narrowed right lower lobe bronchus. Transbronchial needle aspiration biopsy was made (Figure 3). Cytological findings (identified by routine staining with hematoxylin and eozin, HE)-increased number of tumor cells “naked nuclei” type with “salt and pepper” chromatin, with marked crash phenomenon, morphologicaly corresponding to small cell lung cancer cells (Figure 4). |
| The patient began the chemotherapy (Cisplatin/Etoposide) and after six courses of chemotherapy tumor mass in the lung increased. The patient died with clinical features of respiratory failure one month later. |
Discussion
|
| Lambert-Eaton myastenic syndrome is an idiopathic or paraneoplastic syndrome producing antibodies against presynaptic voltage-gated P/Q calcium channels. This decreases calcium entry into presynaptic terminal, which prevents binding of vesicles to the presynaptic membrane and acetylcholine release, resulting in skeletal muscle weakness and autoimmune symptoms [1,2]. LEMS can be divided into two groups depending on whether it is associated with cancer or not [3]. Around 60% patients with LEMS have an inderlying malignancy, most commonly small cell lung cancer, it is therefore regarded as a paraneoplastic syndrome [2,4,5]. LEMS occurs in about 1-3% of patients with small cell lung cancer [3,6]. |
| Clinical features include proximal leg muscle weakness, temporary increase in strenght after voluntary exercise, double vision, ptosis of the eyelids, difficulty swallowing, disruption of the autoimmune nervous system (dry month, blurred vision, orthostatic hypotension) [3]. One of the main clinical symptoms of LEMS is the weakness in the proximal muscle groups, predominantly in the legs. Differential diagnosis of the proximal muscle weakness with slow progression ranges over a variety of nosologic entities – polymyositis, myositis associated with other connective tissue disease, paraneoplastic myositis, myastenia gravis, dystrophia musculorum. Detection of antibodies to the voltage-gated calcium channels and typical electrophysiological features proved the diagnosis LEMS [7,8]. O’Neil et al. indicate that a patient presenting with LEMS has a 62% risk of an inderlying small cell lung cancer, as it is diagnosed most frequently in the first 2 years [9]. We presented a clinical case of patient with small cell lung cancer, which has been diagnosed four years after the developping of LEMS. The paraneoplastic LEMS can be the first manifestation of different cancers (lung, breast, stomach, colon, prostate, pancreas), especially small lung lung cancer. According to Stickler et al. if cancer has not been found in patients presenting with LEMS, they should be screened for small cell lung carcinoma every 6 months with chest imaging for at least 5 years [10]. O’Neil et al. determine that the risk declines sharply after fifth years [9,11]. |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |
|
|









