Keywords
Anticancer; Antioxidants; Analysis; Extraction; Polyphenols
Introduction
Malignancy is a developing health problem all over the world and is the 2nd leading reason for death following heart disease [1]. According to into a recent statement by the Globe Health Business (WHO), coming from a total of 58 million deaths worldwide in 2006, cancer made up 13%. Nowadays there are more than 12 million instances of malignancy peryear globally, including a number of more than 75 diseases including cancer from the Liver, chest, stomach, digestive tract, breast. The most effective way to affect carcinogenesis is by interfering with modulation steps (initiation, promotion, and progression) and also the associated transmission transduction paths. There are many physiological and biochemical cancer causing agents, for example, ultraviolet (UV) and ionizing radiation; asbestos friction material and cigarette smoke; infections simply by virus (e.g., hepatitis B computer virus causing liver organ cancer and human papilloma virus triggering cervical cancer); bacteria (Helicobacter pylori producing gastric cancer) and parasites (schistosomiasis causing urinary cancer) contaminants of meals by mycotoxins (e.g., aflatoxins resulting in liver cancer) Certain kinds of cancer may have due to oxygen-centred free radicals and other reactive oxygen varieties because excessive generation of many of these froe fanciers can cause oxidative damage to biomolecules “e.g., lipids, protein, DNA”.
There are lack strategies to find an active drug to deal with most cancers. There exists a overall demand new medications that are impressive, possess low toxicity, and also have a minor environment impact. New natural items offer possibilities for the development of medication discovery. In fact, natural products have a determining role in tumor prevention and treatment. Numerous antitumor brokers currently utilized in the medical centre are of a natural source. For instance, more than half of almost all anticancer prescription medications approved around the globe between the nineteen forties and 2006 were healthy products or perhaps their derivatives. One of them, plants had been the chief supply of natural ingredients used for medication.
During the sixties, the Countrywide Cancer Company (USA) began to display plant components with antitumor activity. Natural chemical substances isolated coming from medicinal plant life, as rich sources of book anticancer prescription drugs, have been of accelerating interest since that time. Traditional healing herbs have been thoroughly used for pharmaceutics and nutritional therapy for many millennia in East South America, for example, in China, Asia, India, and are presently widely used in cancer remedy. During long-term people practice, a lot of anticancer medicinal herbs and several appropriate medications have been tested and utilized for treating and preventing numerous cancer. Recently, many investigations have reported medicinal plants in treatment and avoidance of cancers.
The previous investigation demonstrated that a common of 35% of general human cancer-related mortality has attributed to diet plant. Considerable evidence coming from population along with laboratory research has exposed an inverse relationship among sufficient usage of fruit and veggies and the risk of specific malignancies, that is, a higher dietary the consumption of fruits and vegetables and whole grains is usually strongly connected with reduced likelihood of cancer. Many trials on the utilization of nutritional supplements and modified diet programs to prevent malignancy are regular. Diet plant such because fruits, fruit and vegetables, spices, cereals, and ready-to-eat tubers/roots-which likewise contain significant levels of bioactive natural substances, may offer social health advantages beyond essential nutrition to lessen the risk of various chronic illnesses including malignancy. The cancer-protective results elicited merely by these nutritional compounds are thought to be because of the induction of cellular protection systems such as the detoxifying and antioxidant digestive enzymes system and also the inhibition
of anti-inflammatory and anti-cell development signaling paths culminating in cell routine arrest and cell-death.
Phytochemicals are thought as bioactive non-essential nutrients by plants (phyto is derived from the Greek phrase phyto, this means plant). They have a variety of individual health effects such as obtaining putative chemo-preventive properties (anti-carcinogenic and anti-mutagenic) and interfering with tumor promotion and progression. The National Tumor Institute, based upon numerous information describing anticancer activity, determined about 45 edible plant Life possessing cancer-preventive properties. Moreover, several studies are discovered more than four hundred species of traditional Chinese healing herbs linked to anticancer. It is estimated that much more than 5000 individual phytochemicals have been identified in fruits, vegetables, grains, and other plants, generally classified since phenolics, carotenoids, vitamins, alkaloid, nitrogen-containing ingredients, and organosulfur compounds. Among the extraordinary structural diversity of phytochemicals, phenolic compounds have got attracted significant interest plus the most focus for their vast array of bioactivities.
Phenolic compounds offer essential features in the duplication and regarding plants; work as defence mechanisms against pathogens, organisms, and potential predators; as well as help the colour of plant life. Additionally, phenolics rich in vegetables and fruits will be reported to carry out an essential part as chemopreventive agents; for instance, the phenolic components of pears have been associated with inhibition of colon malignancy in-vitrο. Various phenolic substances have been reported to possess potent antioxidant activity and to possess anticancer or perhaps anticarcinogenic/ antimutagenic, antiatherosclerosis, antibacterial, viricide and vigorous activities into a greater or lesser degree. With this study, we now have characterized a lot of natural polyphenolic compounds coming from natural medicinal herbs associated with anticancer (e.g., spices, cereals, vegetables, and fruits). Numerous natural phenolic compounds have already been identified for tested healing herbs and dietary crops, mainly incorporating phenolic stomach acids, flavonoids, tannins, stilbenes, curcuminoids, coumarins, lignans, quinones, and phenolic mixes and other phenylethanoids and phenylpropanoids. Their physical and medicinal functions might originate from their antioxidant and free revolutionary scavenging houses and function of regulating cleansing enzymes. Further, these types of antioxidant actions are associated with the constructions of phenolic compounds, generally depending on the quantity and positions of hydroxyl groups and glycosylation or perhaps other substituents.
Antioxidant in plants
Interestingly, plants are notable for creating an assorted cluster of secondary metabolites. Secondary metabolites are classified into terpenes, alkaloids, and phenols. Phenols are categorized as simple phenols with low molecular weight or polyphenols with high molecular weight. The amount and polyphenols type produced by plants differ noticeably among species. Polyphenols are abundant in the plant kingdom as they protect plants against Ultraviolet (UV) radiation. They additionally offer a mechanism to repair the plants upon mechanical damage, through oxidative polymerization using enzymes. Polyphenols play a vital role in plant growth and reproduction, as they provide an effective defence against predators and pathogens. Moreover, they contribute to the nutritional properties of fruits and vegetables and the colour, sensory characteristics. Due to their bioavailability polyphenols human diet, and the potential bioactivities of phenols, their biological influence has been investigated in the past decade in effective manners. Some polyphenols are introduced as therapeutic agents for diversity of diseases or to promote health in general.
Roles of antioxidant in food and human health
There has been growing indication over the past decades, that particular human diseases and oxidative stress may be prohibited by counting plant foods in the diets, that contain enormous quantities of antioxidants, for example, vitamins C, E or natural antioxidants such as tannins, phenolics, coumarins, flavonoids and terpenoids. Dietary antioxidants perform as scavengers of free radicals, a metal ligand, radical chain reaction inhibitors, antioxidant enzyme cofactors, and oxidative enzyme inhibitors.
Consequently, Almajano et al. [1] reported the increment in the interest of broadening the antioxidants that could be utilized as food constituents to avoid food oxidation. Additionally, phenolic extracts obtained from plant substances, for instance, green tea, aromatic herbs and grape seeds are recognized to possess antimicrobial properties to encounter foodborne pathogens.
Antioxidants, for example, BHA, BHT and plant extricates have been broadly utilized as additives, food preservatives. The higher intake of dietary antioxidants could assist in regulating the antioxidant status and sustaining ordinary physiological activities of the human body.
Despite the antioxidant importance as phytonutrients, there is no daily recommendation for “total antioxidant” intake because of their variety and complexity. Therefore, it is still required to investigate the antioxidant activities and influence of foods including fruit and vegetables on both in vitro and in vivo. Health-associated diseases, for example, coronary disease, diabetes, muscular degeneration and cancer are all impacted by damage due to cellular oxidative reactions. There has been growing attention in the response mechanism of antioxidants and whether they precisely interrupt or eradicate free radicals from human cells. Ames conveyed the antioxidants ability to prevent membranes injury of the blood vessel, optimise blood movement to both brain and heart, avert cancer-triggering DNA damage, and lessen the probability of the Alzheimer’s and cardiovascular diseases.
It was stated by Ramsay, that antioxidants might restrain or reduce the oxidative damage associated with several diseases, for instance, atherogenesis, carcinogenesis, and ageing. The cocoa’s flavan-3-cells have been conveyed for its vasodilatory effect that enhanced blood flow. Chlorogenic acid is available in high quantity in coffee and is claimed to have a positive impact on cardiovascular disease and also in reducing type II diabetes.
Its proposed that protective substances which possess the capacity of reactive oxygen species (ROS) inhibition, metalligand or scavenge free radicals, may prevent or impede these diseases. Natural endogenous antioxidant systems in the body have been evolved to encounter the free radical’s production and have been categorized into two groups: enzymatic and non-enzymatic. Enzymatic antioxidants include superoxide dismutase, catalase and glutathione peroxidase. Where nonenzymatic antioxidants include but not limited to β-carotene, vitamin E and vitamin C. Moreover, there is a group called phytochemical antioxidants, for instance, polyphenols, lutein and lycopene that could defend the body against oxidative defect.
Despite the attention given to antioxidant properties of phytochemicals for several years, it is recognized that nonantioxidant influences such as gene expression and cell signaling are vital for health.
Antioxidant activity analytical methods
Various assay techniques for antioxidant activity are used in the literature. According to Prior et al. [2] the First International Congress on Antioxidant Methods in June 2004 was held in Orlando. The discussions included the analytical issues correlated to assessing antioxidant capacity (AOC) in food, nutraceuticals, botanicals and other dietary supplements. It was recommended that standardization of one or more analytical approaches should be executed for regular AOC assessment. Standardization of assays offers (1) guidance for proper application of assays, (2) expressive evaluations of food or commercial products, (3) resources to manage deviations within or between products, also, (4) providing quality standards for health claims and regulative issues. The assay used for standardisation should fall in the analytical range, reproducible, recognizing interfering substances and functional recovery.
It was reported by Huang et al. [3] and Prior et al. [2] that AOC may be classified into two groups referring to their chemical reactions or reaction mechanisms. The two classes are Hydrogen Atom Transfer (HAT) based assay and Single Electron Transfer (SET) based assay. The potential and kinetics for side reactions vary while the result for both reactions is the same. HAT-based methods measure the ability of an antioxidant to suppress free radicals by hydrogen donation (AH=any H donor) to procedure stable compounds: Equation. 1.
X•+AH→XH+A• (1)
The HAT-based procedures consist of an antioxidant, a synthetic generator for free radicals and an oxidable molecular probe.
Mostly, the HAT-based assays employ a competitive scheme, through which both substrate and the antioxidant contest for peroxyl radicals that are thermally produced through azocompounds decomposition. HAT reactions do not depend on pH and solvent and generally accomplished in duration of seconds to minutes.
SET-based methods, on the other hand, detect the ability of a potent antioxidant to transfer one electron to reduce any compound, including metals, carbonyls, and radicals.
X•+ AH→H-+AH+• (2)
AH+• ↔ A•-H3O+ (3)
X- +H3O+→ XH+H2O (4)
M(III)+AH→AH++M(II) (5)
The SET-based assay procedures comprise of one redox reaction with the oxidant as a probe for reaction monitoring) as the reaction endpoint identifier. These assays analyses antioxidant ability to hold an oxidant that alters colour when upon reduction. The colour degree is interrelated with the concentration of the antioxidant present in the sample.
SET reactions depend on pH, somewhat slow and need longer time to come to completion. In contrast to the HAT, SET relies on the solvent, can produce new antioxidants using phenolic compounds polymerization and could undervalue the antioxidant potential when reactions are not approaching completion. Various reaction mechanisms are involved in different assays, among few are:
Assays based on Hydrogen Atom Transfer (HAT)
1. ORAC (Oxygen Radical Absorbance Capacity)
2. TRAP (Total Radical Trapping Antioxidant Parameter)
3. DPPH (Diphenylpicryl hydrazyl)
4. IOU (Inhibited Oxygen Uptake)
5. Linoleic oxidation inhibition.
6. Low-density lipoprotein (LDL) oxidation inhibition.
Assays based on Single Electron Transfer (SET):
1. ABTS (2,2`-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid)
2. FRAP (Ferric Ion Reducing Antioxidant Parameter)
3. DPPH (Diphenylpicryl hydrazyl)
4. Copper (II) reduction capacity
5. TPC (Total Phenol Content, using Folin-Ciocalteu reagent).
It should be stated that the SET and HAT could take place simultaneously dominating mechanism in the system is identified by the antioxidant properties. The characteristics of the five antioxidant assays DPPH, ABTS, ORAC, FRAP and TPC are elaborated below:
Total Phenolic Content (TPC): The Folin-Ciocalteu assay is applied to quantify total phenolics using an oxidation/ reduction (redox) reaction. The assay is based on the (SET) reaction from phenolic compounds to molybdenum in alkaline solution to obtain a blue complex that could be observed at 750-765 nm using spectrophotometer. In current times, the longest-used and most effective assessment for phenolic levels is the Folin-Ciocalteu (FC) method. Another method is an approach designed by Singleton et al. [4] in which phenolics are turned into a molybdotungstphosphate blue by oxidizing the yellow molybdotungstphosphoric heteropolyanion reagent. This method uses a tungstate-multidate solution with 7.5% aqueous Na2CO3. The production of C-2 is a consequence of phenolic oxidization in the essential medium. This then forms Mo04+ upon reacting with multidate, which is highly absorbent (almost 750-770 nm).
The FC method reports phenolic content regarding gallic acid counterparts. Other approaches such as used caffeic acid, and tannic acid used ferulic acid, and used catechin. Roginsky et al. [5] proposed that this method generate a steady blue pigment when mixed with the relevant phenolic. In order to secure consistent findings, it is suggested that the methods use the ideal temperature and experiment time for the colour to appear, the correct ratio of FC and alkali and an optical density of 765 nm. Also, Prior et al. [2] suggested gallic acid to measure the phenolics.
The TPC's main strength is that it is linked with absorbencyemergence. Furthermore, the FC method does not need a structured generalization of the method regulations, and it is also essentially more accurate. Huang et al. [6] argued that although the FC method does not have an identified chemical component, the overall phenol is natural to conduct and replicate. On the other hand, the FC's main weakness is that the overall phenol assessment cannot be applied to lipophilic antioxidants. Also, it identifies both nonphenolics and polyphenols, and therefore, it is not highly discriminative. Nucleic acids, proteins and ascorbic acid can be used to lessen the FC reagent. Because of this, it has been proposed that instead of using the phrase 'total phenolics', the phrase Folin- Ciocalteu Index (FCI) should be adopted instead.
Gheldof et al. [7] Stratil et al. [8] have discovered strong linear relationships between antioxidant animation and total phenolic profiles, based on the various methods proposed earlier, as well as FC. Prior et al. [2] highlighted that the FCI had been utilized for a long time to assess the total phenolics of organic foods. However, it can also be thought of as a technique for identifying antioxidant content, since it’s essential process is to cause a decrease in oxidation Advantages. An excellent linear association with other assays, for instance, FRAP, DPPH, ORAC, TEAC.
The chromophore’s long wavelength diminishes probable interference from the sample matrix, which is frequently coloured.
Acknowledged assay, regularly performed in research laboratories which provides a considerable body of comparative data.
Characterizing and standardizing plant specimens.
Drawbacks
1. The occurrence of interference from aromatic amines, sugar, sulphuric acids, Fe2+.
2. Various non-phenolic organic substances, as well as some inorganic materials, may result in false values.
3. Executed in the aqueous phase and is not pertinent for lipophilic antioxidants.
4. Presence of more than one reacting hydroxy group in standards results in high absorbance backgrounds.
5. Ferric Reducing Antioxidant Power (FRAP): Benzie and Strain (1999) have reported that the assay of FRAP is found on the capacity to reduce the yellow complex, ferric tripyridyltriazine (Fe(III)-TPTZ) into the blue complex, ferrous (Fe(II)-TPTZ) in acidic solution by electron-donating antioxidants.
Advantages:
1. A fast, simple, low-cost and powerful assay that does not demand a specific apparatus and could be completed automatically or manually.
2. It is entirely electron transfer rather than a mixed SET and HAT. It is a very convenient assay to differentiate the dominant mechanisms of different antioxidants, in association with other methods.
Shortcomings:
1. An extended reaction period is required to detect polyphenols that give a slow response.
2. The reactivity order may noticeably differ between various antioxidants. Pulido et al. [9] conveyed that several samples have exhibited this prolonged reaction period, including tannic acid, caffeic acid, ascorbic acid, ferulic acid and quercetin.
3. Fe2+ is a recognised “pro-oxidant” that reacts with H2O2 to yield a hydroxyl radical (OH•) which is the furthermost destructive free radical in vivo.
4. A few antioxidants, for example, uric acid and ascorbic acid could result in the reduction of both Fe3+, moreover, reactive components in the FRAP test, so their capacity to reduce Fe3+ might express their capacity for the reduction of reactive components.
DPPH radical scavenging activity: Locatelli et al. [10] described that the absorption power is reduced and the radical solution (DPPH radicals purple chromogen), in the existence of a hydrogen/electron donor (free radical scavenging antioxidant), is discoloured to a pale-yellow hydrazine in line with the number of captured electrons. Paixao et al. [11] confirmed that DPPH performs in both hydrogen transfer (HAT) as well as electron transfer (SET) systems and grant the assessment of a substance or a complex mixture that contribute with either electrons or hydrogen atoms in a homogeneous medium. For measuring the antioxidant behaviour within the plant, DPPH free radicals are often utilized. The antioxidants that contribute hydrogen to lower the radicals can be accessed via a reduction in their ideal density on a long wavelength. Brand-Williams et al. [12] proposed that the DPPH approach involve the antioxidants sourcing the DPPH radical.
This then generates a lowered absorbency (517 nm): The mechanism of the reaction is described in the equation below where AH represents the antioxidant the free radical is represented as R•:
DPPH (purple) + AH→ DPPH-H (yellowish) A• (6)
The chemical structure of DPPH is shown in Figure 1
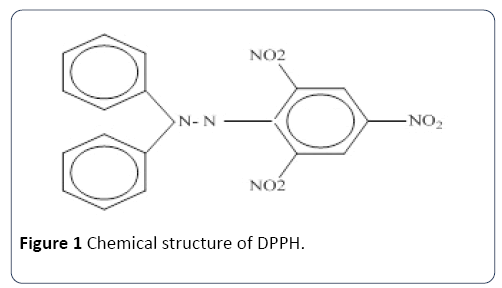
Figure 1 Chemical structure of DPPH.
Fukumoto et al. [13] explain that the DPPH is most likely often used to assess antioxidant content because of its requirement for a UV-Vis spectrophotometer, as well as its speed and lack of complexity. In addition, microplates could be used to assess a high quantity of samples. DPPH is commonly used to assess various food types for antioxidant content due to its numerous strengths. The primary benefit of the DPPH is its lack of volatility, which was supported by a lack of identifiable reduction in its absorbency. Also, DPPH is not susceptible to oxidation, remains consistently absorbent across a broad set of pH levels and stays monomeric when blended with liquids, generates a good level of essential knowledge regarding compound reactivity, and does not require a severe catalyst for a reaction to occur. The DPPH radical is powerful and the DPPH assessment involves a measurement of complicated phenolic antioxidants because these are usually the strongest radical scavenger. Arnao et al. [14] found that the DPPH approach tests this capability.
However, one issue with this is that other compounds can act as catalysts when interacting with free radicals, and this can hinder the antioxidants' ability to hunt the radicals. Arnao et al. [14] developed a high quantity assessment of antioxidant depending on a DPPH dry reagent range. The technique includes a surface coating of a 96 microplate with the DPPH, (dissolved in methanol), by drying it under nitrogen. The DPPH dry reagent array was successfully applied to the antioxidant activity evaluation in actual tasters and executed in addition to the new DPPH solution assay.
Advantages:
1. A fast, effortless and low-cost technique for assessing the antiradical activity.
2. It generates steady organic nitrogen radicals defined by a deep purple colour in the range of 515-520 nm.
Disadvantages:
1. The spectra of some antioxidants like carotenoids overlays at 515 nm with DPPH measurement and results in undesired interference.
2. Discolouring of DPPH from radical reactions (HAT) or reductions (SET) and subsequently irrelevant reactions may result in imprecise data.
3. Only organic solvents like acetone, methanol or ethanol, could dissolve DPPH radical which regarded as a limitation when the function of hydrophilic antioxidants is being interpreted.
4. Several aspects might influence the assay like pH, solvent, the concentration of samples and the period of the reaction.
5. The DPPH radical absorbance at 515-520 nm post the reaction with an oxidant is decreased by light, solvent type and oxygen.
6. Antioxidants that react with peroxyl radicals in a fast manner in vivo might react at low speed or may be deactivated towards DPPH as a result of the steric effects that over the accessibility.
7. It is indicated that there is a non-linear correlation between the DPPH radical scavenging activity and antioxidant concentration.
8. DPPH is stable nitrogen radical. However, it is not reproduced in vivo condition.
ABTS 2, 2’ Azinobis (3-Ethyl-Benzothiazoline-6-Sulfonic): Floegel et al. [15] explained that antioxidants might diminish the production of a significantly stable chromophoric cation radical of ABTS (blue/green) through peroxyl radicals or other oxidants in the occurrence of H2O2. The antioxidant could postpone or reduce its absorbance. In recent adjustments, ABTS reduction which counts on electron transfer is observed. The ABTS assay was first proposed by Miller [16] but it was developed afterwards by Re et al. [17] The variance between the two methods is the generation method of ABTS. The native ABTS assay relied on metmyoglobin activation by means of hydrogen peroxide, in the occurrence of ABTS to generate the radical cation, in the lack or the availability of antioxidants. This procedure was disregarded because the rapid reacting antioxidants may also contribute to the ferry myoglobin radical reduction. The amended procedure for ABTS production includes the re-creation of the radical cation past the antioxidant addition.
Advantages:
1. A fast and simple technique that generates consistent data.
2. ABTS•+ might be solubilized in aqueous as well as organic solvents and is not influenced by ionic strength. It could be utilized to quantify the antioxidant activity of lipophilic and hydrophilic antioxidants.
3. Rapidly reacts with antioxidants within 30 minutes, may be used in a broad range of pH and can be done automatically in micro plates.
Disadvantages:
1. The values of TEAC mark the capability of a specimen to react with ABTS•+ rather than to constrain the oxidative procedure.
2. The reaction amongst specimen may consume a long time to attain an endpoint.
3. An assay with a steady short-time (4-6 minutes) is too short and might result in faulty antioxidant capacity values due to the incomplete reaction
4. The obtained values of TEAC at a fixed endpoint or assessed built on the samples’ kinetic performance yield altered results.
5. Necessitates distinct preparation wherein ABTS•+ requires generation by enzymes or chemical reaction.
6. The applied ABTS•+ in TEAC is a synthetic radical which does not exist in a biological system. Accordingly, the assay is not reproducible in the in vivo conditions.
Cupric reducing antioxidant power (CUPRAC) assay: The CUPRAC method was a novel and can be employed to lipophilic as well as hydrophilic antioxidants synchronously, by engaging their “host-guest” complexes with M-β-CD, a cyclic oligosaccharide, in the acetonated aqueous system. M-β-CD was presented as the water solubility booster for lipophilic antioxidants. M-β-CD at a concentration of 2% (w/v) in a mixture of 90% acetone-10% H2O adequately solubilized vitamin E, β-carotene, vitamin C, oil-soluble synthetic antioxidants, and other phenolic antioxidants. This technique requires for the broad inconsistency in antioxidant abilities of oil and water-soluble antioxidants presentation different levels of accumulation at the interfaces of the emulsion in oil-inwater and water-in-oil. It also allocates an objective value of TEAC to each antioxidant which depends on its chemical property (i.e., H-atom or electron donating capability). To detect polyphenolic compounds using CUPRAC, the recognition of hydroxyl radicals and quantification of hydroxyl radical scavenging activity is vital in bioanalytical chemistry and food technology, as it relates to antioxidant activity and antiradical of food products and antioxidant treatment. At present, the most extensively applied colourimetric and chromatographic approaches for hydroxyl radical identification and) scavenging activity assessment is the TBARS colourimetric assay and HPLC with electrochemical detection of hydroxylated aromatic probes, correspondingly. The suggested CUPRAC/salicylate assay of identification is much more effectual than the conventional TBARS assay since the ratio of conversion of the probe is much higher. The generated hydroxyl radicals attacked both the probe and the watersoluble antioxidants at 37°C for two hours.
The most significant involvement of the developed assay is the Fenton reaction termination in 10 min with catalase deterioration of hydrogen peroxide so that the residual H2H2 would neither give a CUPRAC absorbance nor involve in redox cycle formation of phenolic antioxidants. This enables the fast and accurate determination of the rate constant of scavenging for polyphenolics which does not occur in most other procedures.
Apak et al. [18] and Ozyurek et al. [19] have updated the CUPRAC methods with significant advantages and few drawbacks summarized as follows:
The advantages of the CUPRAC method:
1. The CUPRAC reagent (outer-sphere e-transfer agent) is performing adequately fast in the oxidation of thiol-type antioxidants.
2. As the possesses a low redox potential, it is characterized by selectivity.
3. The potential of the Cu (II, I)-Nc redox pair at standard conditions, is 0.6 V, close to that of ABTS•+/ABTS (E°=0.68 V).
4. CUPRAC reagent does not oxidise citric acid and simple sugars.
5. Its stability and easiness of accessibility characterizes the reagent
6. The CUPRAC assessment for a biological specimen might have an indirect but effectual reflection of the total power of antioxidant although no radicals are taking part in the assay.
7. The technique can be applied merely in typical laboratories thru standard colorimeters with no the necessity of specialized instruments and highly trained machinists.
8. Technique responds evenly to both lipophilic as well as hydrophilic antioxidants.
9. The curves of CUPRAC absorbance vs concentration from a perfect line over a broad range of concentration.
10. The control of CUPRAC reagent and related chemicals enables the assessment of ROS scavenging activity and antioxidants’ TAC.
Disadvantages:
Sophisticated instruments are required which are more expensive.
Antioxidant classification
Antioxidants form various categories of compounds that may obstruct oxidative cycles to impede or retard the biomolecules from oxidative damage. The main categories of substances with antioxidant properties are polyphenols (phenolic acids, flavonoids, lignans and stilbenes), and vitamins (C and E), and carotenoids, (carotenes and xanthophylls).
Phenolic compounds/polyphenols
Polyphenols are omnipresent in all plant components and are, consequently forms an essential part of the human diet. More than of 8000 phenolic structures have been conveyed and are extensively distributed within the plant kingdom. Phenolics compounds vary from pure, low molecular mass, single aromatic ring compounds to the big complex tannins and polyphenol derivative. They are produced by the polyketide, shikimate and mevalonate pathways or mixed pathways, in which, a large variety of plant phenols is yielded. In the late 20th century, the attention towards phenolics derived from food has risen owing to their antioxidant properties and free radical-scavenging capabilities as well as signal transduction modulation, anti-inflammation, antiproliferation and anti-microbial activities.
Polyphenols might exhibit an indirect antioxidant effect by conserving endogenous antioxidant enzymes in the human body; Zhang et al. [20] proposed in their review that polyphenols could offer significant conservation from oxidative damage in vitro at lower levels than the required amount for chemical antioxidant protection. The key mechanisms whereby phenolic substances from diet play a part in averting deteriorating diseases are: NF-κB (nuclear factor kappa B) signalling pathway, activator protein-1 (AP-1- a redox-sensitive transcriptional factor), Phase II enzyme activation and Nrf 2 and mitogen-activated protein kinase (MAPK) signalling pathway.
Phenolic compounds from an extensive diversity of molecules that involve a polyphenol structure (i.e., few hydroxyl groups on aromatic rings), as well as molecules that own one phenyl ring, like phenolic alcohols and phenolic acids. Polyphenols (Figure 2) are categorized into several classes giving the phenol rings number that they hold and the structural components that link these rings together. The primary groups of polyphenol are phenolic acids, flavonoids, tannins (condensed and hydrolysable), lignans and stilbenes.

Figure 2 Tyrosol and hydroxytyrosol: (a) Tyrosine and (b) Hydroxytyrosol.
Because of its abundance in the natural world, the plantderived compound, polyphenol, has been significantly researched. The structure of phenolic compounds differs significantly; while they are all formed of the aromatic ring bearing hydroxyl groups, they can vary in size from simple phenols-simple molecules, up to large oligomers or oligo phenols. Bravo highlights that the profusion of their occurrence is glycosylated [21]. As a result, these phenolic compounds are water soluble, although the larger oligomers have a higher molecular weight, and thus are generally less soluble.
The significance of polyphenols within bioactive compounds, in a functional capacity, is that they have antioxidant capabilities. They are available in some fruits and are thus considered the critical phytochemicals of a balanced diet. Insidious carcinogenic related to the onset of cancer, nitrosamines are targeted by the properties and functions of phenolic acids and flavonoids, which stimulate the human body’s natural abilities to cope with and fight the toxins. In this process, initially, phenolic acids and carotenoids attack the highly-reactive free radicals, which have the potential to lead to the development of cancerous cells.
Leitzmann and Groeneveld [22] highlighted the process of the subsequent phase, also known as the promotion phase, in which active blocking agents diminish the speed with which the cleavage of potentially cancerous cells occurs. This process occurs before or within the process of carcinogenesis. The presence of suppressing agents, in the promotion or progression phase, leads to further interference in the process. Suppressing agents, in the form of carotenoids, and blocking agents, phenolic acids, coumarins and flavones, are considered anti-carcinogens, and are important in the fight against the accumulation of cancerous cells in the body.
The main groups of polyphenols are flavonoids, phenolic acids, phenolic alcohols, stilbenes and lignans. Their chemical structures are presented in Figure 2. The antioxidant activity mechanism of the polyphenols has been projected by Leopoldini et al. [23]. Initially, the polyphenol molecule deactivates free radicals based on the mechanisms of hydrogen atom transfer and single electron transfer. The hydrogen atom transfer mechanism presumes that antioxidant the reaction occurs between Arron, the antioxidant, and the free radical, R, with hydrogen atom transfer:
ArOH + R• → ArO•+ RH (7)
On the other hand, single electron transfer assumes that the antioxidant molecule receives an electron from the oxidant:
ArOH + R• → ArOH•+ + R- (8)
The two mechanisms generate a harmless species (RH), an oxidized radical ArO•, a cation radical ArOH•+, moreover, a dynamically stable species R-.
Flavonoids: Flavonoids many of which are plant pigments are abundant in nature (Figure 3). Flavonoids can be further divided into several subclasses, of which the most representative are: flavones, flavanones, flavonols, flavanols (also called flavan-3-ols or catechins), anthocyanidins and isoflavones. The general structure of flavonoids includes a C15 (C6-C3-C6) skeleton. The flavonoids can be classified in several subgroups which are mainly indicated either by (i) hydroxylation, (ii) O-methylation, (iii) C-methylation, (iv) isoprenylation, or (v) methylenedioxy substitution. The primary sources of flavonoids and their health benefits are presented in Table 1.

Figure 3 Examples of chemical structure of different subclasses of flavonoids: (a) a flavonol, (b) a flavone (c) a dihydroflavonol (d) a flavan-3-ol, (e) a flavanone, (f) an anthocyanidin, (g) a chalcone, (h) a dihydrochalcone, (i) an aurone.
Table 1 The main sources of flavones and their health benefits are presented.
| Antioxidant |
Health benefits |
Natural resources |
| Polyphenols Flavonoids |
The intake of flavonoids is inversely associated with subsequent cancer |
Grains, Honey, Orange juice, Tsaoko, Amomum fruit, Drumstick, Stevia, Rebaudiana leaves [25-31] |
| Flavonols |
Quercetin is the main compound of flavonol to be found in onions with anti-cancer, Anti-Inflammatory, and anti-viral activity.
It may also have the capability of preventing cardiovascular disease in humans. |
Vitis vinifera, Grape berry skins, Green tea, Green prickle, yash, Sichuan pepper, Fennel, Onion [32-34] |
| Flavones |
The flavones have antioxidant anti-cancer, anti-inflammation, anti-diabetes, anti-ulcer and antimicrobial effects. Naringenin as a flavanone demonstrated antidiabetic and antioxidant activity, as well as anti-proliferative properties in cervical cancer. This compound works in isolated animal tissues by inhibiting gastrointestinal smooth muscle contractility, and in isolated colonic epithelia for chloride secretion. |
Citrus peel, Troll flowers, Onion, Parsley, Lemons [35-38] |
| Flavanones |
Flavanones come with a wide spectrum of pharmacological properties, e. g inflammatory, anticarcinogenic, antihypertensive and anti-atherogenic. Moreover, also, exhibits antioxidant properties. |
Limes, Sweet, Oranges, Fruits, Black sorghums [39] |
| Flavanols (flavan-3-ols) |
Flavanols exhibit anti-inflammatory, antiproliferative, antioxidant, and antithrombotic activities, as well as lipid metabolism modulation and pathogenic bacteria inhibition. |
Green coffee beans, Human milk [40] |
| Anthocyanins |
Anthocyanins exert benefits as an anticarcinogenic and antioxidant activity and plays a vital role in preventing cardiovascular and neuronal diseases, diabetes and cancer. Four weeks administration of anthocyanins decreases diabetes, obesity, myopia, and apoptosis symptoms. The antioxidant potential relies on the molecular chemical structure and the phenolic structure to give the antioxidant properties. |
Grapes, tomatoes, Pomegranates, Purple carrots, Green coffee beans, Sweet potatoes [41] |
| Isoflavones |
Isoflavones contain a diversified functional benefits for human, ranging from the reduction of cardiovascular risk, cancer inhibition, and postmenopausal symptoms. |
Soybean, Green, yellow and red lentils, red, Kidney beans, haricot beans, Chickpeas [42,43] |
| Phenolic acids |
The phenolic acids are potent antioxidants demonstrating anti-inflammatory, anticarcinogenic, antiviral, antibacterial, and vasodilatory actions. |
Dried ginger, Fennel, Grains, Green coffee beans, Orange juice [44] |
| Phenolic alcohols |
Hydroxytyrosol has an unusual antioxidation activity, making it a promising alternative to synthetic antioxidants such as 2- and 3-tert-butyl-4-hydroxyanisole (BHA), 2,6- di-tert-butyl-4-hydroxytoluene (BHT), or ethoxyquin, which are typically used as preservatives in food and feed, despite their confirmed toxicity. Hydroxytyrosol and tyrosol exhibit an in vitro protective effect towards the oxidation of low-density lipoprotein (LDL) and protect DNA and erythrocytes from oxidative damages at low concentration. The European Food Safety has approved the compound for its ability to control healthy LDL cholesterol and lipid antioxidation levels. |
Olives and olive oil, Olive oil mill waste water [44,45] |
| Tannins |
Tannins have been reported to exert the properties of anti-thrombotic, antiviral and antibacterial, anti-inflammatory, anti-carcinogenic, anti-diabetic and anti-proliferative effects, as well as anti-mutagenic actions. |
Bean seed coats, Mangosteens, Green coffee beans, Mango kernels, Strawberries [46-48] |
| Stilbenes |
The stilbenes are well-known for preventing cardiovascular illness with antioxidant and antimicrobial efficiency, as well as exerting the properties of arteriosclerosis and cancer acting, executing the role of antiviral and anti-inflammatory agents. |
Almonds, Chocolate, Cocoa Seeds, Grapes [49] |
| Lignans |
Lignans take a function in normal colour functioning as antioxidants and display the growth of experimental mammary cancer inhibition. |
Roots, leaves, seeds, fruits and woody, Parts of vascular plants, vegetables and beverages [50] |
| Vitamin C |
The capability of increasing the plasma resistance to lipid peroxidation in long and short-term supplementation makes Vitamin C a compound of great interest. It works by lowering serum, uric acid levels, thus resulting in lowering incidence of gout significantly, as well as decreasing the risk of having a stroke and reduces the possibility of degenerative and chronic diseases. |
Apple, Banana, Bayberry, Broccoli, Citrus peel, Garlic [51,52] |
| Vitamin E |
Vitamin E has been considered as the primary components for spermatozoa antioxidant system and considered to be one of the main membrane protectants against lipid peroxidation and reactive oxygen species |
Grains, Green tea, Olives and olive oil, Palm oil pumpkin seeds, Sunflower seeds and sunflower oil [53] |
| Carotenoids |
Carotenoids have diversified roles in preventing various health disorders, including cardiovascular disease, metabolic disease, and cancer. |
Grains, Orange juice, Parsley, celery, basil, coriander [54,55] |
| ß-carotene |
Beta-carotene has been reported to be associated with positively influencing some instances of cardiovascular diseases and certain types of cancer. |
Amaranth, dark green leafy vegetables, Gac, Olive oil, Red carrots, Sweet potato [56] |
| Lycopene |
Lycopene has the role of preventing several pathologies, e.g. cardiovascular disease, certain types of cancer, as well as the case of obesity. |
Apricots, Grapefruit, Guava, Watermelon, Papaya, Tomatoes, Gac, Spinach Kale, Carrot [57] |
| Carotenoids- Xanthophylls |
Lutein and zeaxanthin are well-known to have a strong correlation with visual health, as well as being involved in cardiovascular diseases risk reduction at its developing stage. |
Spinach, Kale, Zea mays Carrot [58] |
Markham highlighted the great concentration of flavonoids in the plant tissue of over 4000 groups of plant polyphenols. As highlighted in Figure 3, a number of scholars classify flavonoids into five categories: the predominant, abundant flavoand flavonols, existing in the majority of plant foods; the citrus-based flavanones; the berry-borne purple anthocyanins; and catechins, present in tea. As Hertog et al. [24] stated, flavonoids are generally concentrated in the leaves and external cells of plants. A variety of flavonoids exist in vegetables; the most abundant is quercetin, while kaempferol, luteolin and apigenin also occur.
Strack also highlights the profusion of glycosides present in flora, from which two fundamental linkages occur O-glycosides and C-glycosides. Of the sugar moieties present, the most common are glucose, galactose, rhamnose, xylose and arabinose.
Flavonols: Flavonols are the fullest class in Vitis vinifera grape berry skins. Flavonols are recognised by the position of the alcohol group on the carbon ring (Figure 3). The typical sugar components in flavonols are glucose, galactose, rhamnose and arabinose. The primary dietary flavonols are myricetin, quercetin, isorhamnetin, rutin, and kaempferol. One of the most important flavonols is quercetin. Fruit and vegetables contain quercetin, 3, 3‘,4‘,5,7- pentahydroxyflavone. It exists primarily in leaves and other plant parts like glycosides and aglycones, whereby one or more sugar groups are linked to phenolic groups through a glycosidic bond. The most abundant sugar is glucose, with rhamnose and galactose regularly associated with flavonoids. Commonly, quercetin glycosides comprise of a sugar group at the 3-location. The fundamental wellsprings of flavonols and their medical advantages are exhibited in Table 1.
Flavones: Chemically, flavones are deficient in a 3-hydroxy group. The central part of the flavones synthesis from two flavanones (pinocembrin and naringenin) (Figure 3). Both flavones are produced from the condensation reaction of one molecule of hydroxycinnamoyl-Coenzyme A and the molecules of malonyl-Coenzyme A. Flavones synthetases I and II can convert the flavones to flavones. The numbers of flavones are enormous due to the occurrence of a combination of several modifications. Flavones may be found in all parts of plants; above and below ground, in vegetative and generative organs: stem, leaves, buds, bark, heartwood, thorns, roots, rhizomes, flowers, fruit, seeds, and also in root and leaf exudates or resin. The primary primary of flavones and their health benefits are demonstrated in Table 1.
Flavanones: Flavanones may also exist as oxygen or carbonglycosides and are mainly available in citrus fruit and prunes. Naringenin (4′,5, seven tri-hydroxy flavanone) is a flavanone present mostly in citrus fruit like grapefruit and orange. The flavanones primary sources and their health benefits are found in Table 1.
Flavanols (flavan-3-ols)/procyanidins: The flavanols are susceptible to many deteriorative reactions enhanced by heat, high pH (>5), food ingredients and occurrence of dissolved oxygen or other reactive oxygen. The auto-oxidation of flavan-3-ols ensues by a radical semiquinone formation which is stabilised through several resonance structures existing to the flavan-3-ol.
Anthocyanins: Anthocyanins group is the largest amongst natural pigments, and it is one of the groups accountable for colour in some flowers and fruits. Anthocyanins are a category of natural bioactive substances whose occurrence beverages and food could be anticipated which enables the information transfer from the nutritional as well as a pharmacological exploration into applied assistance to health-concerned consumers. When anthocyanidins are allied with one or more glycosidic components, they are called, anthocyanins. Anthocyanidins are associated with one or more glycosides. For instance, glycosylated polyhydroxy and polymethoxy derivative of 2-phenyl benzo pyrylium salts (flavylium). The glycosidic units may be linked to the anthocyanidin through α or β link at position 3 of the aglycone (Figure 3). When more sugars are existing in the anthocyanin molecules, they are connected to 5 and 7 positions, and less often to 3‘and 5‘. The sugars come upon in anthocyanins may be hexoses (galactose and glucose) and pentoses (arabinose and xylose). The isolated anthocyanins lack the stability and are very vulnerable to degradation by temperature, light, pH, solvents, oxygen, metallic ions, ascorbic acid sulphate and enzymes. The anthocyanins primary sources and their well-being advantages are shown in Table 1.
Isoflavones: Isoflavones (Figure 3) are a cluster of oxygen heterocyclic compounds that allied with phytoestrogens. The most prominent isoflavones are genistein, daidzein and glycitein, which ensue in legumes, particularly in soybean. In plants, the primary forms of isoflavones are glycosides with sugar, for instance, glucose, galactose, rhamnose, malonylglucose and acetylglucose. Isoflavones’ main sources and their health benefits are shown in Table 1.
Phenolic alcohols: The phenolic alcohols (Figure 2) are found as 2-phenylethanol, (3, 4-dihydrozyphenyl) ethanol, and known as hydroxytyrosol, (p-hydroxyphenyl) ethanol, also recognised as tyrosol and (3, 4- dihidrophenyl) ethanolglucodise. Tyrosol and hydroxytyrosol are two crucial odiphenols occur in olives, olive oil and olive mill wastewater. Table 1 elaborates the primary sources of phenolic alcohols and their health benefits.
Phenolic acids: Phenolic acids can be classified into two groups based on their structure: benzoic acid derivatives and cinnamic acid derivatives. They comprise of a benzene ring attached to a carboxylic group (benzoic acids) or a propenoic acid (cinnamic acids). Cinnamic acids, particularly caffeic acid, exist in many vegetables and fruits as ester derivates, for example, chlorogenic acid, other cinnamoyl quinic acids, phenylethanoic glycosides which display great antioxidant properties. Hydroxybenzoic acids contain gallic, protocatechuic, p-hydroxy-benzoic vanillic and syringic acids. The most important hydroxycinnamic acids are caffeic, pcoumaric, ferulic, and sinapic acids (Figure 3).
The occurrence of flavonoids in plants is dependent on their prevalence to act as glycosides. The fundamental structure of flavonoids is that of a phenolic benzopyran; they are formed of a compound with CIS-acting flavone nucleus. As seen in Figure 4, this is formed through a C6-C3-C6 nucleus, and an A and B benzene ring connected through ring C of oxygen-containing pyran or pyrone. Both 3-deoxyflavanoids-flavones, flavanone isoflavones and neoflavones and 3-hydroxyflavonoids, flavonols, anthocyanins, flavan-3, 4-dils and flavan-3-ols, utilize this structure. Kiehne et al. [60] highlights that the differences witnessed here are developed from the existence of a hydroxyl group at position C3.
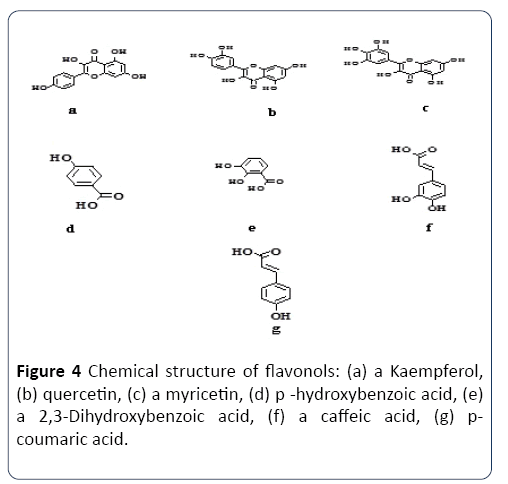
Figure 4 Chemical structure of flavonols: (a) a Kaempferol, (b) quercetin, (c) a myricetin, (d) p -hydroxybenzoic acid, (e) a 2,3-Dihydroxybenzoic acid, (f) a caffeic acid, (g) pcoumaric acid.
The inherent characteristics of flavonoids suggested that a considerable number of variations of a single flavonoid must exist. These variations exist as a result of discrepancies in intra and intermolecular structures and their different susceptibility to undertaking transformation and redox processes; the fluctuating position and amount of the hydroxyl groups; and the polymerization and structures seen in the correlation with saccharides. Some scholars have acknowledged that these differences have confirmed the value of these groups of organics, in particular through their biological structure. For example, polyphenolic-structured flavonoids have strong antioxidant capabilities and can be absorbed rapidly by organisms.
A reduction in the prevalence of heart disease can be attributed to the antioxidant capacity of flavonoids. Other studies, in particular, epidemiological and in vivo studies, have confirmed that flavonoids can defend the human body from the development of cancerous cells, cerebrovascular accidents and atherosclerotic heart disease.
In particular, flavonols are suspected to have higher antioxidant capacities than both vitamin C or E, and thus are highly beneficial for living organisms. Despite the perceived benefits, particularly in the reduction of carcinogenic activity, juxtaposed these advantages with results that suggested flavonoids had both mutagenic and carcinogenic effects.
The primary sources of phenolic acids and their health benefits are accessible in Table 1.
Figure 3 shows both flavonols and flavones, respectively. Both of these are considered important flavonoids as a result of their high concentration of antioxidants, and free radical scavenging ability. Netzel et al. [61] refute Crossett’s claim of carcinogenicity, stating that epidemiological studies have highlighted that flavonols and flavones consumption will consistently lead to a decrease in the prevalence of both cancer and cardiovascular disease. This is because the oxidation of low-density lipoprotein and lipid peroxidation is inhibited by the antioxidant abilities of flavonols, including quercetin, myricetin, isorhamnetin, and kaempferol and the corresponding flavones, apigenin and luteolin. Along with the antioxidant nature of flavonoids, these compounds also exude other advantageous characteristics as confirmed by various studies. The antihepatotoxic, anti-angiogenesis, antiplatelet, anticarcinogenic and antimutagenic abilities of flavonoids have been explored by Cook et al. [62].
Tannins: Tannins are formed by plants as secondary metabolites and have a molecular weight of up to 30,000 Da (Figure 5). Giving their structure, tannins could be categorized into two types of macromolecules, marked as hydrolysable and condensed tannins. The first class possesses a molecular mass fluctuating amongst 500 and 5000 Da. In addition to their bitterness, they own important antioxidant property. The second class, condensed tannins or proanthocyanidins are polymers with a high molecular mass up to 30,000 Da. The monomer is a flavan-3-ol such as catechin or epicatechin with a flavan-3, 4-diol or a leucoanthocyanidin molecule as its ancestor. Oxidative condensation takes place between C- 4 of the heterocycle and C- 6 or C- 8 of adjacent units. The primary sources of tannins and their health benefits are enlisted in Table 1.
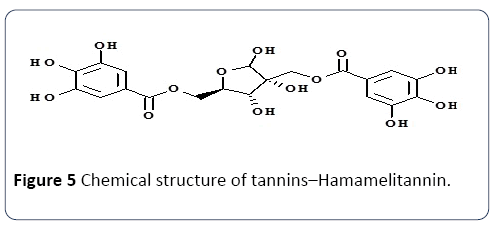
Figure 5 Chemical structure of tannins–Hamamelitannin.
Stilbenes: Oroian et al. [63] defined stilbenes to be categorized under the class of phenolic compounds. Kostadinovic et al. [64] clearly defined stilbenes has/with one ethane bridge linking two displayed aromatic rings, that exist in the form of oligomeric as stilbenes oligomers (e.g., polymers, trimers, or dimers of resveratrol) or other stilbenes (e.g., pallidol, epsilon-viniferin), as well as monomeric (resveratrol and oxyresveratrol) form. Stilbenes are essential due to its health effects that appear to be at lower concentrations than other phenolic compounds. Resveratrol is an essential and an vital stilbene (Figure 6), noted to be produced by vines as an infection response towards Botrytis and other fungal attacks. The natural phenol exists in two isomeric forms, the isomers with trans and cis-configuration where light affects the cis/trans isomerization. The glycosylated forms of resveratrol are trans-piceid and cispiceid. Table 1 provides the significant sources of stilbenes and their health benefits explanation.
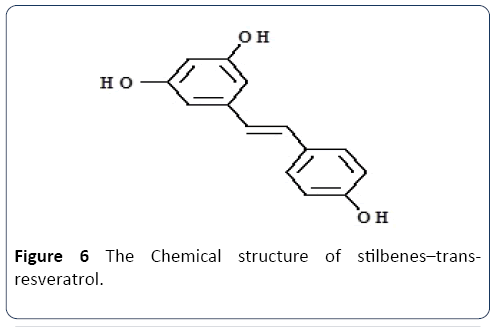
Figure 6 The Chemical structure of stilbenes–transresveratrol.
Lignans: Lignans can be considered as a group of phenolic compounds that remain in flaxseed and other seeds with high concentrations (Figure 7), and also to be found in leaves, fruits, roots, other woody parts of vascular plants and grains. The group has ‘structures built on C6-C3 units of aa propyl benzene skeleton that is derived from cinnamyl units. Table 1, represents the significant sources of lignans and the health benefits they can offer.
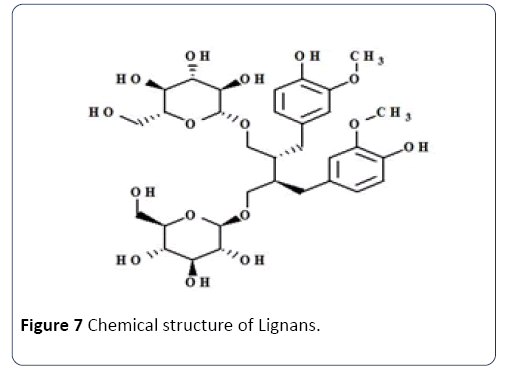
Figure 7 Chemical structure of Lignans.
Vitamins
Vitamin C
Vitamin C, also known as L-ascorbic acid (Hence ‘ascorbic acid’ refer to ‘L-(+)-ascorbic acid’ and ‘ascorbate’ refer to ‘Lascorbate’) (Figure 8) is a peculiar vitamin for multiple reasons compare to the others. This organic compound is considered as one of the most critical hydrophilic antioxidant Bahadoran et al. [65]. Efficient in scavenging hydroxyl radicals, superoxide radical anions, hydrogen peroxide, singlet oxygen and reactive nitrogen species. Vitamin C catalyzes the oxidation of muscle tissue in a low-level application (<100 mg/kg), but oppositely inhibits the oxidation and scavenging reactive oxygen species (ROS) at high-level application (>1000 mg/kg). Vitamin C has the capability of donating hydrogen from it is 4-OH groups to an oxidising system at the structural level. Linster et al. [66] highlighted that the vitamin C which has lost one electron could be written in the resonance forms resulting in a considerably more stable radical semi-dehydroascorbate (SDA) with much less reactivity compare to other free radicals.

Figure 8 Chemical structure of Vitamin C.
Bendich et al. [67] proposed the vitamin C antioxidant mechanism. They explained the ascorbic acid (AH2) acidic nature leads to ascorbate anion (AH-) as the predominant form present at pH 7. This compound has the capability for reversible oxidation process, and with ascorbyl radical formation (A-•) form a dehydroascorbic acid (A). The relatively unreactive nature of the ascorbyl radical can assist the reactions with other free radicals, thus stopping their reaction propagations (eqn. (9)):
2A-• + H+ → AH- + A• (9)
Bendich et al. [67] proposed the reaction between the ascorbate and the peroxyl radicals as:
ROO•+AH- → ROOH+ A-• (10)
A-•+ O2→A+O2-• (11)
O2-•+ ROO• →H+ + O2 + ROOH (12)
O2-• + AH- →H+ H2O2 + A+ (13)
A-• + ROO• →H+ + A + ROOH (14)
In the early report ofin vivo cellular assays, ascorbate efficiency was observed to be higher at low concentrations (one ascorbate molecule can trap two peroxyl radical) but decreases exponentially to zero (0) at higher concentrations. A peroxyl radical reacts with the ascorbyl radical (reaction 6) or the superoxide anion (reaction 4) in the presence of low ascorbate concentrations. High concentrations of ascorbate lead to the competition of reaction four propagation and the reactions of chain termination 4 and 6. Thus the ascorbate is wasted, and its efficiency is lowered. The European Food Safety Authority recommends vitamin C intake based on the age but in the range of 25 to 45 mg/day. The significant sources of vitamin C and the corresponding health benefits are presented in Table 1.
Vitamin E
Vitamin E, belongs to tocotrienols and tocopherols group of chemical compounds. It is broadly known as the primary lipidsoluble antioxidant source in humans and works by two primary mechanisms as an antioxidant: (i) chain-breaking acceptor (CB-A) and (ii) chain-breaking electron donor (CB-D) mechanisms. The capacity of α-tocopherol as an antioxidant has been studied through the observation on storage, parboiling and cooking as well as genotype that may affect its activity (Figure 9). Vitamin E is composed by four tocotrienols and four tocopherols with the activity of chain-breaking antioxidant exhibited from both groups and the tocopherols group expressing the antioxidant activity in particular. It was suggested by Burton et al. [68] as well as Acker et al. [69] that the phenol (Ar OH) number towards (ROO.), the trapped radicals number per phenol (n), and the chain transfer occurrence may affect the self-initiated autoxidation:

Figure 9 Chemical structure of a-Tocopherol.
ArO•+RH → ArOH+ R• (15)
The peroxyl radicals are being trapped:
ROO• + ArOH (→K1) ROOH+ ArO• (16)
Phenoxyl radicals discontinue the chain (ArO•) which are resonance stabilised.
A second peroxyl radical may, in some cases, react with the phenoxyl radicals:
ROO•+ArO• (→K2) molecular products (17)
Where: k1 and k2 reaction rate constants.
Using age as a baseline, the European Food Safety Authority (EFSA, 2010) recommend vitamin E nutrient intake at the range of 8 to 25 mg/day. Table 1 presents the significant sources of vitamin E combined with its health benefits.
Carotenoids
The importance of a healthy diet has been accentuated by the increasing focus on nutrition and well-being that has been garnered in contemporary society. Fruit and vegetables constitute an integral part of a healthy diet and thus are considered as functional foods due to their ability to increase health benefits. Such benefits include reducing the prevalence and delaying the possibility of developing chronic diseases, as well as more fundamental day-to-day nutritional issues. The sufficient consumption of fruit and vegetable maintains healthy levels of phytochemicals, carotenoids, and nutrients within the body.
Plant-based functional foods have been developed by nutritionists and dietetics to establish more regular healthy eating habits amongst people. In particular, a key focus has rested on geometric carotenoid isomers and the carotenoids in fruit and vegetable, as a result of the functional properties they possess, and the health benefits they offer. Some 600 variations of carotenoids have been confirmed. Carotenoids differ between plants and animals, in that they are not synthesized in the latter. Subcellular organelles or plastids for example chloroplasts and chromoplasts encompass carotenoids, although in different forms and with different functions. Carotenoids in chloroplasts act as crucial pigments in the accessibility of photosynthesis, as a form of protein; while carotenoids in chromoplasts take the structural form of crystals or oil drops. In the case of chlorophyll-present carotenoids, xanthophylls, the presence of these compounds catalyses photosynthesis by emphasizing energy transfer.
Carotenoids alter the colour of fruit and vegetable, in mainly accentuating the yellow tinge often found. Alongside yellow, red and orange also highlight the existence of carotenoids. Minguez-Mosquera et al. [70] highlighted that colour intensity in fruit and vegetables is often witnessed during the ripening of fruit, as a result of the esterification of carotenoids in the presence of fatty acids.
β-Carotene: Beta-carotene (β-carotene), is a lipid-soluble provitamin, composed of two retinyl groups, where it is broken down into a form of vitamin A, called as retinal, in the human small intestine mucosa (Figure 10). Beta-carotene is well known as one of the most potent antioxidants, having the capacity of lipid oxidation inhibition and singlet oxygen quenching.
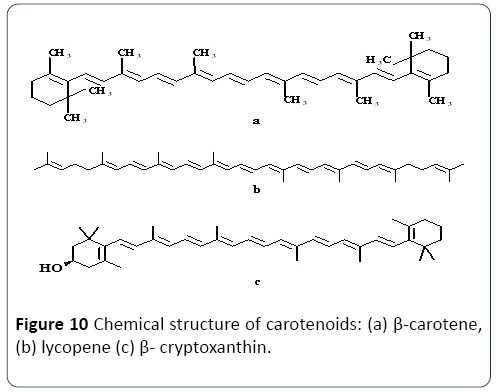
Figure 10 Chemical structure of carotenoids: (a) ß-carotene, (b) lycopene (c) ß- cryptoxanthin.
As previously discussed, carotenoids are present in the cells and tissues throughout plants. The consumption of fruit and vegetable leads to the most substantial contribution of carotenoids into the human body. Carotenoids are coloured pigments, with fat-soluble attributes. The coloured characteristics of carotenoids also affect plant colourings as a result of their isolation from the grana present in chloroplasts. The resulting isolated carotenoids are labelled as carotenoprotein complexes.
The colour of plants forms from the absorption of light by the conjugated double bonds of carotenoids; in particular, red wavelengths are absorbed in a higher concentration, in the presence of increased numbers of double bonds. Carotenoids do not exist in plants as single compounds. Chlorophyll-binding occurs amongst carotenoids, resulting in carotene-chlorophyll and xanthophyll-chlorophyll compounds, and it is this process that provides the variations in colours seen in plants and fruit. The ripening of fruit leads to a reduction in chlorophyll concentration and discolours the carotenoid pigment. Markus et al. [71] have undertaken experiments in order to increase the colour retention abilities of carotenoids in fruit, as the fruit ripens. The concentration of xanthophyll differs between fruits and vegetables.
In general, fruits have a considerably lower concentration, although persimmon (Diospyros spp.), as well as papaya (Carica papaya L), can be considered as an exception with xanthophylls, zeaxanthin and lutein levels consistent with those in vegetables. Wieruszewski [72] highlighted the formation of the blue, green and purple colouration of fruit, resulting from the orange and red light absorption by the chlorophyll-carotenoid complexes, formed by the binding of β- carotene with either chlorophylls or xanthophylls. The inherent nature of chlorophyll-carotenoid complexes, formed from β- carotene, reduces the bioavailability of β-carotene; this results in reduced bioefficacy when converting to vitamin A. Through the process of saponification, this reduction in bioefficacy can be reversed; all-trans-β-carotene can be yielded in free form through the saponification of plant extracts. Bioavailability of carotenoids differ between fruit and vegetables: levels of provitamin A carotenoids are much lower in vegetables than fruit. It is often regarded that this is as a result of the proteincomplex structures of pro-vitamin A carotenoids in chloroplasts.
Figure 10 depicts the chemical structure of β-carotene, lycopene and β-cryptoxanthin – three carotenoids that are pVA active, and that occur naturally. These three carotenoids are also considered nutritionally active, as a result of the unsubstituted p-ionone ring present at either or both ends of the molecule. As β-ionone rings encompass the β-carotene, it is considered the molecule that can be transformed into retinol most effectively. In contrast, the s-cyclic ring of acarotene and the hydroxylated β-cyclic ring of β - cryptoxanthin reduced the efficiency of converting these carotenoids to retinol. Table 1 shows the primary sources of β- carotene and the corresponding health benefits.
Lycopene
Lycopene was noted as the most efficient natural carotenoids singlet oxygen quencher with two non-conjugated and eleven conjugated double bonds (Figure 10). The many conjugated double bonds of lycopene make it a potentially powerful antioxidant, the beneficial effects arise from its unique characteristic compare to others. The capacity of lycopene antioxidant activity is featured by its entrapment ability of peroxyl radicals. Table 1 outlines the major sources of lycopene and its corresponding health benefits.
Hydroxy-carotenoids (Xanthophylls)
Xanthophylls fall under the group of oxygenated carotenoids; frequently found as leaves yellow pigments that are produced within the cell plastids of plants. Pasaporte et al. [73] reported that zeaxanthin and lutein are described as structural isomers with a single difference at one of the cyclohexene moieties configuration that flanks the two compounds at their terminal ends. Zeaxanthin is chemically structured as β, β - carotene -3, 3‘-diol, while separately lutein is chemically written as β, ε – carotene -3, 3‘– diol. Table 1 presents the significant sources of xanthophylls and their health benefits.
Purification and fractionation of carotenoids
The extracts of plant crude accommodate vast amounts of lipoidal material and carbohydrates with the taint of polyphenol-rich fractions before analysis. The approaches that include phenolics sequential extract concentration in the crude extract are probably low. The commonly used method to concentrate the compounds is by solid phase extraction and liquid-liquid partitioning by using acidity and polarity as a reference. The utilisation of non-polar solvents, e.g., hexane, to wash the crude extract may generally eliminate the lipoidal material, dichloromethane or chloroform.
Solid phase extraction (SPE) is the typical process to carry when the removal of polar non-phenolic compounds (e.g., organic acids, sugars) needs to be carried out. The economical, rapid and sensitive characteristics of SPE make the technique popular, yet to add the capability to use a great variety of sorbents through different discs and cartridges. Improving the performance, now this technique can also be automated. Separation of phenolic compounds uses C18 cartridges for most of the time. The process involves the aqueous sample passing through the preconditioned C18 cartridges, where the cartridges are later washed to remove organic acids, sugar, and other water-soluble constituents with acidified water. Absolute methanol or aqueous acetone is later used to elute the polyphenols.
Adjustment of the pH of the sample and the eluents, as well as the eluents polarity, may further separate the phenolic compounds. Related to this method, Pinelo et al. [74] adjusted alcoholic wine sample to the pH of 7.0 and eluting water and phenolic acids in the first fraction. In the successive step, 0.01 M HCl was used for the acidification of the C18 cartridge and ethyl acetate was used for the elution of nonpolymeric phenols such as flavonols, catechins and anthocyanins. The final stage involved the elution of polymeric phenols by the mixture of methanol, acetone and water. Purification of phenolic compounds by other sorbents, e.g. Amberlite XAD-2, have also successfully been recorded for wine samples and crude extracts. In a comparison between several SPE cartridge including silica-based C8, Amberlite, copolymer-based ENV+, HLB, PH, and MCX against silica-based C18 for wine phenolic compounds isolation at low concentration provided a result that a higher reproducibility, higher sensitivity and loading capacity was attained by the SPE method with HLB cartridge compare to the C18 cartridge. This result may also indicate that the HLB cartridge can be a decent substitute for the C18 cartridge in the wine phenolic compounds isolation.
Another method of column chromatography for the phenolic extracts fractionation has also been used. Despite the solvent-consuming and labour-intensive nature of the method, it offers greater fractions count for subsequent pure substances identification and isolation. In this case, the typically-used column sorbents are RP-C18.
The combinations of methanol, ethanol, acetone and water are frequently used as eluents. On a specific occasion, the Sephadex LH-20 column chromatography has been routinely employed for proanthocyanidins (condensed tannins) isolations. The process involved the application of the crude extract towards the column which was washed with ethanol or methanol for eluting the non-tannin compounds, consecutively followed by alcohol-water or acetone-water elution to obtain proanthocyanidins. Methanol was found to be frequently used than ethanol when it comes to non-tannin compounds elution by LH-20 column chromatography. For procyanidins elution, particularly polymeric procyanidins, acetone-water is much better than ethanol-water as a solvent for this process. In some instances of polyphenol sample purification, a preparative scale HPLC has also been regularly used.
It is an infrequent case to employ a traditional liquid-liquid extraction procedure as it is considered to be time-consuming and tedious, but with low recoveries and high solvent costs. A phenolic compounds extraction from cider apple tissues can be an excellent example of a sequential extraction process. Hexane (for lipids, chlorophyll and carotenoids removal), aqueous acetone (polymerised polyphenols) and methanol (organic acids, sugars and low molecular weight phenolic compounds) were employed to extract the freeze-dried apple tissue powder. Another alternative for liquid chromatography, which is known as Countercurrent Chromatography (CCC), was developed and has been used with good efficiency for various classes of phenolic compounds fractionation. CCC can be considered as a preparative all-liquid technique chromatography with a basis on a liquid mobile, and stationary phases, as well as the two immiscible liquid phases, compounds partitioning. Using the hydrophobicity characteristic, the solutes are separated based on their coefficients of partition. With the absence of a solid matrix, CCC is an advantage, since the role of the liquid mobile and stationary phases can be altered during a run. Thus, it is possible to obtain a 100% recovery with no irreversible sample adsorption.
High-speed counter-current chromatography (HSCCC) was studied by Degenhardt et al. [75] for the anthocyanins separation from pigment mixtures extraction obtained from black currant, red cabbage, black chokeberry, blackcurrant and roselle. In a similar study, Andersen et al. [76] noted that a fractionation of anthocyanins based on their polarities was successfully performed with a conversion to the biphasic mixture of tert-butyl methyl ether/n-butanol/acetonitrile/ water (2:2:1:5, v/v/v/v) acidified with trifluoroacetic acid (TFA). HSCCC can also be used for tea catechins isolation and other food-related polyphenols such as phenolic acids, procyanidins, and flavonol glycosides by utilizing tert-butyl methyl ether/ acetonitrile/0.1% aqueous TFA (2:2:3, v/v/v).
Fuleki et al. [77] made a coupling of multilayer countercurrent chromatography (MLCCC), and preparative High-Performace Liquid Chromatography (HPLC) in the effort of obtaining pure flavonoids extracted from Rooibos tea. The employment of this method enabling material isolation up to one gram for verification of known structures of polyphenol, as well as to explore the previous unpublished structures.
According to Silverman et al. [78] purification and identification of natural products or bioactive compounds having cytotoxic activity against cancer cells have several advantages, including:
1. Pure bioactive compounds having inhibitory activity can be administered in reproducible and accurate doses, with apparent benefits from the scientific work or traditional therapeutic point of view.
2. It leads to the development of an analytical assay for particular anti-cancer compounds or classes of compounds. This is necessary for potential toxicity and quality control of food for human or animal consumption.
Preparative chromatography covers a variety of techniques that resolve solutes by differential migration through a porous medium. The system depends on the differential distribution of compounds between two phases called mobile and stationary phase. In this process, solute components are separated as a result of the differential affinity of the components.
The quality and efficiency of any chromatographic separation are highly dependent on the characteristics of the compounds to be separated. Phenolic compounds have a wide range of polarities; from the most polar (hydroxybenzoic acids) to the least polar (polymethoxylated flavonoids) compounds. Phenolics are also weak acids that can be separated as neutral, relatively hydrophobic compounds in the weak acid matrix. In the present attempt, gas chromatography (GC), thin layer chromatography (TLC), high-performance thin layer chromatography (HPTLC) and both normal phase and reverse phase column chromatography are a few options that will be attempted for the purification process of bioactive agarwood compound (s).
GC will provide the general information on several compounds (s) to be expected from the sample. GC works on analysing the compounds which can be vaporized without decomposition based on the principle of separation between the stationary phase and gas phase. TLC is a common practice used to separate organic compounds based on differential rates of migration.
Separated compounds are usually scraped from the plate and eluted with suitable solvents for further testing. HPTLC is an improved type of TLC in which the TLC plate that is coated with the fine microparticles of silica that are used in the columns for HPLC. Usually, it gives most efficient and rapid separations than on conventional silica layers. Both standard and reverse phase column chromatography will help to separate both polar and non-polar components from the crude extract.
Manipulating the mobile phase polarity will allow the separation of compounds from the stationary phase in the column setup. For instance, Ediriweera et al. [79] have eluted a dried chloroform-partitioned extract using 100 ml hexaneethyl acetate (8:2, 7:3, 6:4, 1:1, 4:6, 3:7, 2:8, 1:9, v/v), ethylacetate-methanol (1:1,v/v) and methanol in a silica gel column chromatography procedure. Then, active fractions were collected and monitored using normal-phase TLC using hexane-ethyl acetate as the mobile phase. All fractions gave a bright spot in which all active fractions were pooled and concentrated to give T1. T1 was subjected to reverse phase procedure (reverse phase column, C18, and reverse phase TLC). Fractions obtained were assessed using cytotoxic bioassays to determine bioactivity against cancer cells. The primary sources of xanthophylls and its health benefits are presented in Table 1.
Properties of natural phenolics and solvents
Extraction compatibility: There are various characteristics to be considered to use the solvent system (SS) e.g. Methanol, Ethanol and Acetone for the extraction and production of the phenolic compound. Some are presented in Table 1. Properties of SS depend mainly on the mechanism of the polarity. It was suggested that SS containing a different polarizing cation is a better choice for antioxidant extraction from Gum Arabic. Plant phenolics compounds are the most abundant polyphenols in human diets, ranging from phenolic acids, tannins (Figure 11), flavonoids, and the less common stilbenes and lignans (Figure 12). The flavan nucleus is the basic flavonoid structure containing 15 carbon atoms in the arrangement of three rings (C6-C3-C6) with A, B and C as labels. Six subgroups are dividing the flavanoid according to the central C ring oxidation state, which is: flavanols, flavones, flavanones, isoflavones and anthocyanins.
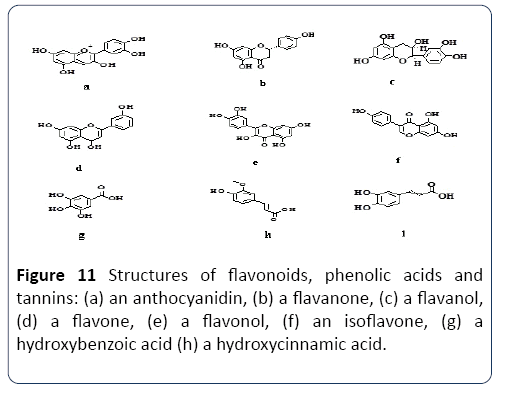
Figure 11 Structures of flavonoids, phenolic acids and tannins: (a) an anthocyanidin, (b) a flavanone, (c) a flavanol, (d) a flavone, (e) a flavonol, (f) an isoflavone, (g) a hydroxybenzoic acid (h) a hydroxycinnamic acid.
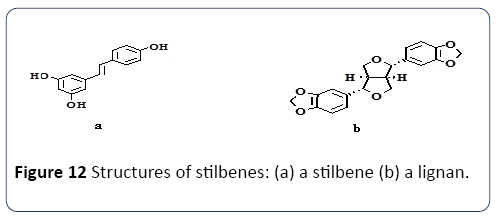
Figure 12 Structures of stilbenes: (a) a stilbene (b) a lignan.
Phenolic acids can be categorised into two different derivative classes of cinnamic acid derivatives such as coumaric, ferulic acids and caffeic, as well as benzoic acid derivatives such as gallic acid. Caffeic acid is the main phenolic compound in coffee and can be found in many fruits and vegetables as the most abundant phenolic acid often esterified with quinic acid, e.g. chromogenic acid, Ferulic acid becomes another common compound of phenolic acid which is esterified in the cell wall to hemicelluloses and presents in cereals crops.
Another main group of polyphenols for humans’ diet are tannins that come in two groups of condensed tannins and hydrolysable tannins. Hydrolysable tannins are substances with glucose or another polyol central core esterified with either gallic acid, also known as Gallo-tannins, or with hexahydroxydihenic acid, also termed as ellagitannins. The many possibilities to create oxidative linkage make these groups come with a high number of structural variations. Many oligomeric compounds with a molecular weight from 2,000 to 5,000 Daltons created from these intermolecular oxidation reactions. Condensed tannins are a linkage of polymers or oligomers of flavan-3-ol by an interflavan carbon bond. The heating process in acidic alcohol solutions may decompose these compounds through the reaction of acidcatalysed oxidation turning them into anthocyanidin. Thus it is common to refer them as proanthocyanidins as illustrated in Figure 12.
These compounds are widely distributed, but the dietary polyphenols health effects gain the attention from nutritionists just in the recent years. Due to their antioxidant properties potential, the likely effects in preventing many oxidative stress-associated illnesses, and their affluence amount in diet, food manufacturers and researchers began to focus their interests in polyphenols.
Similarly, epidemiologic data with in vitro and in vivo testimonies deduce these plant metabolites preventive effects regarding cancer, neurodegenerative and cardiovascular diseases prevention with an outcome in the particular nutritional recommendations. Polyphenols were also found modulating a wide range of cell receptors and enzyme activity. In this way, polyphenols have more specific biological activity capable of treating and preventing diseases added to their antioxidant properties.
Antioxidant extraction and characterization
Extraction with solvents: An observation was conducted by Gonzalez-Montelongo et al. [80] with a note highlighting that the conditions in which the liquid-solid extraction process is carried out may significantly influence the antioxidant compounds extraction yield. The resulting material-solvent system came up with unpredictable behaviour when solvents are added together due to the unique composition and structure properties of each plant material.
The first step in the phytochemicals utilization for the preparation of nutraceuticals and dietary supplements, pharmaceutical, food ingredients and cosmetic products is the bioactive compounds extraction from the plant materials. Plant samples, either in the dried, fresh or frozen form, can be the source for phenolics extraction. It is common to treat the plant samples by grinding, milling and homogenization with freeze-or air-drying possibly preceding the whole treatment processes. Compare to air-drying; freeze-drying has so far retained a higher amount of phenolics content in plant samples.
The most frequently used method for the preparations of plant materials extract is solvent extractions, due to the efficiency, ease of use, and broad applicability nature of the technique. It is also widely known that the type of solvents with variations on extraction time, polarities and temperature, combined with samples physical characteristics and chemical contents as well as the sample-to-solvent ratio, may significantly affect the yield of the chemical extraction process.
The solvents polarity and the plant sample chemical nature regulate the solubility of the phenolics. Plant materials contain different quantities of phenolics, varying from the highly polymerised substances (such as tannins) to the simple one (such as phenolic acids and anthocyanins). Furthermore, there is an association between phenolics and the other parts of the plants, such as protein and carbohydrates. Thus, it is hard to derive a universal procedure for the phenolics extraction process that suitable for all type of plants.
The extraction of phenolics from the plant materials involves various solvents mixed with different proportions of water, from ethanol, methanol, ethyl acetate, acetone, and their combinations. The appropriate selection of solvent type also affects the rate and the amount of polyphenols extra. Along the process, some non-phenolic substances, such as sugar, fats and organic acids, can be found in the content. Thus, resulting in the requirement of additional steps to eliminate the unwanted components.
Solvent extraction can be defined as a process of separation by applying a solvent to extract the targeted component (solute) from the solid food. The mutual solubility is determined through the intermolecular interaction between the solute molecules and the solvent. Murphy et al. [81] found that the total efficiency of extraction can be optimised by adding a certain amount of water in the range of 5-10 mL, depending on the matrix, to different organic solvents. Boulekbache et al. [82] defined that the anthocyanins are observed to be polar molecules. Thus, the typical most efficient solvents for extractions are aqueous mixtures of methanol, ethanol and acetone.
Chandrasekhar et al. [83] have mentioned that acidified ethanol and methanol are among the most common extraction solvents for the extraction process. Ramirez-Lopez et al. [84] have confirmed that the acid content of the solvents causes the cell membranes rupture, thus releasing anthocyanins. However, despite the action, this chemical treatment is harsh and may break down the structure of natural anthocyanin. It is accordingly critical to acidify the solvents, not with mineral acids, e.g., 0.1% HCl, but rather with organic acids (e.g., acetic or formic acid).
Due to its cheap, reusable and non-toxic nature, Chew et al. [85] reported that ethanol is among the most used solvents in the food industry for antioxidant extraction. Moreover, Durling et al. [86] demonstrated that phenolics fractionation by polarity basis is attainable with the utilisation of aqueous ethanol at different concentrations. There are many advantages offered by the utilisation of aqueous ethanol solutions, such as synergistic interactions inside the medium, and the collective recovery of hydrophilic and lipophilic active compounds in different proportions. Therfore, solvents are extremely effective in extraction techniques.
A study on the phenolic content extraction of olives with ethanol concentrations differed from 20, 50, 70 and 100 ml per 100 ml showed that the highest yields for total phenolic compounds (absorbance band of 725 nm and 280 nm), flavonols and o-diphenols, and hydroxycinnamic acids was reached at the ethanol concentration of 70 ml/100 ml.
Strati et al. [87] classified that ethanol is preferred over other alcohols as it has generally been recognised as safe by the American Food and Drug Administration (U.S. FDA), cheaper with usable extracts in the food industry. Even though methanol is cheaper than ethanol regarding price, its toxicity is not something tolerated in the food industry. Also, carotenoids compounds like tomato lycopene are considered to be more liposoluble, and therefore, non-polar or polar aprotic solvents (especially acetone or ethyl acetate, subsequently) are preferred.
Galanakis said that before any re-utilisation attempt in food products, the solvent should be wholly separated from the extract. The phenols, flavourings and carotenoids extraction are frequently occurred in combination with distillation and pressurised processes, accelerating the process and helping to extract the volatile compounds, respectively. Galanakis [88] analysed the activity coefficients of fifteen natural phenols in three different extraction temperatures and seven solvents. The observation showed that the aliphatic fragment of alcohols and the non-polar part of methanol and ethanol could be the cause of phenols preference over the two substances. The molecules with bigger size, such as rosmarinic acid, oleuropein, p-hydroxyphenyl, p-hydroxyphenyl acetic acid, phydroxybenzoic, tyrosol and hydroxytyrosol have favoured ethanol for the extraction process since the compound has a better covering capability for the gaps between the hydrogen bonds. The recovery of tyrosol and hydroxytyrosol are performed better in the polar aprotic ethyl acetate compare to non-polar solvents, while at the opposite, extraction yield is improved with the average polarity increase.
An observation made by Do et al. [89] for phenol extraction of Limnophila aromatica showed that extraction yields decreased, from higher to lower concentrations, in the application of various solvents with the order as follows: 50% aqueous acetone, 50% aqueous ethanol, 75% aqueous methanol, 50% aqueous ethanol, 75% aqueous ethanol, 100% methanol, water, 100% ethanol, and 100% acetone, respectively. It is obvious to see that pure methanol gave a higher extraction yield compare to pure ethanol and pure acetone. It can be concluded that the yield increases as the polarity of the solvent increases. It was also suggested that the increase in solvent water concentration enhanced the extraction yield. The extraction of the chemicals that are soluble in organic solvent and water may be facilitated by the combined use of organic solvent and water. For example, the anthocyanins extraction from red cabbage with HCl (1% v/v) provided a better result than the same substance extraction with acidified ethanol (50% v/v).
Increasing the temperature of the solvent may improve the extraction of antioxidants. The higher temperatures favour the extraction process by making the diffusion coefficient is higher. Galanakis et al. [90] studied the extraction of anthocyanin and total phenol from olive waste by varying the solvent temperature through ethanolic extraction process at four different temperatures of 25, 50, 60 and 85°C. Their observation showed that the yield decreases in total phenol extractions (at 725 nm) with the order of: 25>80>60>50°C, while the decrease in anthocyanin concentration occurred at: 50>25>60>80°C. Despite the insignificant difference, the flavonols and hydroxycinnamic acids extracts concentrations decreased as the temperature risen. Preservation becomes one of the major issues for antioxidants extracts.
A report from Galanakis et al. [91] showed that the total phenol content (at 725 nm) extracted from olive waste remained constant for the least of 18 weeks period, while total phenols concentration (at 280 nm) with room-temperature treatment expressed a significant reduction, compare to 50, 60 and 80°C temperature treatments, in terms of total phenols content. The treatment on high temperature of 80°C seems to have a critical role in flavonol and hydroxycinnamic acid preservation.
Methods of extraction and techniques of antioxidant: Extraction is one of the most essential phases in obtaining compounds of interest. Extraction is defined as a separation process whereby the distribution of the chemical constituents between two immiscible phases is systematically made in order to arrive at the appropriate distribution coefficient. Musa et al. [90] reported that some of the extraction techniques that are widely used might be summarized as follows:
1. High-performance disperser homogenization (T25 digital ULTRATURRAX®, IKA, Germany) with high speed (24,000 rpm) for 1 minute.
1. Minimum of 12 hours sample maceration in the extraction solvent.
2. Magnetic stirrer is mixing at 1,000 rpm for 1 hour (Heidolph, MR3001, K, Germany).
3. Shaker extraction at 300 rpm for 1 hour (Intertech, Taiwan).
4. Ultrasonic extraction in the 12-kHz ultrasonic bath (Soniclean, Thebarton, Australia) for 1 hour.
The most important methods of extraction technique are ultrasonic sonication which is known as one of the most frequently used industrial methods to improve the phenomena of mass transfer. Cavitation forces enhanced the ultrasonic extraction by increasing the mass transfer rate, where plant tissue rupture was occurred by the explosive collapse bubbles that generate localised pressure onto the tissue, thus improving the condition for the intracellular to be released into the solvent. An example of the use of ultrasonic extraction can be seen from the case of anthocyanin extraction from grapes eggplant.
Hydrodynamic and acoustic cavitation methods are among the diversified modes for generating cavitation, and they are regularly accounted for the easiness of operation and for the cavitation required intensities generation, which is suitable for different chemical and physical transformations. However, only ultrasound-based cavitation was reported to be successful for natural products extraction. The examples may cover vanillin, ginseng saponins, ginger, almond oils, herbal extracts (e.g., fennel, marigold, hops, mint), soy protein, soy isoflavones, rosemary carnosic acid, polyphenols, green tea amino acid and caffeine, as well as flowers pyrethrines extraction.
Advantages:
1. Compare to conventional methods; ultrasonic extraction is simple, inexpensive and efficient.
2. Compounds chemical degradation can be avoided since there is no chemical involved in the process.
3. Thermal damage avoidance can be done by carrying out the process at a lower temperature, evading damage to the extracts and preventing loss by boiling towards the volatile components.
4. Environmentally friendly.
Disadvantages:
Sometimes, long time application is required (1 hour) at a considerably high temperature (70°C) to significantly increase the polyphenols yield.
The solvent system selected for the extraction process also affects the type of compound being extracted by the process as shown in (Figure 13). Solvent selection is made based on the solubility parameters and polarity of the target component and the solvent. In order to reach a maximized yield of the target component, its solubility parameters should be as close as possible to the corresponding parameters of the selected solvent. Other than the extraction yield, the selectivity of a solvent for the target compared to side components is a significant factor, as this will have a considerable influence on further purification phases.
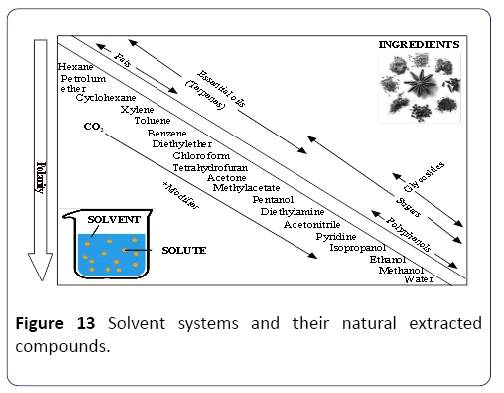
Figure 13 Solvent systems and their natural extracted compounds.
Choice of solvents: Tiwari et al. [92], Eloff [93] have explained that the type of the solvent used for the extraction process determines the successfulness of the biologically active compounds from the plant material extraction. There are several good properties for a solvent to have in-plant extractions, which may include low toxicity, promotion on the extract rapid physiologic absorption, ease for low heat evaporation, preservative action, and the inadequacy to cause complex or dissociation of the extract. The factors for selecting solvents will depend heavily on the extraction quantity of phytochemicals, its extraction rate, extracted compounds diversity, the variance on extracted inhibitory compounds, easiness on the extracts consecutive handling, extractants potential health hazard, and solvent toxicity during the bioassay process. Remington et al. [94] have mentioned that the solvent selection is profoundly affected by the actions which are intended for the extract. It is essential to use a nontoxic solvent and to avoid any interference with the bioassay since the final products always contain the traces of residual solvent. The targeted compounds may also affect the preference of the solvent selections, as illustrated in Table 2.
Table 2 Solvents used for active component extraction.
| Acetone |
Methanol |
Ethanol |
Chloroform |
Ether |
Water |
| Phenol |
Anthocyanins |
Tannins |
Terpenoids |
Alkaloids |
Anthocyanins |
| Flavonols |
Terpenoids |
Polyphenols |
Flavonoids |
Terpenoids |
Starches |
| |
Saponins |
Flavonol |
|
Coumarins |
Tannins |
| |
Tannins |
Terpenoids |
|
Fatty acids |
Saponins |
| |
Xanthoxyllines |
Sterols |
|
|
Terpenoids |
| |
Quassinoids |
Alkaloids |
|
|
Polypeptides |
| |
Totarol |
|
|
|
Lectins |
| |
Lactones |
|
|
|
|
| |
Flavones |
|
|
|
|
| |
Polyphenols |
|
|
|
|
Factors affecting of antioxidant extraction: Temperature and extraction time may as well influence the phenolic compounds recovery from the plant materials that emulate the oxidation analyte degradation and solubilisation conflicting actions. The higher extraction temperature promotes an increase in the analyte solubility through the increase in its mass transfer rate and solubility. Additionally, higher temperature also leads to the decrease in solvents surface tension and viscosity, which ease the sample matrices to be reached by the solvents, thus improving the rate of extraction. However, numerous phenolic compounds are susceptible to hydrolyzation and oxidation. The chances for oxidation are increased with higher extraction temperature and longer extraction times, thus decreasing the phenolics yield in the extracts. As an illustration, anthocyanins conventional extraction and concentration are frequently regulated with temperature ranges of 20 to 50°C and higher temperatures beyond 70°C have been known to degrade anthocyanin rapidly. Accordingly, the selection of extraction procedures and methods are critical, as well as maintaining the stability of the phenolic compound.
Purification and fractionation: Plant crude extracts phenolics concentrations are typically low due to the more considerable amount of carbohydrates and lipoidal materials. This leads to the use of strategies for solid phase extraction (SPE) and liquid-liquid partitioning or sequential extraction that based on acidity and polarity, to concentrate and acquire polyphenol-rich fractions before analysis. Commonly, using the non-polar solvents (e.g. hexane) to wash the crude extract may eliminate the lipoidal material, dichloromethane or chloroform.
The removal of polar and non-phenolic compounds (e.g., sugar, organic acids) can usually be carried out by SPE. The SPE process itself has been widely used as it is economical, rapid and sensitive, as well as the easiness for using a great variety of sorbents via different cartridges and discs. Moreover, an automation function can now be added to this technique. The widest application for phenolic compound separation uses C18 cartridges. The aqueous sample should be passed through the preconditioned C18 cartridges, and acidified water should be used to wash the cartridges with separate organic acids, sugar and other water-soluble constituents. Elution will then be performed for the polyphenols by absolute methanol or aqueous acetone.
Other methods of phenolic compounds separation can be attained by adjusting the sample pH and the eluents pH and polarity. As an example, Pinelo et al. [74] adjusted the alcoholic wine sample pH to 7.0 and eluted phenolic acids in the first fraction with water. Subsequently, 0.01 m HCl can be used for acidifying the C18 cartridge, and nonpolymeric phenols (e.g., anthocyanins, catechins and flavonols) can be eluted with ethyl acetate.
Finally, polymeric phenols elution can be done by a mixture of water, methanol, and acetone. More sorbents such as Oasis HLB, Amberlite XAD-2, have also shown a successful result in purifying wine samples and crude extracts phenolic compounds. Comparing the SPE cartridges variation of Amberlite, copolymer-based HLB, silica-based C8, ENV+, PH and MCX with silica-based C18 for the phenolic compounds isolation of wine at low concentration showed that, the SPE method equipped with HLB cartridge has a better loading capacity, higher sensitivity, and improved reproducibility compare to the C18 cartridge. It is probably a good alternative to use HLB cartridge in the place of the C18 cartridge for the wine phenolic compounds isolation.
According to Silverman et al. [78] purification and identification of natural products or bioactive compounds having cytotoxic activity against cancer cells have several advantages, including:
1. Pure bioactive compounds have an inhibitory activity that can be administered in reproducible and accurate doses, with apparent benefits from the scientific work or traditional therapeutic point of view. It leads to the development of an analytical assay for anti-cancer compounds or classes of compounds. This is necessary for potential toxicity and quality control of food for human or animal consumption.
2. Preparative chromatography covers a variety of techniques that resolve solutes by differential migration through a porous medium. The system depends on the differential distribution of compounds between two phases called mobile and stationary phase. In this process, solute components are separated as a result of the differential affinity of the components.
Characterization of antioxidants: Harborne et al. [95] have defined that from the viewpoints of astringency, antioxidants, browning reactions, bitterness, colour and many others, natural phenolics are attractive and of interest. Purposes of the study, as well as the sample and analyte natures, influence the selection of the strategy for proper analysis in studying plant materials phenolics. Khoddami et al. [96] have confirmed that, in analysing the phenolics, the used assays are generally classified as either those for a specific group or class phenolic compounds classification or those for total phenolics content measurement.
The heterogeneity of natural phenolics and the possibility of interference from plant materials oxidised substances has led to the use of several methods for determining total phenolics content with none of the methods anywhere near to perfect. The Folin-Ciocalteu method (F-C), Folin-Denis method (FD), iron salts colourimetry, permanganate titration, and ultraviolet absorbance are some of the methods that have been tried. For most of the cases, the F-C method has been preferred form most occasions as compared to the other methods.
Apart from that, High-performance liquid chromatography (HPLC) currently gains the popularity for its decent and dependable technique in the phenolic compounds analysis. There has been many supports and mobile phases available for the phenolics analysis of anthocyanins, hydrolysable tannins, proanthocyanidins, flavonols, flavan-3-ols, flavones, flavanones, and phenolic acids from various food samples and plant extract. Furthermore, the methods offer the possibility of simultaneously analysing all the components of interest, altogether with their degradation products and possible derivatives as illustrated in Figure 14.
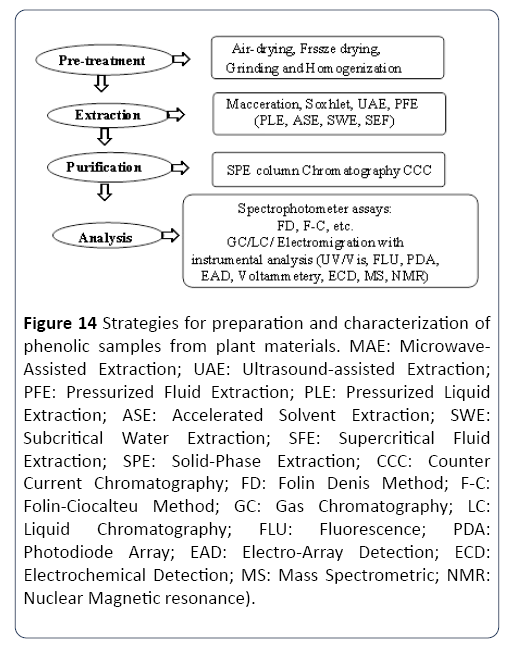
Figure 14 Strategies for preparation and characterization of phenolic samples from plant materials. MAE: Microwave-Assisted Extraction; UAE: Ultrasound-assisted Extraction; PFE: Pressurized Fluid Extraction; PLE: Pressurized Liquid Extraction; ASE: Accelerated Solvent Extraction; SWE: Subcritical Water Extraction; SFE: Supercritical Fluid Extraction; SPE: Solid-Phase Extraction; dihydrochalcone: Counter Current Chromatography; FD: Folin Denis Method; F-C: Folin-Ciocalteu Method; GC: Gas Chromatography; LC: Liquid Chromatography; FLU: Fluorescence; PDA: Photodiode Array; EAD: Electro-Array Detection; ECD: Electrochemical Detection; MS: Mass Spectrometric; NMR: Nuclear Magnetic resonance).
Natural antioxidant and cancer
Cancer can be defined as a multi-step disease with the incorporation of physical, chemical, environmental, metabolic and genetic factors that play a role directly and indirectly, in the induction and deterioration stages of cancers. The high consumption of fruits and vegetables with antioxidant-rich contents can significantly lower the risk of many cancers, as shown consistently by epidemiology evidence, suggesting the idea that certain dietary antioxidants can effectively prevent cancer incidence and mortality. For their general acceptance, safety and low toxicity aspects, these antioxidants present in the diet are considered to be promising. As a result, the identification and development of these antioxidant agents have become the central area of much experimental research in the last few years. Phenolic compounds are plant metabolites with most numerous and ubiquitous contents, being an integral part of the human dietary plan. Aside from their primary antioxidant activity, it was found that this group of substances has a full spectrum of biological functions that are directly related to the modulation of carcinogenesis. With all the potentials, many in vivo and in vitro systems have been employed for determining the natural phenolic compounds and extracts capacity of anticancer and anticarcinogenic agents.
In-vivo effect of natural antioxidant
Many studies have been conducted on the effects of isolated polyphenols or phenolic extracts towards several cancer cell lines at each various evolutionary cancer stages. One example illustrates the growth inhibition of human oral (KB, CAL-27), prostate (LNCaP, DU-145), breast (MCF-7), and colon (HT-29, HCT-116) tumor cells by the extracts of berry which were prepared from cranberry, blueberry, raspberry, blackberry, strawberry and isolated strawberry polyphenols i.e., coumaric acid esters, ellagic acid, anthocyanins, quercetin and kaempferol, which were applied throughout the different cell lines sensitivities and in a dose-dependent manner.
McDougall et al. [97] observed that the extract of bilberry has the highest efficiency on HCT-116 cells and HL-60 human leukaemia cells among ten other edible berries, regarding ethanol extracts antiproliferative activity. Ross et al. [98] observed that raspberry extract character on the antiproliferative activity towards human cervical cancer (Hela) cells was predominantly associated with ellagitannins.
However, in the correlation between the antiproliferative effectiveness and phytochemical diversity of berry extracts, McDougall et al. [97] narrated that the critical part on the growth inhibition of cancer cells would be ellagitannins compounds from the Rubus plant family (e.g. raspberry, cloudberry and arctic bramble) as well as strawberry, while procyanidins primarily caused lingonberry antiproliferative activity.
Other intensive studies on the phenolic compounds and extracts have been conducted on curcumin from the spice turmeric, olives, apples, legumes and citrus. As an example, the growth inhibition of various cancer cell lines such as breast, head and neck cancer cells, leukaemia, prostate, and lymphoma can be administered by isoflavone genistein isoflavone. HL-60 leukaemia cells were also effectively inhibited by citrus flavonoids. Meanwhile Cavazos-Garduño et al. [99] examined the apple phenolic extract influence through the utilization of genotoxicity (HT-29), colonic barrier function (CaCo-2), and metastatic and invasion potential (HT-115) cell models on the critical stages of colorectal carcinogenesis, resulting the beneficial effects observed from the extract of apple on all three stages of the carcinogenesis.
Mechanism action of natural antioxidants
Cancer develops in a multistage manner, involving a series of individual steps from the initiation, promotion, followed by progression, invasion and metastasis. Separately, a tumour can be initiated when DNA, in a population of cells or an individual cell, is impaired by carcinogens exposure that can be derived from typically three significant causes of nutrition and diet, infection or inflammation, or cigarette smoking, as illustrated in Figure 15.
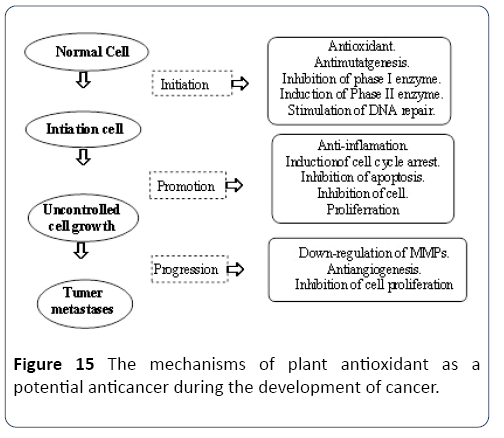
Figure 15 The mechanisms of plant antioxidant as a potential anticancer during the development of cancer.
The failure of the repair of the DNA damage leads to genetic mutation. The reproduced somatic mutation can trigger the rise of the mutated cells clone in the damaged cell during cell’s mitosis. In this case, tumour promotion can be defined as the development of premalignant tumour cell population with active multi-cellular proliferation which was initiated from the cells selective clonal expansion. This has been well known as a reversible and a long-term interruptible process. The premalignant cells are expanding into tumours by the clonal expansion process during the progression of the whole stages. The tumour cells then detached from the first mass when metastasis and invasion occur at the late stages of the cancer development, moving through towards lymphatic cells or blood vessels through the enclosing tissues, creating a second lesion. Thus, metastasis is well-known as the leading cause of cancer mortality.
Natural antioxidants have been expected to interfere all stages on the development of cancer. Adding to the capability of the antioxidant action, the capacity of phenolic compounds on inhibiting the cancer development relies on several basic mechanisms of cells, suggesting basic cellular machinery of the particular spectrum. Furthermore, with the utilization of Gum Arabic extraction, more hints can be obtained through the extensive studies on these compounds in the field of oncology and the study may provide more clues on the possibility of their pharmaceutical exploration.
Conclusion
Phenolic compounds will be ubiquitous and abundant in medicinal herbs and dietary crops (e. g., fruits, fruit and vegetables, spices, cereals, and beverages). Various phenolic compounds include a diverse selection of beneficial natural activities, which usually contribute to their very own potent results on suppressing carcinogenesis. Considerable research has been conducted in-vitro or in faster on antioxidant and anticancer activities of phenolic materials from healing herbs and dietary plants. Overwhelming medical evidence indicates that chemoprevention by phenolic phytochemicals is usually an inexpensive, readily applicable, suitable and available approach to cancer control and management. However, more info about the benefits, as well as the possible dangers of health supplement or herbal supplements, is needed to make sure their effectiveness and security. Harmful flavonoids and drug relationship, liver falling, dermatitis and anaemia had been reported for a few cases of polyphenol chemo preventive use. These potential toxic associated with phenolic substances are highly dependent on all their concentration and chemical environment, which should be thoroughly comprehended before any preventive or perhaps therapeutic to consider. However, information about safety evaluation of organic phenolic substances is still missing. In general, heading herbs and dietary vegetation are a excellent sources of bioactive phenolic compounds in cancer tumour prevention, in support of limited elements have been investigated. There are nonetheless many functions needed to look for novel and useful anticancer phenolic substances from even more medicinal and dietary vegetation, to further uncover mechanistic procedure, and to carry out confirmatory human being clinical trials of those phenolic ingredients in tumours prevention and treatment.
23589
References
- Almajano MP, Carbo R, Jimenez JAL, Gordon MH (2008) Antioxidant and antimicrobial activities of tea infusions. Food Chem 108: 55-63.
- Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53: 4290-4302.
- Huang, Ingber DE (2005) Cell tension, matrix mechanics, and cancer development. Cancer Cell 8: 175-176.
- Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16: 144-158.
- Roginsky V, Lissi EA (2005) Review of methods to determine chain-breaking antioxidant activity in food. Food Chem 92: 235-254.
- Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53: 1841-1856.
- Gheldof N, Wang XH, Engeseth NJ (2002) Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem 50: 5870-5877.
- Stratil P, Klejdus B, Kuban V (2006) Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J Agric Food Chem 54: 607-616.
- Pulido R, Bravo L, Saura-Calixto F (2000) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48: 3396-3402.
- Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, et al. (2009) Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol 20: 645-654.
- Paixao N, Perestrelo R, Marques JC, Camara JS (2007) Relationship between antioxidant capacity and total phenolic content of red, rose and white wines. Food Chem 105: 204-214.
- Brand-Williams W, Cuvelier M, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28: 25-30.
- Fukumoto L, Mazza G (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48: 3597-3604.
- Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 73: 239-244.
- Floegel A, Kim DO, Chung SJ, Koo SI, Chun OK (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compost Anal 24: 1043-1048.
- Miller D (1996) Configurations revisited. Strategic Manag J 17: 505-512.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26: 1231-1237.
- Apak R, Guclu K, Ozyurek M, Karademir SE (2004) Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 52: 7970-7981.
- Ozyürek M, Guclu K, Apak R (2011) The main and modified CUPRAC methods of antioxidant measurement. Trends Analyt Chem 30: 652-664.
- Zhang C, Fu J, Wang Y, Gao S, Du D, et al. (2015) Glucose supply improves petal coloration and anthocyanin biosynthesis in Paeonia suffruticosa ‘Luoyang Hong’cut flowers. Postharvest Biol Technol 101: 73-81.
- Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism and nutritional significance. Nutr Rev 56: 317-333.
- Leitzmann C, Groeneveld M (1997) One can eat health-bioactive substances in food. Munich: Deutscher Taschenbuch Verlag.
- Leopoldini M, Russo N, Toscano M (2011) The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem 125: 288-306.
- Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, et al. (1995) Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 155: 381-386.
- Taleon V, Dykes L, Rooney W, Rooney L (2014) Environmental effect on flavonoid concentrations and profiles of red and lemon-yellow sorghum grains. J Food Compost Anal 34: 178-185.
- Okarter N, Liu CS, Sorrells ME, Liu RH (2010) Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem 119: 249-257.
- Alvarez-Suarez JM, Giampieri F, Gonzalez-Paramas AM, Damiani E, Astolfi P, et al. (2012) Phenolics from monofloral honeys protect human erythrocyte membranes against oxidative damage. Food Chem Toxicol 50: 1508-1516.
- Fernandes L, Hagen KB, Bijlsma JW, Andreassen O, Christensen P, et al. (2013) EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 72: 1125-1135.
- Yuan L, Lu XH, Xiao X, Zhai T, Dai J, et al. (2011) Flexible solid-state supercapacitors based on carbon nanoparticles/MnO2 nanorods hybrid structure. ACS Nano 6: 656-661.
- Periche A, Castello ML, Heredia A, Escriche I (2015) Influence of drying method on steviol glycosides and antioxidants in Stevia rebaudiana leaves. Food Chem 172: 1-6.
- Liang M, Chen J (2013) Arylamine organic dyes for dye-sensitized solar cells. Chem Soc Rev 42: 3453-3488.
- Tran V, Soklaski R, Liang Y, Yang L (2014) Layer-controlled band gap and anisotropic excitons in few-layer black phosphorus. Phys Rev B 89: 235-319.
- Alcalde-Eon C, Garcia-Estevez I, Martin-Baz A, Rivas-Gonzalo JC, Escribano-Bailon MT (2014) Anthocyanin and flavonol profiles of Vitis vinifera L. cv Rufete grapes. Biochem Syst Ecol 53: 76-80.
- Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, et al. (2014) STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res 1003.
- Kuhn S, Wollseifen H, Galensa R, Schulze-Kaysers N, Kunz B (2014) Adsorption of flavonols from onion (Allium cepa L.) processing residues on a macroporous acrylic resin. Food Res Int 65: 103-108.
- Sun Y, Yuan H, Hao L, Min C, Cai J, et al. (2013) Enrichment and antioxidant properties of flavone C-glycosides from trollflowers using macroporous resin. Food Chem 141: 533-541.
- Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, et al. (2013) NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest 123: 700-711.
- Voicescu M, Nistor CL, Meghea A (2015) Insights into the antioxidant activity of some flavones on silver nanoparticles using the chemiluminescence method. J Lumin 157: 243-248.
- Khan MK, Dangles O (2014) A comprehensive review on flavanones, the major citrus polyphenols. J Food Compost Anal 33: 85-104.
- Alshatwi AA, Periasamy VS, Athinarayanan J, Elango R (2016) Synergistic anticancer activity of dietary tea polyphenols and bleomycin hydrochloride in human cervical cancer cell: Caspase-dependent and independent apoptotic pathways. Chem Biol Interact 247: 1-10.
- Song BJ, Jouni ZE, Ferruzzi MG (2013) Assessment of phytochemical content in human milk during different stages of lactation. Nutrition 29: 195-202.
- Santos-Buelga C, Mateus N, De Freitas V (2014) Anthocyanins. Plant pigments and beyond. In: ACS Publications.
- Park SJ, Ahn JM, Kim YH, Park DW, Yun SC, et al. (2015) Trial of everolimus-eluting stents or bypass surgery for coronary disease. New Eng J Med 372: 1204-1212.
- Virk-Baker MK, Barnes S, Krontiras H, Nagy TR (2014) S-(−) equol producing status not associated with breast cancer risk among low isoflavone-consuming US postmenopausal women undergoing a physician-recommended breast biopsy. Nutri Res 34: 116-125.
- Rahmanian N, Jafari SM, Galanakis CM (2014) Recovery and removal of phenolic compounds from olive mill wastewater. J Am Oil Chemists Soc 91: 1-18.
- Tourtoglou C, Nenadis N, Paraskevopoulou A (2014) Phenolic composition and radical scavenging activity of commercial Greek white wines from Vitis vinifera L. cv. Malagousia. J Food Compost Anal 33: 166-174.
- Luo F, Fu Y, Xiang Y, Yan S, Hu G, et al. (2014) Identification and quantification of gallotannins in mango (Mangifera indica L.) kernel and peel and their antiproliferative activities. J Funct Foods 8: 282-291.
- Alvarez-Fernandez MA, Hornedo-Ortega R, Cerezo AB, Troncoso AM, Garcia-Parrilla MC (2014) Effects of the strawberry (Fragaria ananassa) purée elaboration process on non-anthocyanin phenolic composition and antioxidant activity. Food Chem 164: 104-112.
- Ntchapda F, Barama J, Azambou DRK, Etet PFS, Dimo T (2015) Diuretic and antioxidant activities of the aqueous extract of leaves of Cassia occidentalis (Linn.) in rats. Asian Pac J Trop Med 8: 685-693.
- Xie L, Bolling BW (2014) Characterisation of stilbenes in California almonds (Prunus dulcis) by UHPLC–MS. Food Chem 148: 300-306.
- Su GY, Wang KW, Wang XY, Wu B (2015) Bioactive lignans from Zanthoxylum planispinum with cytotoxic potential. Phytochem Lett 11: 120-126.
- Rybarczyk-Plonska A, Hansen MK, Wold AB, Hagen SF, Borge GIA, et al. (2014) Vitamin C in broccoli (Brassica oleracea L. var. italica) flower buds as affected by postharvest light, UV-B irradiation and temperature. Postharvest Biol Technol 98: 82-89.
- Yamagata K, Tagami M, Yamori Y (2015) Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 31: 28-37.
- Aladedunye F, Przybylski R (2013) Frying stability of high oleic sunflower oils as affected by composition of tocopherol isomers and linoleic acid content. Food Chem 141: 2373-2378.
- Mellado-Ortega E, Hornero-Mendez D (2015) Carotenoid profiling of Hordeum chilense grains: the parental proof for the origin of the high carotenoid content and esterification pattern of tritordeum. J Cereal Sci 62: 15-21.
- Wibowo S, Vervoort L, Tomic J, Santiago JS, Lemmens L, et al. (2015) Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem 171: 330-340.
- Zaccari F, Cabrera MC, Ramos A, Saadoun A (2015) In vitro bioaccessibility of β-carotene, Ca, Mg and Zn in landrace carrots (Daucus carota, L.). Food Chem 166: 365-371.
- Choi SH, Kim DS, Kozukue N, Kim HJ, Nishitani Y, et al. (2014. Protein, free amino acid, phenolic, β-carotene, and lycopene content, and antioxidative and cancer cell inhibitory effects of 12 greenhouse-grown commercial cherry tomato varieties. J Food Compost Anal 34: 115-127.
- Arnold C, Jentsch S, Dawczynski J, Bohm V (2013) Age-related macular degeneration: Effects of a short-term intervention with an oleaginous kale extract-a pilot study. Nutrition 29: 1412-1417.
- Kiehne A, Engelhardt UH (1996) Thermospray-LC-MS analysis of various groups of polyphenols in tea. J Food Examination Res 202: 48-54.
- Netzel M, Strass G, Herbst M, Dietrich H, Bitsch R, et al. (2005) The excretion and biological antioxidant activity of elderberry antioxidants in healthy humans. Food Res Int 38: 905-910.
- Cook N, Samman S (1996) Flavonoids—chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 7: 66-76.
- Oroian M, Escriche I (2015) Antioxidants: characterization, natural sources, extraction and analysis. Food Res Int 74: 10-36.
- Kostadinovic S, Wilkens A, Stefova M, Ivanova V, Vojnoski B, et al. (2012) Stilbene levels and antioxidant activity of Vranec and Merlot wines from Macedonia: Effect of variety and enological practices. Food Chem 135: 3003-3009.
- Bahadoran Z, Mirmiran P, Ghasemi A, Carlstrom M, Azizi F, et al. (2017) Vitamin C intake modify the impact of dietary nitrite on the incidence of type 2 diabetes: A 6-year follow-up in Tehran Lipid and Glucose Study. Nitric Oxide 62: 24-31.
- Bendich A, Machlin L, Scandurra O, Burton G, Wayner D (1986) The antioxidant role of vitamin C. Adv Free Radical Biol Med 2: 419-444.
- Burton G, Ingold K (1981) Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J Am Chemical Soc 103: 6472-6477.
- Van Acker SA, Koymans LM, Bast A (1993) Molecular pharmacology of vitamin E: structural aspects of antioxidant activity. Free Radical Biol Med 15: 311-328.
- Minguez-Mosquera MI, Hornero-Mendez D (1994) Changes in carotenoid esterification during the fruit ripening of Capsicum annuum cv. Bola. J Agric Food Chem 42: 640-644.
- Markus F, Daood H, Kapitany J, Biacs P (1999) Change in the carotenoid and antioxidant content of spice red pepper (paprika) as a function of ripening and some technological factors. J Agric Food Chem 47: 100-107.
- Wieruszewski JB (2002) Astaxanthin bioavailabity, retention efficiency and kinetics in Atlantic salmon (Salmo salar) as influenced by pigment concentration and method of administration (kinetics only). Master Thesis, Simon Fraser University, Ottawa, Canada.
- Pasaporte MS, Rabaya FJR, Toleco MM, Flores DM (2014) Xanthophyll content of selected vegetables commonly consumed in the Philippines and the effect of boiling. Food Chem 158: 35-40.
- Pinelo M, Laurie VF, Waterhouse AL (2006) A simple method to separate red wine nonpolymeric and polymeric phenols by solid-phase extraction. J Agric Food Chem 54: 2839-2844.
- Degenhardt A, Knapp H, Winterhalter P (2000) Separation and purification of anthocyanins by high-speed countercurrent chromatography and screening for antioxidant activity. J Agric Food Chem 48: 338-343.
- Andersen O, Francis G (2004) Techniques of pigment identification. Davies K editor. Annual Plant Reviews-Plant Pigments and Their Manipulation 14: 293-341.
- Fuleki T, Francis F (1968) Quantitative Methods for Anthocyanins. J Food Sci 33: 266-274.
- Silverman RB, Holladay MW (2014) The organic chemistry of drug design and drug action: Academic press.
- Ediriweera MK, Tennekoon KH, Samarakoon SR, Thabrew I, Dilip De Silva E (2016) A study of the potential anticancer activity of Mangifera zeylanica bark: Evaluation of cytotoxic and apoptotic effects of the hexane extract and bioassay-guided fractionation to identify phytochemical constituents. Oncol Lett 11: 1335-1344.
- Gonzalez-Montelongo R, Lobo MG, Gonzalez M (2010) The effect of extraction temperature, time and number of steps on the antioxidant capacity of methanolic banana peel extracts. Sep Purif Technol 71: 347-355.
- Murphy PA, Song T, Buseman G, Barua K, Beecher GR, et al. (1999) Isoflavones in retail and institutional soy foods. J Agric Food Chem 47: 2697-2704.
- Boulekbache-Makhlouf L, Medouni L, Medouni-Adrar S, Arkoub L, Madani K (2013) Effect of solvents extraction on phenolic content and antioxidant activity of the byproduct of eggplant. Ind Crops Prod 49: 668-674.
- Chandrasekhar J, Madhusudhan M, Raghavarao K (2012) Extraction of anthocyanins from red cabbage and purification using adsorption. Food Bioprod Process 90: 615-623.
- Ramirez-Lopez L, McGlynn W, Goad C, DeWitt CM (2014) Simultaneous determination of phenolic compounds in Cynthiana grape (Vitis aestivalis) by high performance liquid chromatography–electrospray ionisation–mass spectrometry. Food Chem 149: 15-24.
- Chew K, Khoo M, Ng S, Thoo Y, Aida W, et al. (2011) Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int Food Res J 18: 4.
- Durling NE, Catchpole OJ, Tallon SJ, Grey JB (2007) Measurement and modelling of the ternary phase equilibria for high pressure carbon dioxide–ethanol–water mixtures. Fluid Phase Equilib 252: 103-113.
- Strati IF, Oreopoulou V (2011) Effect of extraction parameters on the carotenoid recovery from tomato waste. Int J Food Sci Technol 46: 23-29.
- Galanakis CM (2012) Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. Trends Food Sci Technol 26: 68-87.
- Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, et al. (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 22: 296-302.
- Galanakis C, Goulas V, Tsakona S, Manganaris G, Gekas V (2013) A knowledge base for the recovery of natural phenols with different solvents. Int J Food Prop 16: 382-396.
- Musa KH, Abdullah A, Kuswandi B, Hidayat MA (2013) A novel high throughput method based on the DPPH dry reagent array for determination of antioxidant activity. Food Chem 141: 4102-4106.
- Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H (2011) Phytochemical screening and extraction: a review. Int Pharmaceut Sci 1: 98-106.
- Eloff J (1998) Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol 60: 1-8.
- Remington JP, Troy DB, Beringer P (2006) Remington: The science and practice of pharmacy: Lippincott Williams & Wilkins.
- Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochem 55: 481-504.
- Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Mol 18: 2328-2375.
- McDougall GJ, Ross HA, Ikeji M, Stewart D (2008) Berry extracts exert different antiproliferative effects against cervical and colon cancer cells grown in vitro. J Agric Food Chem 56: 3016-3023.
- Ross HA, McDougall GJ, Stewart D (2007) Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochem 68: 218-228.
- Cavazos-Garduno A, Serrano-Nino JC, Garcia-Varela R, Garcia HS (2017) Anticarcinogenic Phytochemicals. Fruit Veg Phytochem Chem Human Health 2: 1410-1448.




















