Sandeep Kumar Kar*, Anusua Banerjee, Chaitali Sen Dasgupta and Anupam Goswami
Department of Cardiac Anaesthesia, Institute of Postgraduate Medical Education and Research, Kolkata, India
*Corresponding Author:
Dr Sandeep Kumar Kar
Assistant Professor, Department of Cardiac Anaesthesia, Institute of Postgraduate Medical Education and Research, Kolkata, India
Tel: 919477234900
E-mail: sndpkar@yahoo.co.in
Received Date:December 08, 2015; Accepted Date: January 28, 2016; Published Date: February 05, 2016
Citation: Kar SK, Banerje A, Dasgupta CS, et al. Challenges of Percutaneous Balloon Mitral Valvuloplasty in Paediatric Patients with Rheumatic Mitral Stenosis: A Case Report. Transl Biomed. 2016, 7:1. DOI: 10.21767/2172-0479.100042
Keywords
Rheumatic mitral stenosis; Rheumatic fever; Pulmonary venous congestion
Introduction
Rheumatic fever is still the commonest cause of mitral stenosis in developing countries. This is attributable to predominance of overcrowding, unhygienic lifestyle and poor socio-economic status [1]. Usually rheumatic carditis sets in by 10-20 years after an attack of rheumatic fever. However juvenile rheumatic carditis has a fulminant course and requires prompt intervention [2]. In case of a pliable valve and minimal mitral regurgitation, percutaneous balloon mitral valvuloplasty has shown to produce good immediate and long term outcome in paediatric patients [3-8].
Case Report
A 4 year 3month old patient presented to the Department of Cardiology of our institution in February 2014 with complaints of dyspnea since past 18 months. The dyspnea was progressive (NYHA grade 4) and the patient had two episodes of paroxysmal nocturnal dyspnea in the past 6 months. He also had an episode of hemoptysis approximately a month back. Past history revealed an episode of rheumatic fever 26 months back, as evident from history of fleeting migratory polyarthritis and antecedent streptococcal sore throat infection. The child weighed 12 kgs and had a height of 100 cm. Complete haemogram showed haemoglobin level of 12 gm/dl, total count of 7300/mm3 with predominant lymphocytosis and adequate platelets. Serum urea was 21 mg/dl and creatinine 0.9 mg/dl. Anti-Streptolysin O titre and C-Reactive Protein were not raised and electrolytes, coagulation profile were within normal limits. ECG showed presence of sinus rhythm, right axis deviation, bi-atrial enlargement and right ventricular hypertrophy. Chest X-ray showed features of pulmonary venous congestion. 2D transthoracic echocardiography revealed severe mitral stenosis with a mitral valve area of 0.78 sqcm, with commissural fusion, good pliability of leaflets and no leaflet thickening or calcification and mild subvalvular fusion. The mean pressure gradient across mitral valve was 27 mm Hg and there was mild mitral regurgitation.
The tricuspid valve had normal leaflets with no ring dilatation and grade 2+ tricuspid regurgitation. The pulmonary arterial pressure was 96 mm of Hg. The left atrium measured 3.10 cm (normal range 1.5-2.3 cmm-2 of BSA) with no evidence of left atrial clot [9]. The Wilkins score in this case was 9. This score is based on echocardiographic imaging of the mitral valve Criteria for scoring include leaflet thickening, leaflet calcification, mobility of the leaflets and thickening of subvalvular apparatus. Patients with a score <8 are ideal candidates for balloon mitral valvuloplasty [10].
The patient was on syrup frusemide (2 mg/kg) and syrup digoxin (10 mcg/kg) once daily.
Procedure
The procedure was carried out under general anaesthesia. The child was given premedication with oral midazolam at a dose of 0.3 mg/kg 30 minutes prior to induction. Eutectic mixture of local anaesthesia (EMLA) cream, which contains 2.5% lignocaine and 2.5% prilocaine, was applied at the intravenous cannulation site. After applying standard monitors, induction of anaesthesia was done using 8% sevoflurane in oxygen. With loss of eyelash reflex, intravenous cannulation was done and 2 mcg/kg fentanyl was given. To facilitate intubation intravenous atracurium at a dose of 0.5 mg/ kg was given. Anaesthesia was maintained using oxygen and sevoflurane. Nitrous oxide was avoided as the patient had severe pulmonary arterial hypertension. Local infiltration was done with 2% lignocaine in groin at the site of puncture. Selection of an appropriate sized Inoue balloon is very important. In this case it was done before the procedure was embarked upon, by measuring the distance between two commissures of mitral valve in the apical 2 chamber view (Figure 1). It can also be calculated using the formula [Height in cm/10+10]. This formula does not take into account the degree of mitral commissural fusion and hence overestimates the annular size. This can lead to damage to the mitral valve leaflets and chordae and the patient may subsequently develop haemodynamically significant mitral regurgitation [11]. Mitral valve annular diameter, in this case, was calculated using echocardiography by measuring the distance between two commissures in 2 chamber view and was found out to be 20 mm.
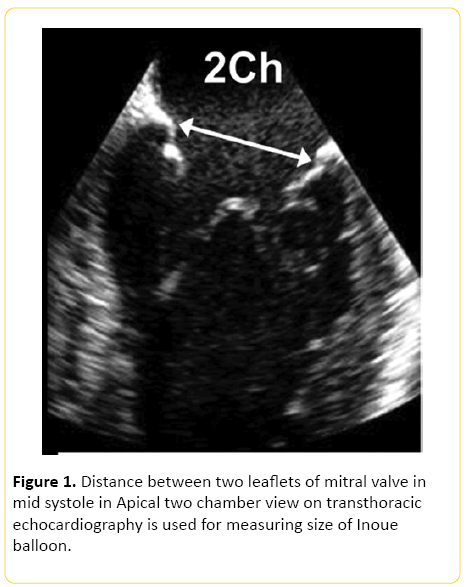
Figure 1: Distance between two leaflets of mitral valve in mid systole in Apical two chamber view on transthoracic echocardiography is used for measuring size of Inoue balloon.
After administration of 1000 U of heparin, right heart catheterization was performed. This was followed by right atriography and puncture of the inter-atrial septum with a standard Brochenbrough needle. The catheter was advanced into left atrium and directed towards orifice of mitral valve. A 20 mm Inoue balloon was found to be appropriate for this case (Figure 2). The distal balloon was then inflated with contrast media. After the distal balloon floated past the mitral valve, the proximal balloon was inflated. The whole assembly was pulled out until resistance was met. The middle waist portion was inflated to a length of 16 mm. After each dilatation the gradient between left atrium and left ventricle was measured using the middle port of Inoue balloon and pigtail catheter respectively. Serial dilation was done at 1 mm increments until the pressure gradient between left atrium and left ventricle decreased (Figure 3).
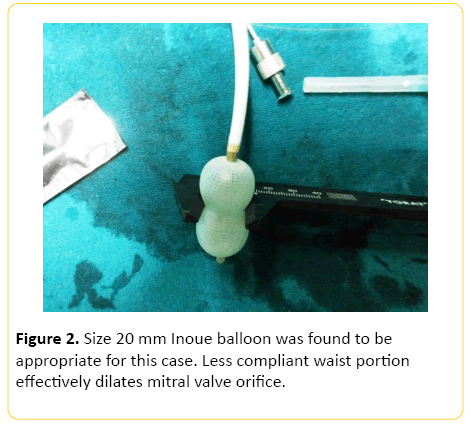
Figure 2: Size 20 mm Inoue balloon was found to be appropriate for this case. Less compliant waist portion effectively dilates mitral valve orifice.
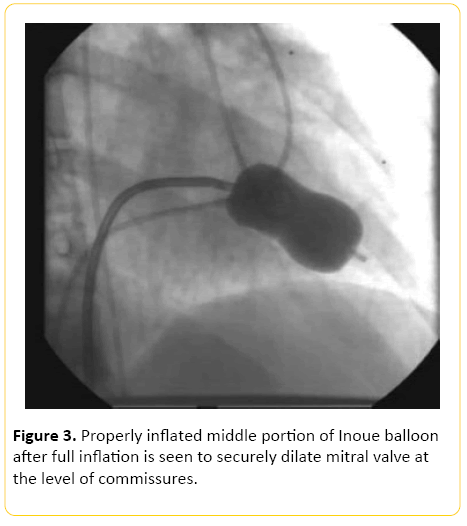
Figure 3: Properly inflated middle portion of Inoue balloon after full inflation is seen to securely dilate mitral valve at the level of commissures.
Each dilatation was followed by measurement of mitral valve area by planimetry on transthoracic echocardiography. Any worsening of mitral regurgitation was also observed closely. The entire procedure was uneventful. 2D transthoracic echocardiography at the end of procedure revealed a mitral valve area of 1.7sq cm (more than twice the initial orifice area) and there was evidence of grade 2+ mitral regurgitation.
The patient was reversed using neostigmine 50 mcg/kg and glycopyrrolate 10 mcg/kg and shifted to ICU. The stay in ICU was uneventful and the patient was discharged at the end of 4 days.
The patient came for a follow up at the end of 3 months and 1 year. The patient was in NYHA class I and the mitral regurgitation had not progressed beyond grade 2.
Discussion
Juvenile rheumatic mitral stenosis has a fulminant course, especially in developing countries. This has been attributed to persistence of predisposing factors to acute rheumatic fever and inadequate penicillin therapy. Moreover, many patients lack access to secondary prophylaxis and most of them fail to adhere to it [12].
Echocardiographic evaluation is gold standard for differentiating between congenital and rheumatic mitral stenosis. Commissural fusion is hallmark of rheumatic process. Congenital mitral stenosis may be associated with other abnormalities. There may be complete or incomplete supravalvular mitral ring, annular hypoplasia, leaflet abnormalities, chordae abnormalities or single papillary muscle (parachute mitral valve). PBMV produce better results in rheumatic rather than in congenital mitral stenosis [13].
Percutaneous balloon mitral valvuloplasty offers definite advantages by obviating the need for lifelong anticoagulation which is mandatory in case of prosthetic valves. In young patients PBMV is the procedure of choice circumventing disadvantages of patient prosthesis mismatch as the child grows.
Percutaneous balloon mitral valvuloplasty by different routes are possible, namely antegrade and retrograde technique. Antegrade technique using the Inoue balloon is most commonly used. Other techniques are double ballon, multi-track and Cribier’s metallic dilator technique. The advantages of double balloon method is a smaller sheath can be used [14] (Figure 4).
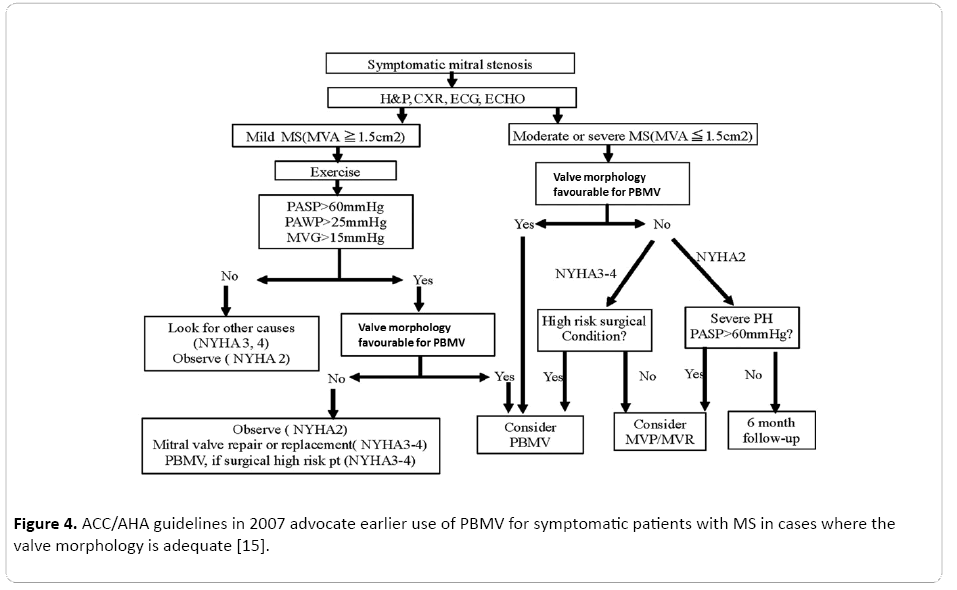
Figure 4: ACC/AHA guidelines in 2007 advocate earlier use of PBMV for symptomatic patients with MS in cases where the valve morphology is adequate [15].
Percutaneous balloon mitral valvuloplasty in paediatric patients needs special considerations. Firstly, the procedure has to be carried out under general anaesthesia because complete immobility is required during the procedure. The patients are usually sick with severe pulmonary arterial hypertension and very sensitive to effects of anaesthetic agents [16].
Care must be taken regarding the quantity of contrast that is used and must not exceed 4 ml/kg [17].
Occult blood loss is very common during these procedures especially during vascular puncture. All the paraphernalia designed for PMBV is mostly for adult patients. Inadvertent injury to vessels may occur because of the rigidity of the Inoue balloon catheter. Packed cells should be kept in reserve and it is desirable that the procedure is carried out in a hybrid OR where cardiac surgery back up is available.
Serious intraoperative complications may occur during transeptal puncture with Brockenbrough needle. If done too anteriorly, it might cause damage to ascending aorta and when done too posteriorly, the needle might enter post-atrial space. There may be perforation of left atrial appendage, pulmonary veins and left ventricular apex with guide wires or balloon catheter leading to cardiac tamponade [18,19]. The balloon may get entrapped in the subvalvular apparatus and this will be evident by distortion in shape of balloon. In that case proper positioning is required before balloon inflation. Overzealous balloon inflation may cause severe mitral regurgitation [20,21]. Other considerations include limiting the size of sheath because of small caliber of blood vessels in paediatric patients. Procedures may be difficult to perform in children <5 kgs. Also, risk of air embolism due to air entrapped in the sheath.
During follow up at the end of 3 months, the functional capacity showed to be improved drastically but there was evidence of mild mitral regurgitation. At the end of 1 year the child was in NYHA grade 1, with mild mitral regurgitation. The pulmonary arterial pressures had regressed and the patient had no evidence of pulmonary hypertension.
Kapoor et al. reported successful balloon mitral valvuloplasty in a 4 year old child [22]. Till date there is only one single report of successful balloon mitral valvuloplasty in such a small child in India.
Adel Zaki followed 46 children and adolescents in the age group 7-19 years for a period of 5 years and they found out that BMV produced excellent intermediate term results in patients with relatively low mitral valve scores [23].
Conclusion
Percutaneous balloon mitral valvuloplasty is now considered the procedure of choice in paediatric patients with rheumatic mitral stenosis with favourable valve morphology. This technique forms a cost effective alternative which is minimally invasive and thus suits to the need of the children in developing countries where rheumatic fever is prevalent.
8543
References
- Nobuyoshi M, Arita T, Shirai S, Hamasaki N,Yokoi H, et al. (2009) Percutaneous Balloon Mitral Valvuloplasty A Review. Circulation 119: e211-e219
- Tandon R, Potti S, Mathur VS, Roy SB (1972) Critical rheumatic mitral stenosis in children. Indian Pediatr 9: 171-173
- Gamra H, Betbout F, Ben Hamda K, Addad F, Maatouk F, et al. (2003) Balloon mitral commissurotomy in juvenile rheumatic mitral stenosis: a ten-year clinical and echocardio-graphic actuarial results. Eur Heart J24: 1349-1356.
- Sinha N, Kapoor A, Kumar AS, Shahi M, Radhakrishnan S, et al. (1997) Immediate and follow up results of Inoue balloon mitral valvotomy in juvenile rheumatic mitral stenosis. J Heart Valve Dis 6: 599-603.
- Gotsman MS, Van der Horst RL (1975) Surgical management of severe mitral valve disease in childhood. Am Heart J 90: 685-687.
- Kothari SS, Ramakrishnan S, Kumar CK, Juneja R, Yadav R (2005) Intermediate-term results of percutaneous transvenous mitral commissurotomy in children less than 12 years of age. Cath-eter Cardiovasc Interv 64: 487-490.
- Krishnamoorthy KM, Tharakan JA (2003) Balloon mitralvalvulotomy in children aged
- Essop MR, Govendrageloo K, Du Plessis J, van Dyk M, SareliP (1993) Balloon mitral valvotomy for rheumatic mitral stenosis in children aged
- Wyman W, Geva T, Shirali GS (2006) Guidelines and Standards for Performance of a Pediatric Echocardiogram: A Report from the Task Force of the Pediatric Council of the American Society of Echocardiography. Journal of the American Society of Echocardiography Volume 19: 1413-1430.
- Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF (1988) Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 60: 299-308.
- Vahanian A, Cormier B, Iung B (1994) Percutaneous transvenous mitral commissurotomy using the Inoue balloon: international experience. Cathet Cardiovasc Diagn 8-15.
- Tadele H, Mekonnen W, Tefera E (2013) Rheumatic mitral stenosis in Children: more accelerated course in sub-Saharan Patients. BMC cardiovascular disorders 13: 95.
- Krapf L, Dreyfus J, Cueff C(2013) Anatomical features of rheumatic and non-rheumatic mitral stenosis: Potential additional value of three-dimensional echocardiography. Archives of Cardiovascular Disease 106: 111-115.
- Bahl VK, Chandra S, Goswami KC (1998) Combined mitral and aortic valvuloplasty by antegrade transseptal approach using inoue balloon catheter. Int JCardiol63: 313-315.
- Bonow RO, Carabello BA, Kanu C, de Leon AC Jr, Faxon DP, et al. (2006) ACC/AHA 2007 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists. Circulation 114: e84-e231.
- Roy SB, Bhatia ML, Lazaro EJ, Ramalingaswami V (1963) Juvenile mitral stenosis in India. Lancet 2: 1193-1195.
- Kern MJ, Deligonul U (1996) The interventional cardiac catheterization handbook, St Louis, Mosby.
- Nobuyoshi M, Hamasaki N, Kimura T, Nosaka H, Yokoi H, et al. (1989) Indications, complications, and short-term clinical outcome of percutaneous transvenous mitral commissurotomy. Circulation 80: 782-792.
- Martinez-rios MA, Tovar S, Luna J, Eid-Lidt G (1999) Percutaneous mitral commissurotomy. Cardiol Rev 7: 108-116.
- Chen CR, Cheng TO (1995) Percutaneous balloon mitral valvuloplasty by the Inoue technique: a multicenter study of 4832 patients in China. Am Heart J 129: 1197-1203.
- Palacios IF, Sanchez PL, Harrell LC, Weyman AE, Block PC (2002) Which patients benefit from percutaneous mitral balloon valvuloplasty? Prevalvuloplasty and postvalvuloplasty variables that predict long-term outcome. Circulation 105: 1465-1471.
- Kapoor A, Moorthy N, Kumar S (2012) Inoue Balloon Mitral valvotomy in a 4-Year-Old Boy toTreat Fulminant Rheumatic Mitral Stenosis. Tex Heart Inst J 39: 108-111.
- Zaki A, Salama M, El Masry M, Elhendy A (1999)Five-year follow-up after percutaneous balloon mitral valvuloplasty in children and adolescents. Am J Cardiol 83: 735-739.









