Key words
|
| |
| Azetidin-2-one, HMG CoA, Atherosclerosis, Lipid lowering, Cholesterol |
| |
 |
| |
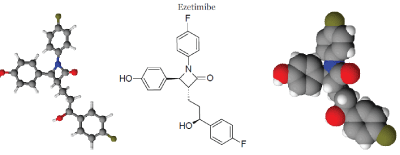
| IUPAC nomenclature: (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4- hydroxyphenyl)azetidin-2-one |
| |
| Molecular Formula = C24H21F2NO3 |
| |
| Formula Weight = 409.4252464 |
| |
| Composition = C(70.41%) H(5.17%) F(9.28%) N(3.42%) O(11.72%) |
| |
| Molar Refractivity = 108.27 ± 0.3 cm3 |
| |
| Molar Volume = 306.7 ± 3.0 cm3 |
| |
| Parachor = 834.5 ± 6.0 cm3 |
| |
| Index of Refraction = 1.623 ± 0.02 |
| |
| Surface Tension = 54.7 ± 3.0 dyne/cm |
| |
| Density = 1.334 ± 0.06 g/cm3 |
| |
| Polarizability = 42.92 ± 0.5 10-24cm3 |
| |
| Monoisotopic Mass = 409.14895 Da |
| |
| Nominal Mass = 409 Da |
| |
| Average Mass = 409.4252 Da |
| |
| CAS number = 163222-33-1 |
| |
| ATC code = C10AX09 |
| |
| MP =164–166 |
| |
| Pharmacokinetics: Bioavailavility =35–65% |
| |
| Protein binding >90% |
| |
| Metabolism Intestinal wall, hepatic |
| |
| Half life =19-30 hours |
| |
| Excretion = Renal 11%, Fecal 78% |
| |
Introduction
|
| |
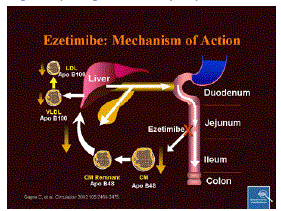
| Ezetimibe localises at the brush border of the small intestine, where it inhibits the absorption of cholesterol from the intestine. Specifically, it appears to bind to a critical mediator of cholesterol absorption, the Niemann-Pick C1-Like 1 (NPC1L1) protein on the gastrointestinal tract epithelial cells] as well as in hepatocytes. In addition to this direct effect, decreased cholesterol absorption leads to an upregulation of LDL-receptors on the surface of cells and an increased LDL-cholesterol uptake into cells, thus decreasing levels of LDL in the blood plasma which contribute to atherosclerosis and cardiovascular events.1 |
| |
|
Clinical use
|
| |
|
Indications
|
| |
| Ezetimibe is indicated as an adjunct to dietary measures in the management of: |
| |
| · Hypercholesterolaemia |
| |
| · Homozygous sitosterolemia (phytosterolemia) |
| |
| On 9 June 2006, US regulators approved the use of ezetimibe in combination with fenofibrate to treat mixed hyperlipidaemia. |
| |
|
Adverse effects
|
| |
| Common adverse drug reactions (≥1% of patients) associated with ezetimibe therapy include: headache and/or diarrhea (steathorrea). Infrequent adverse effects (0.1–1% of patients) include: myalgia and/or raised liver function test (ALT/AST) results. Rarely (<0.1% of patients), hypersensitivity reactions (rash, angioedema) or myopathy may occur. In 2005, the manufacturer of Ezetrol, Merck Frosst/Schering Pharmaceuticals issued a warning through Health Canada associating Ezetrol (ezetimibe) with "myalgia, rhabdomyolysis, hepatitis, pancreatitis, and thrombocytopenia". Also noted were possible changes in liver function tests as described above and longer clotting times if the patient is currently on warfarin while on the cholesterol-lowering medication.2 |
| |
|
Dosage forms
|
| |
| Ezetimibe is available as 10 mg tablets in most markets. A combination preparation ezetimibe/simvastatin, which combines ezetimibe with a statin, is also available. |
| |
|
Side effects
|
| |
| Side-effects include gastro-intestinal disturbances; headache, fatigue; myalgia; rarely arthralgia, hypersensitivity reactions (including rash, angioedema, and anaphylaxis), hepatitis; very rarely pancreatitis, cholelithiasis, cholecystitis, thrombocytopenia, raised creatine kinase, myopathy, and rhabdomyolysis. Get emergency medical help if you have any of these signs of an allergic reaction to ezetimibe: hives; difficulty breathing; swelling of your face, lips, tongue, or throat. |
| |
| Call your doctor at once if you have a serious side effect such as: |
| |
| · unusual muscle weakness, tenderness, or pain; |
| |
| · nausea, stomach pain, low fever, loss of appetite, dark urine, clay-colored stools, jaundice (yellowing of the skin or eyes); |
| |
| · chest pain; |
| |
| · pancreatitis (severe pain in your upper stomach spreading to your back, nausea and vomiting, fast heart rate); or |
| |
| · fever, sore throat, and headache with a severe blistering, peeling, and red skin rash. |
| |
| · Less serious ezetimibe side effects may include: |
| |
| · numbness or tingly feeling; |
| |
| · mild stomach pain, diarrhea; |
| |
| · tired feeling; |
| |
| · headache; |
| |
| · dizziness; |
| |
| · depressed mood; |
| |
| · runny or stuffy nose, cold symptoms; |
| |
| · joint pain, back pain; or |
| |
| · cough. |
| |
|
Efficacy
|
| |
| A clinical study, results of which were presented at the 2009 annual meeting of the American Heart Association and published in the New England Journal of Medicine suggest that in combination with statins, Niaspan, a slow-release form of niacin, is more effective than ezetimibe at reducing arterial plaque buildup. |
| |
|
Pharmacokinetics
|
| |
| Ezetimibe is available as 10 mg tablets. The recommended dose of ezetimibe is 10 mg once daily without regard to meals for all its approved indications. Within 4–12 hours of the oral administration of a 10 mg dose to fasting adults, the attained mean ezetimibe peak plasma concentration (Cmax) was 3.4–5.5 ng/ml. Following oral administration, ezetimibe is absorbed and extensively conjugated to a phenolic glucuronide (active metabolite). Mean Cmax (45–71 ng/ml) of ezetimibe-glucuronide are attained within 1–2 hours. The concomitant administration of food (high-fat vs. non-fat meals) has no effect on the extent of absorption of ezetimibe. However, co-administration with a high fat meal increases the Cmax of ezetimibe by 38%.5 The absolute bioavailability cannot be determined since ezetimibe is insoluble in aqueous media suitable for injection. Ezetimibe and its active metabolite are highly bound to human plasma proteins (90%). |
| |
| Ezetimibe is primarily metabolized in the liver and the small intestine via glucuronide conjugation with subsequent renal and biliary excretion. Both the parent compound and its active metabolite are eliminated from plasma with a half-life of approximately 22 hours allowing for once daily dosing. Ezetimibe lacks significant inhibitor or inducer effects on cytochrome P-450 isoenzymes which explain its limited number of drug interactions (Table 4). No dose adjustment is needed in patients with renal insufficiency or mild hepatic dysfunction (Child-Pugh score 5–6). Due to insufficient data, the manufacturer does not recommend ezetimibe for patients with moderate to severe hepatic impairment (Child-Pugh score 7–15). In patients with mild, moderate, or severe hepatic impairment, the mean AUC values for total ezetimibe are increased approximately 1.7-fold, 3–4 fold, and 5–6 fold respectively, compared to healthy subjects.3 |
| |
|
Clinical trial controversy
|
| |
| The ENHANCE trial of Vytorin (ezetimibe and simvastatin) was designed to show that ezetimibe could reduce the growth of fatty plaques in arteries. Instead, it reported in 2008 that ezetimibe resulted in growth of plaque. The ENHANCE trial was not a clinical-outcome trial, but an imaging study of the thickness of plaque in arteries. The American College of Cardiology (ACC) maintains that ezetimibe may be a reasonable option for patients who cannot tolerate a statin or cannot be controlled on a high dose statin. The primary outcome in the treatment of hypercholesterolemia is prevention of cardiovascular events such as death from cardiovascular disease. While the ENHANCE trial did not have the power to detect significant differences in death, it measured the difference in artery thickness (in the carotid and femoral intima-media) to detect reductions in atherosclerotic plaque. At the end of two years, there was no significant difference in artery thickness between patients taking simvastatin and ezetimibe versus patients taking simvastatin alone. Since in the ENHANCE trial ezetimibe didn't reduce cardiovascular events, atherosclerosis or death, despite the reduction in LDL, doctors have been trying to figure out whether it has any use. Doctors have also concluded that reducing LDL doesn't always reduce atherosclerosis. Reviewers of the ENHANCE trial have raised the possibility that it did not last long enough for ezetimibe to work. Also, many patients had already been treated on statins for a long time, so their artery thickness was already lower. Perhaps if they had not used statins, ezetimibe might have had a greater effect. Results from the trial have provoked three large clinical-outcome trials.4 The ARBITER 6–HALTS trial enrolled patients with coronary artery disease, or an equivalent risk condition such as diabetes, who were already taking statins. They were randomized to additionally take either extended-release niacin or ezetimibe, and the primary end point was change in artery wall thickness. Both drugs reduced LDL cholesterol levels. Niacin reduced artery wall thickness, but ezetimibe paradoxically increased artery wall thickness. Patients on ezetimibe also had more major cardiovascular events. The trial was terminated early after 208 volunteers had completed the study. The results from the other trials will be presented in the next 3 years. However even before completion of ARBITER 6–HALTS, a March 30, 2008 meeting of the ACC resulted in negative press for drugs like Zetia as Yale University Cardiologist Harlan Krumholz and concurring colleagues called into question the efficacy of such drugs. Krumholz' statements maintained that such pharmaceuticals should not be the first or even second option for prescribing doctors. Definitive conclusions of the efficacy and safety of Zetia can be made such a time when the results of more substantial and comprehensive trials are released, such as the upcoming SHARP Trial which has an enrollment of 9000 patients and will report in 2010 and IMPROVE-IT Trial which has an enrollment of 18,000 patients and will report in 2012. Results of the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial (ClinicalTrials.gov number, NCT00092677 [ClinicalTrials.gov] ) showed a potential increase in cancer in association with the use of these drugs together. (www.nejm.org September 2, 2008 (10.1056/NEJMe0807200). The actual significance has yet to be determined. Trials of ezetimibe in combination with simvastatin have proven more favourable than those for ezetimibe alone, however. A 2010 study found that this combination was more effective at lowering lipid levels than alternative agents rosuvastatin and atorvastatin. In the SHARP study, cardiovascular events were lowered by combining ezetimibe and simvastatin.5 |
| |
|
Chemical properties
|
| |
| Solubility: Ezetimibe is highly soluble in alcohols (methanol, ethanol, 1-propanol, 2-propanol, etc.) and insoluble in water. |
| |
| Degradation behaviour: HPLC studies on ezetimibe under different stress conditions suggested the following degradation behaviour. |
| |
| Acidic condition: The drug gradually decreased with time on heating at 80°C in 1 M HCl by forming degradation products. The rate of hydrolysis in acid was slower as compared to that of alkali or water. |
| |
| Neutral (water) condition: Upon heating the drug solution in water at 80°C for 1 h, almost complete degradation of the drug was observed. |
| |
| Degradation in alkali: The drug was found to be highly labile to alkaline hydrolysis. The reaction in 0.1 M NaOH at 80°C was so fast that whole of the drug was degraded in 0 min. Subsequently, studies were performed in 0.01 M NaOH at 40°C and complete degradation of the drug was observed in 4 h. |
| |
| Oxidative conditions: The drug was stable to hydrogen peroxide (3 and 20%) at room temperature. |
| |
| Photolytic conditions: No major degradation product was observed after exposure of drug solution in 1 M HCl to sunlight for 2 d, only minor degradation products were formed. The nature of degradation in light and dark was found to be similar, indicating that light had no effect on the degradation of the drug in acid. On the other hand, the samples in water degraded under sunlight for 2 d. Corresponding rate of degradation in dark was much slower. |
| |
| Solid-state study: The solid-state studies showed that ezetimibe was stable to the effect of temperature. When the drug powder was exposed to dry heat at 50°C for 45 d and at 60°C for 7 d, no decomposition of the drug was seen.6 |
| |
|
Synthesis
|
| |
| The key to the construction of this compound involves formation of an azetidone. Imine formation between p-benzyloxybenzaldehyde and pfluoroaniline provides one of the required reactants. This compound is then treated with the depicted acid chloride in the presence of triethylamine. In all likelihood, this first dehydrohalogenates under reaction conditions to form the substituted ketene. The transient intermediate reacts with the imine in a 2 + 2 cycloaddition to afford a four-membered ring. The reaction proceeds to give the trans isomer almost exclusively. The ester group is then hydrolyzed by means of lithium hydroxide. Condensation with the zinc reagent formed in situ from p-fluoromagnesium bromide and zinc chloride affords the ketone. The carbonyl group is then reduced with diborane to afford the alcohol. Removal of the benzyl protecting group by hydrogenolysis over palladium finally affords ezetimibe.7-10 |
| |
|
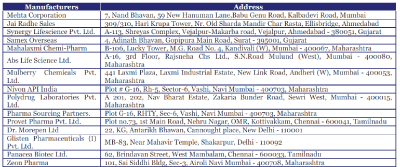
Ezetimibe Manufacturers & Exporters of India
|
| |
| Below are the listings of manufacturers and exporters of ezetimibe. You can view company details & contact them directly through email, refine your search by product keywords, browse trade leads posted by ezetimibe manufacturers and view several other products and trade shows related to ezetimibe. |
| |
 |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Scheme 1 |
|
| |










