Research Article - (2022) Volume 0, Issue 0
Chronic Renal Insufficiency and Its Relation to the Development of Skeletal Lesions.
1Physician, Pontificia Universidad Católica del, Ecuador
Physician, Universidad de las Americas, Ecuador
Physician, Universidad Católica del, Ecuador
Physician, Universidad de Cartagena, Colombia
Physician, Universidad del Sinu, Cartagena, Colombia
Physician, Fundacion Universitaria San Martin, Colombia
Physician, Universidad del Sinu, Colombia
*Correspondence:
Jordan Gonzalo Llerena Velastegui, Physician, Pontificia Universidad Católica del,
Physician, Pontificia Universidad Católi,
Ecuador,
Email:
Received: 05-Mar-2022, Manuscript No. IPHSJ-22-12582;
Editor assigned: 07-Mar-2022, Pre QC No. Preqc No. IPHSJ-22-12582 (PQ);
Reviewed: 21-Mar-2022, QC No. QC No. IPHSJ-22-12582;
Revised: 27-Mar-2022, Manuscript No. IPHSJ-22-12582(R);
Published:
04-Apr-2022, DOI: 10.36648/1791-809X.16.S6.920
Abstract
Background: When there is a glomerular filtration rate lower than ml/min/160.73mt2, it is considered that there is chronic kidney disease; it must persist for 3 months or more regardless of the cause. Depending on the geographical location and the environment where the person is, the cause of this pathology will vary. The prevalence of CKD is around 10% to 14% in the general population. Renal osteodystrophy is a broad term that incorporates all biochemical abnormalities and skeletal manifestations in patients suffering from chronic kidney disease or end-stage renal disease. Methodology: A narrative review was carried out through various databases from January to2002 February 2022; the search and selection of articles was carried out in journals indexed in English. The following keywords were used: Chronic renal failure, renal osteodystrophy, skeletal lesions, and terminal renal failure. Results: Chronic kidney disease leads to a variety of systemic complications that endanger human health. One of the main complications presented by this pathology is renal osteodystrophy, which leads to skeletal and extra skeletal manifestations. Within the histopathological findings of osteodystrophy we can find states of high bone turnover such as osteitis fibrosa and hyperparathyroidism and states of low bone turnover, such as dynamics bone disease or heavy metal- induced osteomalacia. Osteitis fibrosa is considered the predominant histological bone pattern for the development of renal osteodystrophy. Conclusions: This review offers updated and detailed information on the important components of renal osteodystrophy, which pathophysiological mechanisms have been proposed between the association between skeletal lesions and chronic kidney disease, and the pathways of formation of secondary hyperparathyroidism as the cause of osteodystrophies.
Keywords
Chronic renal failure, Renal osteodystrophy, Skeletal lesions, Terminal renal failure.
Introduction
When there is a glomerular filtration rate of less than 60 ml/ min/1.73mt2, chronic kidney disease is considered to be present, which must persist for 3 months or more regardless of the cause. [1, 2] Dialysis or transplantation is the therapeutic approaches available to date in the presence of this chronic disease. Renal damage refers to pathologic abnormalities suggested by imaging studies or renal biopsy, urinary sediment abnormalities, or increased urinary albumin excretion rates [3, 4]. Depending on the geographical location and the environment where the person is, the cause of this pathology will vary, among the most frequent and recognized worldwide we can find the following: Diabetes mellitus type 2, diabetes mellitus type 1 hypertension, and primary glomerulonephritis, and chronic tubulo interstitial nephritis, hereditary or cystic disease, among other causes [5, 6]. Since chronic kidney disease is asymptomatic in nature the true incidence and prevalence is difficult to identify [7, 8].
The prevalence of CKD is about 10% to 14% in the general population. Likewise, albuminuria (micro albuminuria or A2) and GFR less than ml/min/160.73 mt2 have a prevalence of 7% and 35%, respectively. This pathology also presents some risk factors that can be modified and others that cannot [9, 10]. Among the modifiable risk factors we can find advanced age, male gender, African- American, African-Caribbean, Hispanic and Asian, as well as single nucleotide polymorphisms in the TCF7L2 and MTHFS genes were associated with diabetic nephropathy and progression of CKD. Among the modifiable risk factors we can find arterial hypertension, proteinuria and metabolic factors [11]. Renal osteodystrophy is a broad term that incorporates all biochemical abnormalities and skeletal manifestations in patients with chronic kidney disease or end-stage renal disease. Among the main components that we can find in this condition are the disorders in serum levels of calcium, phosphorus, PTH, vitamin D, together with their effects on bone turnover, mineralization and extra skeletal calcifications [9, 11, 10,]. Given the increase in the number of cases of patients with chronic kidney disease, it is convenient to perform this study in order to identify or prevent skeletal complications in a timely manner. This work offers updated information on the important components of renal osteodystrophy, which pathophysiological mechanisms have been proposed between the association between skeletal lesions and chronic kidney disease and the pathways of formation of secondary hyperparathyroidism as a cause of osteodystrophy
Materials and Method
A systematic review was carried out, searching PubMed, Scielo and Science Direct databases, among others. The collection and selection of articles was carried out in English-language indexed journals from 2002 to 2022. As keywords, the following terms were used in the databases according to DeCS and MeSH methodology: Chronic renal failure; renal osteodystrophy; skeletal lesions; renal failure end. In this review, 71 original and review publications related to the subject studied were identified, of which 27 articles met the specified inclusion requirements, such as, articles that were in a range not less than 2002, that were full-text articles and that reported on The development of skeletal lesions in patients with chronic kidney disease.The exclusion criteria were that the articles did not have sufficientinformation and that they did not present the full text at the time of review.
Results
What Is Renal Osteodystrophy?
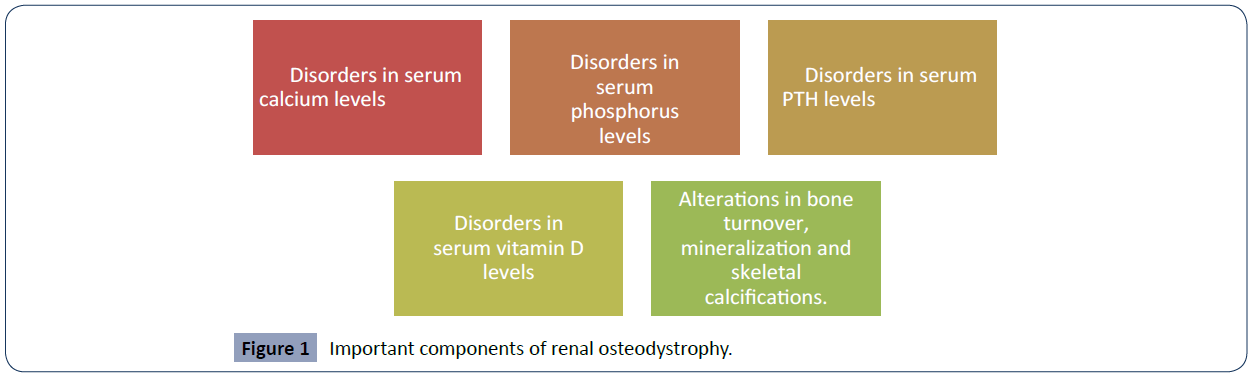
Chronic kidney disease leads to a variety of systemic complications that endanger human health. One of the main complications presented by this pathology is renal osteodystrophy, which leads to skeletal and extra-skeletal manifestations [12]. One of the ways to be able to buffer or reduce the adverse effects of this pathology, renal osteodystrophy, is through haemodialysis, although sometimes it may be inevitable to develop complications, appropriate and timely interventions can help alleviate the symptoms experienced by patients and also reduce comorbidities related to osteodystrophy [13]. But, in order to continue with this approach we must ask ourselves the question, what is renal osteodystrophy? This is a broad term that incorporates a variety of biochemical abnormalities and even skeletal manifestations in patients with or suffering from chronic kidney disease or end-stage renal disease. In Figure 1 we can identify all the important components of this condition [14- 16] (Figure 1).
Figure 1 Important components of renal osteodystrophy.
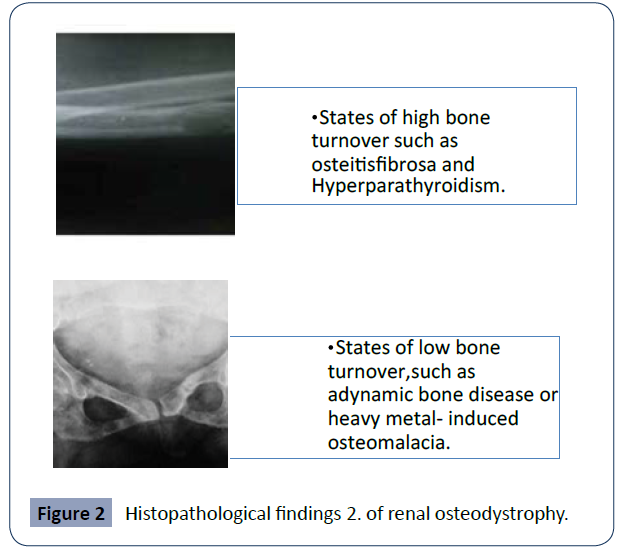
It should be taken into account that osteodystrophy will not occur in all stages of chronic kidney disease; there are reports that show that it is much more frequent when the GFR is less than ml/min/160.73m2 . [17] The histopathology findings of renal osteodystrophy are commonly used to further classify this condition into; in Figure 2 we can identify them [15-18] (Figure 2).
Figure 2 Histopathological findings 2. of renal osteodystrophy.
How Are The Development Of Cheletal Lesions And Chronic Kidney Disease Related?
As previously stated, through the different studies performed to date, renal osteodystrophy is invariably observed in patients with chronic kidney disease, although the disease processes may differ in patients [14]. As shown in Figure 2, histologically, there is a classification into states of high or low bone turnover [18].States of high bone turnover: lead to increased rates of boneresorption and bone formation. Elevated levels of parathyroid hormone (PTH) play an important role in the pathogenesis of high bone turnover states. Hyperparathyroidism can be primary, secondary or tertiary. Parathyroid gland neoplasms that autonomously secrete PTH, an example of tertiary hyperparathyroidism, can lead to a state of high bone turnover [17].
Among the predominant causes of osteodystrophy we find secondary hyperparathyroidism, in Table 1 we can see how chronic kidney disease can lead to the development of hyperparathyroidism and at the same time lead to the development of renal osteodystrophy or skeletal conditions [19- 22] (Table 1).
| Factors |
Summary |
| Phosphate retention |
Stimulation of PTH secretion can be given by elevated phosphate levels, increasing PTH mRNA levels or at the same time decreasing calcium and calcitriol levels, indirectly causing increased blood PTH levels. |
| Calcium |
A decrease in serum calcium will also stimulate PTH secretion. |
| Role of calcitriol |
Both calcitriol and PTH increase serum calcium levels and, in cases of decreased calcitriol in the body, secondary hyperparathyroidism occurs due to decreased calcium absorption through the intestine and a reflex increase in PTH. |
| Fibroblast growth factor 23 |
FGF-23 is responsible for decreasing phosphate levels in the body, and a decrease in FGF-23 can lead to secondary hyperparathyroidism. |
Table 1. Factors 1 involved in the pathway of secondary hyperparathyroidism formation
Osteitis fibrosa is considered the predominant histologic bone pattern for the development of renal osteodystrophy [23]. Osteitis fibrosa is the result of overproduction of parathyroid hormone (PTH) in the context of hyperparathyroidism especially of the secondary type. PTH binds to receptors on osteoblasts, resulting in the expression of RANK ligand receptor activator. RANK ligand binds to RANK on osteoclast precursors, promoting osteoclast formation [20, 23].
Activated osteoclasts cause bone resorption, destruction of cortical bone and fibrous cyst formation. Osteoclast-like giant cells and vascularized fibrous tissue may replace bone marrow and produce brown Tumors, which are non-neoplastic lesions. [15] During the process of Brown's tumour, bone demineralization promotes osteoclast activation. Eventually, bone resorption may cause micro fractures and micro haemorrhages [18].
In order to understand or clarify this pathophysiological process of the development of skeletal lesions, it is useful to know the two main cell types, the osteoclasts that cut or resorb bone and the osteoblasts that accumulate or form new bone [24]. Osteoblasts are activated by a RANK-RANK ligand complex, leading to activation of osteoclasts, and here bone resorption begins.
All these factors are regulated by several factors such as PTH, Vitamin D, and osteoprotegerins [25]
Discussion
The study by Hartmun et al, in which they performed bone biopsies between 2003 and 2008 on patients who volunteered to participate in various research protocols. There were patients 316 from the United States and 314 Europe. Only baseline biopsies were included in the study. All patients received regular dialysis support medication for at least 6 months, including phosphorus chelators, active vitamin D or calcimimetics at the discretion of the treating nephrologists. This study concluded that low bone turnover is a common feature in patients with stage 5 CKD on chronic dialysis, especially in white patients. Treatment of renal osteodystrophy is mainly focused on suppression of elevated bone turnover (secondary hyperparathyroidism) [26].
Conclusion
Chronic kidney disease leads to a variety of systemic complications that endanger human health. One of the main complications presented by this pathology is renal osteodystrophy, which leads to skeletal and extra-skeletal manifestations. Within the important components of renal osteodystrophy we can find disorders in serum calcium levels, disorders in serum phosphorus levels, disorders in serum PTH levels, disorders in serum vitamin D levels, alterations in bone turnover, mineralization and skeletal calcifications.
Within the histopathological findings of osteodystrophy we can find high bone turnover states such as osteitis fibrosa and hyperparathyroidism and low bone turnover states such as dynamics bone disease or heavy metal-induced osteomalacia.
There are some factors involved in the pathway of formation of secondary hyperparathyroidism as a trigger of osteodystrophies, among which we can highlight the retention of phosphate, calcium, as well as calcitriol increasing serum calcium levels and, in cases of decreased calcitriol in the body, secondary hyperparathyroidism occurs due to decreased absorption of calcium through the intestine and a reflex increase in PTH, Osteitis fibrosa is considered the predominant histologic bone pattern for the development of renal osteodystrophy.
Another study by Goto et al, in which they identified crosssectional or cohort studies that investigated the association between chronic kidney disease, falls and fractures, concluded that the lower the glomerular filtration rate is associated with a higher risk of fracture, the most pronounced being hip fractures, this study agrees with ours in stating that chronic kidney disease is associated with skeletal lesions, developing the pathology known as osteodystrophy.
These studies are in agreement with each other in proposing chronic kidney disease as one of the main causes of the development of skeletal lesions by different mechanisms already mentioned. Strength of the current study is the methodology implemented, with respect to the literature search, and steps in the selection of relevant articles, quality assessment and data extraction. However, this study has several limitations, which should be taken into account before reaching a conclusion; among these are the little evidence of clinical trial analysis and studies that demonstrate the pathophysiological mechanisms by which to consider CKD as the trigger of all skeletal injuries, so more studies are needed to answer these questions.
REFERENCES
- Inker LA, Astor BC, Fox CH, Isakova T, Lash JP et al. (2012) clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63:713-735.
Indexed at
, Google Scholar
, Crossref
- Webster AC, Nagler EV, Morton RL, Masson P (2017) Chronic Kidney Disease. Lancet 389:1238-1252.
Indexed at
, Google Scholar
, Cross Ref
- Aeddula NR, Bardhan M, Baradhi KM (2021) Stat Pearls Publishing; Treasure Island (FL): Sickle. Cell Nephropathy.
Indexed at
, Google Scholar
- Khanna R (2011) Clinical presentation & management of glomerular diseases: haematuria, nephritic & nephrotic syndrome. Mo Med 108:33-36.
Indexed at
, Google Scholar
- Aeddula NR, Baradhi KM (2021) Stat Pearls Stat Pearls Publishing; Treasure Island (FL). J Reflux Nephro.
Indexed at
, Google Scholar
- Madero M, GarcÃa-Arroyo FE, Sánchez-Lozada LG (2017) Pathophysiologic insight into Meso American nephropathy. Curr Opin Nephrol Hypertens 26:296-302.
Indexed at
, Google Scholar
, Cross Ref
- Levey AS, Coresh J (2012) chronic kidney disease. Lancet. 379:165-1680.
Indexed at
, Google Scholar
, Cross Ref
- Johnson RJ, Nakagawa T, Jalal D, Sanchez-Lozada LG, Kang DH (2013) Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant 28:2221-2228.
Indexed at
, Google Scholar
, Crossref
- Aeddula NR, Cheungpasitporn W, Thongprayoon C, Pathireddy S (2019) Epicardial Adipose Tissue and Renal Disease. J Clin Med 8.
Indexed at
, Google Scholar
, Crossref
- Sen A, Callisen H, Libricz S, Patel B (2019) Complications of Solid Organ Transplantation: Cardiovascular, Neurologic, Renal, and Gastrointestinal. Crit Care Clin 35:169-186.
Indexed at
, Google Scholar
, Crossref
- Thongprayoon C, Chokesuwattanaskul R, Bathini T, Khoury NJ, Sharma K et al. (2018) Epidemiology and Prognostic Importance of Atrial Fibrillation in Kidney Transplant Recipients: A Meta-Analysis. J Clin Med 19:7.
Indexed at
, Google Scholar
, Crossref
- Ho LT, Sprague SM (2002) renal osteodystrophy in chronic renal failure. Semin Nephrol 22:488-493.
Indexed at
, Google Scholar
, Crossref
- Legg V (2005) Complications of chronic kidney disease: a close look at renal osteodystrophy, nutritional disturbances, and inflammation. Am J Nurs 105:40-49.
Indexed at
, Google Scholar
, Cross Ref
- Brandenburg VM, Floege J A (2008) dynamic bone disease-bone and beyond. NDT Plus 1:135-47.
Indexed at
, Google Scholar
, CrossRef
- Martin KJ, Olgaard K, Coburn JW, Coen GM, Fukagawa M (2004) Bone Turnover Work Group. Diagnosis, assessment, and treatment of bone turnover abnormalities in renal osteodystrophy Am J Kidney Dis. 43:558-565.
Indexed at
, Google Scholar
, Crossref
- Schwarz C, Sulzbacher I, Oberbauer R (2006) Diagnosis of renal osteodystrophy. Eur J Clin Invest. 2:13-22.
Indexed at
, Google Scholar
, Cross Ref
- Messa P, Macário F, Yaqoob M, Bouman K, Braun J et al. (2008) The Optima assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol 3:36-45.
Indexed at
, Google Scholar
, Crossref
- London GM (2009) Bone-vascular axis in chronic kidney disease a reality? Clin J Am Soc Nephrol 4:254-257.
Indexed at
, Google Scholar
, Crossref
- Li C, Chen XM, Li Y, Zhou YL, Yan JN (2019) Factors and Outcome of Renal Osteodystrophy-Associated Initial Fragility Fracture in End-Stage Renal Disease Patients. Kidney Dis 5:118-125.
Indexed at
, Google Scholar
, Crossref
- Ghimire S, Lee K, Jose MD, Castelino RL, Zaidi STR (2019) Adherence assessment practices in haemodialysis settings: A qualitative exploration of nurses and pharmacists' perspectives. J Clin Nurs 28:2197-2205.
Google Scholar
, Crossref
- Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A (2004) The burden of kidney disease: improving global outcomes. Kidney Int 66:1310-1314.
Indexed at
, Google Scholar
, Cross Ref
- Carol M, Jerry Y, Hartmut M, Rao DS, Monier-Faugere M C (2009) Relationship between bone histology and markers of bone and mineral metabolism in African-American haemodialysis patients. Clin J Am Soc Nephrol 4:1484-1493.
Indexed at
, Google Scholar
, Crossref
- Kazama JJ, Koda R, Yamamoto S, Narita I, Gejyo F (2010) Cancellous bone volume is an indicator for trabecular bone connectivity in dialysis patients. Clin J Am Soc Nephrol 5:292-298.
Indexed at
, Google Scholar
, Crossref
- K. Bembem, T Singgh, N Pal (2017)Â one Histo-Morphology in Chronic Kidney Disease Mineral Bone Disorder. Indian J Hematol Blood Transfus 33:603-610.
Indexed at
, Google Scholar
, Crossref
- Levin A, Bakris GL, Molitch M, Smulders M, Tian J (2007) Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71:31-38.
Indexed at
, Google Scholar
, Crossref
- H Hartmut W, Hanna M, Marie-Claude (2011) Renal Osteodystrophy in the First Decade of the New Millennium: Analysis of 630 Bone Biopsies in Black and White Patients. J Bone Miner Res 26:1368-1376.
Indexed at
, Google Scholar
, Crossref
Citation: Citation: Velasteguí JGL, Zurita ASV, López ACT, Hernández FAT, Gil LIN, et al. (2022) Chronic Renal Insufficiency and Its Relation to the Development of Skeletal Lesions. Health Sci J. Vol. 16 No. S6: 920.