Keywords
Bloodstream infections, length of stay, cost, Intensive Care Unit
Introduction
During the past decades, intensive care practice has become more complex, with increased use of invasive devices and immunosuppressive therapy, resulting to a two-fold to fivefold increase in the risk for healthcare associated infections (HAIs) [1]. Bacteremia is 1 of the 4 commonest types of HAIs, with an incidence approaching 17.4 per 1000 intensive care unit admissions [2].
Nosocomial bloodstream infections originating from central venous catheters (CVCs) continue to pose a significant problem for patients hospitalized in the United States and European intensive care units (ICUs), despite aggressive educational and behavior modification efforts [3-6]. Several millions of intravascular devices are purchased each year by hospitals and clinics as they are indispensable for administering lifesaving therapies to critically ill patients. However, their use may put patients at risk of local and systematic infectious complications, including both localized site infections and central line-associated bloodstream infections (CLABSI). Nearly 1 of 4 catheterized patients with a central line in place for an average of 8 days is expected to develop catheter colonization, which increases the risk of more serious bacteremia [7,8]. An estimated 80000 CVC associated nosocomial bloodstream infections (catheter related bloodstream infection [CRBSI]) occur annually in the United States. Although there is a debate whether these infections are independent risk factors for mortality, they are causing significant morbidity, increased length of hospital stay, and medical expense. Estimates on the cost of a single episode of CRBSI range from $4000 to $56000 due to increased length of hospital stay and excess cost related to therapy [9-10].
Decreasing the rate of CRBSI in critically ill patients requires a multifaceted and multidisciplinary approach including behavioral and educational interventions as well as the use of new technologies [11,12]. There have been reports of outbreaks of bloodstream infection traced to contaminated infucate associated with the use of open intravenous infusion systems [13-15]. The switching from an open to a closed infusion container resulted in lower CLA-BSI rates (3.5 vs. 8.2 CLA-BSI per 1000 central line) without posing additional burden on hospital budgets [16] and decreased significantly the ICU mortality from 22.0 to 16.9 deaths per 100 patients (P<0.001) [17].
Furthermore, the Center for Medicare and Medicaid Services (CMS) in the United States has recently discontinued hospital reimbursement for costs incurred as a result of nosocomial bloodstream infection. This ruling will alter the economic fundamentals of CRBSI prevention [18].
The objective of this study was to determine the crude excess mortality, the length of stay and the cost of antibiotics of patients with BSIs in two medical/ surgical ICUs.
Methodology
This prospective observational study was carried out in two medica/surgical ICUs, in Athens, Greece, from January to December 2010. The units are part of the referral center that serves mainly the region of Athens.
During the surveillance period, all patients admitted to ICUs and ventilated for at least 48 hours were actively monitored for BSIs until their discharge or death. It included daily clinical examination of patients and daily reviewing of the patient’s medical records and nursing charts. Data collected for each patient were recorded on a standardized survey record form. Patient data included demographic characteristics, disease severity determined by the Acute Physiology and Chronic Health Evaluation II (APACHEII) score on admission, co-morbidity determined by the weighted Charlson co-morbidity index on admission, date of BSI onset, type and duration administrated antibiotics. The diagnosis of BSIs was based on definitions proposed by Centers for Disease Control and Prevention (CDC) [19].
The study protocol was approved by the institutional review board at each hospital and patient confidentiality was protected.
For all patients standard laboratory methods were used to identify microorganisms. Antimicrobial susceptibilities were determined by disk diffusion method and an automated method (bioMerieux, Vitek II, France). Isolates resistant to imipenem and meropenem (minimum inhibitory concentration>8mg/L) were considered carbapenem-resistant regardless of their susceptibility to other antibiotics. Intermediately susceptible strains were accepted as resistant.
Adult patients hospitalized during the study period with BSI were considered cases, while uninfected patients were considered controls Comparisons were made between patients with BSI and patients admitted without HAI who did not acquire BSI in ICU (uninfected patients). Crude excess mortality in the ICU was defined as the difference between the crude overall case fatality of patients with BSI and that of patients admitted without HAI who did not acquire BSI in ICU during the same period. We also calculated the excess LOS and antibiotic cost subtracting average LOS and antibiotic cost of patients with BSI and uninfected patients.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median (interquartile range) in case of skewed data, while categorical variables are expressed as a percentage of the total number of patients analyzed. The Kolmogorov-Smirnov test and graphs (histograms and normal Q-Q plots) were used to test the normality of the distribution of the continuous variables. Differences between groups were evaluated using χ2 test or Fisher’s exact test for categorical variables and Student’s t test for continuous variables following a normal distribution or the Mann-Whitney U-test for those who failed the normality test. All tests of statistical significance were two-tailed, and p-values of less than 0.05 were considered significant. Statistical analysis was performed using the Statistical Package for Social Sciences software (IBM SPSS Statistics 20.0 for Windows, SPSS Inc., Chicago, IL, USA).
Results
During the study period, a total of 234 patients with 4551 patient days were hospitalized in two ICUs. The mean APACHE II score at admission of patients with BSI and without BSI was 16.78±5.74 and 15.99±5.25 respectively. The rates of diabetes melitus and coma on admission were greater among patients with BSI compared to patients without BSI as seen at table 1.
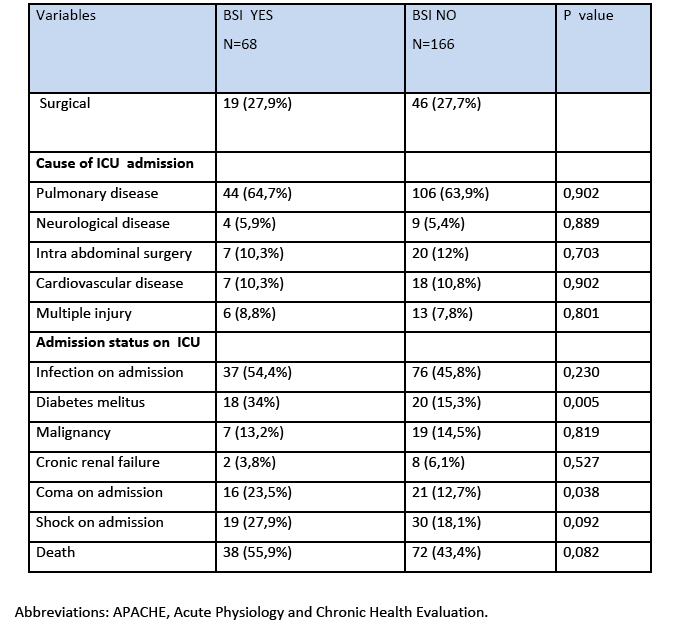
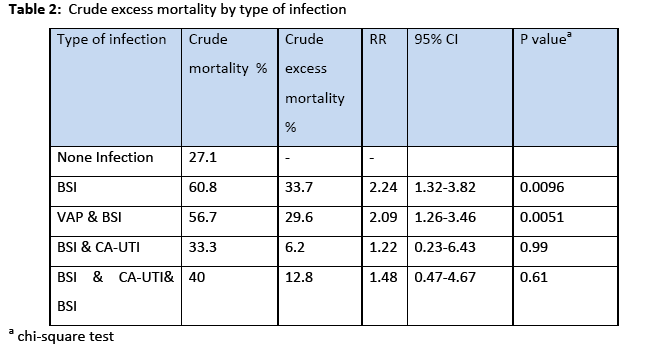
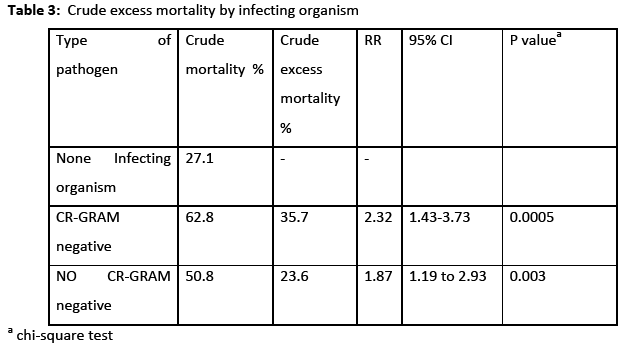
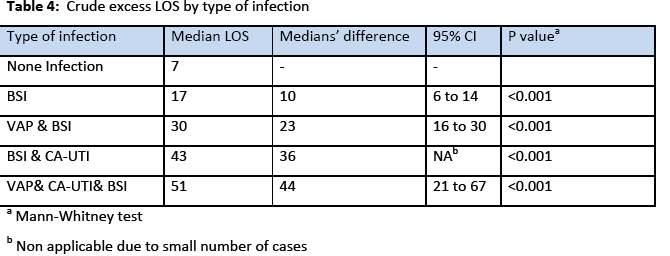
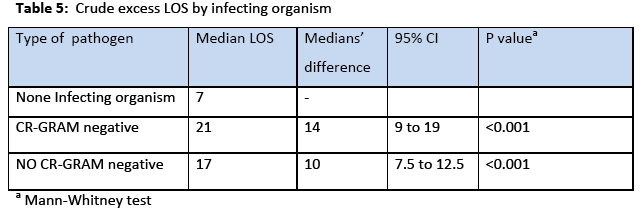
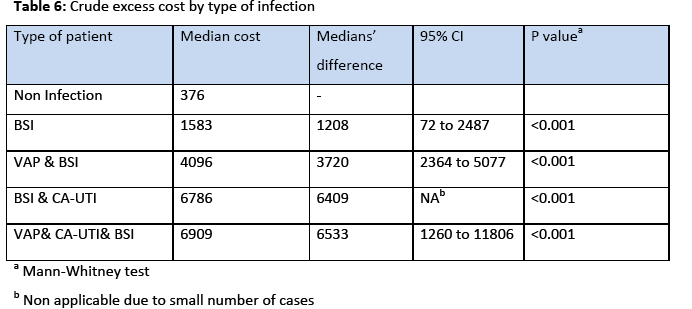
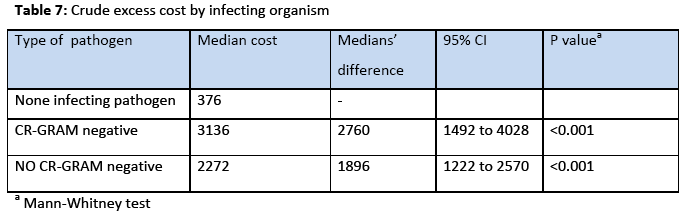
The incidence rate of BSI was 7.7% 29% and the incidence density rate was 14.9 cases/1000 patient days. Among the participants, 151 (64.5%) males and 83 (35.5%) were females. The crude unadjusted attributable mortality was 33.7% among patients with BSI and in patients with BSI caused by CR-CRAM negative pathogens was 35.7%, (Table 2 & 3). The crude unadjusted attributable LOS was 10 days among patients with BSI, and reached 44 days in patients with BSI &VAP& CA-UTI, while in patients with BSI caused by CR-GRAM negative pathogens and no CR-GRAM negative pathogens was 14 and 10 days, respectively, (Table 4 & 5). The crude unadjusted attributable antibiotics cost was 1208€ among patients with BSI and reached 6553€ in patients with BSI &VAP & CA-UTI, while in patients with BSI caused by CR-GRAM negative pathogens and no CR-GRAM negative pathogens was 2760€ and 1896€ , respectively, (Table 6 & 7). The overall excess antibiotic cost among patients with BSI was 3463.48€ per case.
Eight (11.8%) of the BSIs were polymicrobial, twelve (17.6) were secondary and fifty six (82.4%) were central line associated (CLA ) BSI. Overall, 82.3% of isolates were Gram-negative pathogens.
Forty three (76.8%) of fifty six Gram negative pathogens were resistant to Carbapenems.
The most frequently isolated pathogen was Acinetobacter baumannii (35,5%) following by Klebsiella pneumonia (25%).
Discussion
This study revealed that BSIs constitute a significant problem in our ICUs with an overall incidence rate 29% or density rate 14.9 cases/1000 patient-days which is considerably higher than those reported by NHSN report for 2010 and INICC report for 2004-2009 (1.1 and 6.8 per 1000 central line-days, respectively [20,21]. These differences could be attributed to many reasons: disease severity, limited funds and resources for infection control, insufficient numbers of experienced nurses, lack of closed infusion containers, and insufficient compliance with central line insertion bundles and catheter audit programs [11,22,23].
According to the Centers for Disease Control and Prevention (CDC ) report for 2001, 2008 and 2009 crude associated mortality rates of nosocomial bloodstream infection range from 12%-25%. This report provides national estimates of the number of CLA-BSI among patients in ICUs, inpatient wards, and outpatient hemodialysis facilities in 2008, and 2009 and compares ICU estimates with 2001 data [22]. This information was obtained from the approximately 260 hospitals participating in the National Nosocomial Infections Surveillance System (NNIS) in 2001 and the approximately 1,600 hospitals participating in National Healthcare Safety Network (NHSN) in 2009. Surveillance data reported to NNIS and NHSN are collected by trained personnel using standard methodologies and definitions [22].
In other studies [1,24] the mortality rate of BSI range from 12% to 80%, mostly depending on the microbial etiology involved, source of infection, appropriateness of antimicrobial therapy, type of underlying conditions, and associated organ failures. In the present study the crude unadjusted attributable mortality was 33.7% among patients with BSI and among patients with BSI caused by CR-CRAM negative pathogens was 35.68%. Our results highlight that the interventions to reduce the BSI and ICU mortality should be a priority in our ICUs. Additionally, focusing on antibiotic-resistant pathogens can be especially important given the increased risk for mortality associated with these pathogens [17].
Patients with CLA-BSI tend to stay longer in the ICU than patients who avoid infection. Although there is a debate whether these infections are independent risk factors for mortality, they are causing significant morbidity, increased length of hospital stay, and expense. Numerous estimates of the excess length of stay in hospital due to of CLA-BSI have been reported in the literature and range between 2.7 days [25] and 48.5 days [26]. Estimates on the cost of a single episode of CLA-BSI range from $4000 to $56000 due to increased length of hospital stay and excess cost related to therapy [10]. In accordance with these studies in our patients the crude excess LOS was 10 days among patients with BSI, and reached 44 days in patients with BSI &VAP& CA-UTI, while in patients with BSI caused by CR-GRAM negative pathogens and no CR-GRAM negative pathogens was 14 and 10 days.
Another interesting finding in the present study was the high crude excess antibiotics cost. Among patients with BSI crude excess antibiotics cost was 1208€ and reached 6533 € in patients with BSI &VAP &CA-UTI, while in patients with BSI caused by CR-GRAM negative pathogens and no CR-GRAM negative pathogens was 2760€ and 1896€, respectively. The excess antibiotic costs reported from developed and developing countries are significantly lower than costs found in our study. Laupland et al., [27] conducted a matched cohort study within the population of all critically ill patients in a large Canadian health region over a three year period and found that the excess antibiotics cost due to ICU acquired BSI was $288 (expressed as 2003 Canadian dollars). Huguera et al., [28] conducted a prospective matched analysis in adults ICUs in three hospitals in Mexico and found that the mean extra length of stay was 6.1 days and the mean extra cost of antibiotics was $598. Orsi et al., [29] conducted a retrospective cohort study in a university hospital in Italy and found that the extra antibiotic cost to laboratory confirmed BSI (expressed in Euros) was 943€. There has been dramatic variability in the estimates of excess costs associated with the development of BSI in the previous studies. There are a number of potential reasons for major variability among studies including small sample sizes, with resultant imprecise estimates in some reports, differences in patient populations and foci of infection studied, and varying healthcare costs for comparable services internationally and between regions in countries [29].
Additionally, studies of nosocomial infection have revealed that the use of antibiotics is increasing and contributes to increasing of antibiotics cost. The excess use of antibiotics has important consequences for patients in the ICU setting, for whom the risk of acquiring drug-resistant nosocomial pathogens may be higher than that for patients hospitalized in other settings [30]. Thus, prevention of CLA-BSI may reduce healthcare costs through reducing LOS, antibiotic use, and the antibiotic pressure that is driving the selection of resistant microorganisms in hospitals [31].
Finally, the overall excess antibiotic cost among patients with BSI was 3463.48€ per case. Because as many as 65%-70% of cases of BSI are preventable [32] the overall antibiotic cost attributable to BSI (170.769,72€) could have been reduced considerably. Our study shows the possible benefits, in terms of health and cost savings, when BSI is prevented.
In contrast to the medical-surgical ICUs participating in the NHSN, [33] A. baumannii, was the leading pathogen for CLA-BSI followed by K. pneumonia. Likewise, the antimicrobial resistance rates found in our ICUs for CR-GRAM negative pathogens were significantly higher than those reported in NHSN ICUs [36,33] and INICC ICUs [21].
Our results are important in clinical practice. Increasing evidence suggests that interventions other than antimicrobial catheters, including staff education, multifaceted infection control programs, and performance feedback and compliance with best practices may be useful for reducing risks of CLA-BSI in the ICU setting [11,34,35]. An emphasis on adherence to best practices including a combination of hand washing, full barrier precautions, chlorhexidine for skin antisepsis, avoiding femoral catheters when possible, and removing unnecessary catheters helped reduce median incidence of CLABSI to zero in one set of ICUs [11]. Associated with this intervention were the identification of physician and nurse team leaders and an overall ICU safety program. Nurse awareness and implementation of appropriate risk reducing strategies is important. The strategies that recommend are relatively low-cost, pose minimal risks for patients and constitute good nursing practice. Finally, good decision-making requires that the cost-effectiveness of all interventions decision making requires that the cost-effectiveness of all interventions be assessed and compared, but understanding their effectiveness is an important first step [36].
Our study suggests that the price of BSI is considerable and that neither patients, payers, nor hospitals benefit. Given that these infections are largely preventable, the elimination of CLA-BSI carries moral and economic imperative [37].
These results should be interpreted in the context of several potential limitations. First, our study did not include a sufficiently large number of patients. Second, this study was performed in two medical/surgical ICUs and our results might not be generalized to other hospitals, given differences in staffing, patient populations and resources.
In conclusion, our study confirms the clinical and economic burden of BSI in ICU patients and support allocation of scarce healthcare resources to infection prevention and control programs and quality improvement initiatives. The successful implementation of such programs would not only significantly reduce patient mortality but also result in considerable cost saving and reduce LOS.
2618
References
- Blot S, Cankurtaran M, Petrovit M, Vandijck D, Lizy C, Decruyenaere J, et al. Epidemiology and outcome of nosocomial bloodstream infection in elderly critically ill patients: A comparison between middle aged, old, and very old patients. Crit Care Med 2009;37(5):1634-1641.
- Suljagic V, Cobeljic M Javcovic S, Mirovic V,Marcovic-Denil L, Romic P, et al. Nosocomial bloodstream infections in ICU and non-ICU patients. Am J Infect Control 2005;33(6):333-340.
- Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med 2000;132(5):391-402.
- Coello R, Charlett A, Ward V, Wilson J, Pearson A, Sedgwick J, et al. Device-related sources of bacteraemia in English hospitals—opportunities for the prevention of hospital-acquired bacteraemia. J Hosp Infect 2003; 53(1):46-57.
- Van der Kooi TI, de Boer AS, Mannien J, Wille JC, Beaumont MT, Mooi BW, et al. Incidence and risk factors of device-associated infections and associated mortality at the intensive care in the Dutch surveillance system. Intensive Care Med 2007;33(2):271-278.
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004;39(3):309-31714.
- McGee DC, Gould MK: Preventing complications of central venous catheterization. N Engl J Med 2003; 348(12):1123-1133.
- Mermel LA, Farr BM, Sherertz RJ, Raad II, O’Grady N, Harris JS, et al Guidelines for the management of intravascular catheter-related infections. J Intraven Nurs 2001; 24(3):180-205.
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection n adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006;81(9):1159-1171.
- Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1994;271(20):1598-1601.
- Pronovost P, Needham D, Berenholtz S. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355(26):2725-2732.
- Yilmaz G, Caylan R, Aydin K, Topbas M, Koksal I. Effect of education on the rate of and the understanding of risk factors for intravascular catheter-related infections. Infect Control Hosp Epidemiol 2007;28(6):689-694.
- Garrett DO, McDonald LC, Wanderly A. An outbreak of neonatal deaths in Brazil associated with contaminated intravenous fluids. J Infect Dis 2002;186(1):81-86
- Moore KL, Kainer MA, Badrawi N. Neonatal SEPSIS in Egypt associated with bacterial contamination of Glucose-containing intravenous fluids. Pediatr Infect Dis J 2005;24(7):590-594.
- Macias AE, Munoz JM, Bruckner DA. Parenteral infusions bacterial contamination in a multi-institutional survey in Mexico: considerations for nosocomial mortality. Am J Infect Control 1999;27(3):285-290.
- Tarricone R, Torbica A, Franzetti F, Rosenthal V. Hospital costs of central line-associated bloodstream infections and cost-effectiveness of closed vs. open infusion containers. The case of Intensive Care Units in Italy. Cost Effectiveness and Resource Allocation 2010; 8:8 Available froma:https://www.resource-allocation.com/content/8/1/8.
- Maki D, Rosenthal V, Reinaldo S, Franzetti F. Impact of Switching from an open to a closed infusion system on rates of central line associated bloodstream infection: A metanalysis of time Sequence cohort studies in 4 countries. Infect Control Hosp Epidemiol 2011;32(1)50-58.
- Pronovost PJ, Goeschel CA, Wachter RM. The wisdom and justice of not paying for ‘‘preventable complications.’’ JAMA 2008;299(18):2197-2199.
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36 (5):309-32.
- Dudeck M, Horan T, Peterson K, Allen-Bristol K, Morrell G, Pollock D, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control 2011;39(10):798-816.
- Rosenthal V, Bijie H, Maki D, Mehta Y, Apisarnthanarak A, Medeiros E, et al. International nosocomial infection control consortium report data summary of 36 countries, for 2004-2009. Am J Infect Control 2012;40(5):396-407.
- Centers for Disease Control and Prevention. Vital Signs: Central line-associated Bloodstream infections-United States, 2001, 2008, and 2009. MMWR 2011;60 (8):243-247.
- Elliott TS, Faroqui MH, Tebbs SE, Armstrong RF, Hanson GC: An audit programme for central venous catheter-associated infections. J Hosp Infect 1995;30(3):181-191.
- Gastmeier P, Menzel K, Sohr D. Usefulness of severity-of illness scores based on admission data only in nosocomial infection surveillance systems. Infect Control Hosp Epidemiol 2007;28(4):453-454.
- Beyersman J. Gastmer P, Grundmann H. Use of multistate models to assess prolongation of intensive care unit stay due to nosocomial infection. Infect Control Hosp Epidemiol 2006;27(5):493-495.
- Stone PW, Gupta A, Loughter M. Attributable costs and length of stay of an extended-spectrum beta-lactamase-producing Klebsiella pneumonia outbreak in a neonatal intensive care unit. Infect Control Hosp Epdemiol 2003;24(8):601-606.
- Laupland K, Lee H, Gregson D, Manns B. Cost of intensive care unit acquired bloodstream infections. J Hosp Infect 2006;63(2):124-1322.
- Higuera F, Rosenthal V, Soto J, Castanon J, Tabal-Galan N, Ruiz J, et al. Atributable cost and length of stay for patients with central venous catheter associated blood stream infection in Mexico city ICUs: a prospective, matched analysis. Infection Control Hospital Epidemiol 2007;28 (1):31-35.
- Orsi G, Stefano L, Noah N. Hospital acquired laboratory confirmed bloodstream infection: Increased hospital stay and direct costs. Infect Control Hosp Epidemiol 2002;23(4):190-197.
- Kollef MH. Antibiotic use and antibiotic resistance in the intensive care unit. Are we curing or creating disease? Heart Lung 1994;23:363-367
- Crnich CJ, Maki DG. The promise of novel technology for the prevention of intravascular device-related bloodstream infection. II. Long-term devices. Clin Infect Dis 2002;34(10):1362-1368.
- Umscheid C, Mitchell M, Doshi J, Agarwal R, Williams K , Brennan P. Estimating the proportion of Healthcare-associated infections are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol 2011;32(2):101-114.
- Hidron A, Edwards J, Patel J, Horan T, Sievert D, Pollock D, et al. Antimicrobial-resistant pathogens associated with healthcare associated infections: Annual summary of data report to the national health care safety network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol 2008;29(11):996-1011.
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection n adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 2006;81(9):1159-1171.
- Walz J, Memtsoutis S, Heard S. Prevention of central venous catheter bloodstream infections. Journal of Intensive Care Medicine 2009;25(3):131-138.
- Ramritu P, Halton K, Cook D, Whitby M, Cravens N. Catheter related bloodstream infections in intensive care units: a systematic review with metanalysis. JAN 2008;62(1):3-21.
- Shannon R, Patel B, Cummins D. Shannon A, Ganguli G. Economics of central line associated bloodstream infections. Am J Med Quality 2006;26(6):7-16












