Zohre Soltani1, Rasool Ghorbani1, Seyed Aliakbar Hedayati1, Hamed Ghafari Farsani2, Mohammad Hasan Gerami3*
1Department of Fisheries, Gorgan University of Agricultural and Natural Resources, Gorgan, Iran
2Young Researchers and Elite Club, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran
3Young Researchers and Elite Club, Shiraz Branch, Islamic Azad University, Shiraz, Iran
Corresponding Author:
Mohammad Hasan Gerami
Young Researchers and Elite Club
Shiraz Branch, Islamic Azad University, Shiraz, Iran
Tel: +98 9122884418
E-mail: m.h.gerami@gmail.com
Received Date: 18.05.2016; Accepted Date: 04.06.2016; Published Date: 15.06.2016
Keywords
Fish; Nanometals; Toxic Concentration; Hematology
Introduction
Heavy metals are highly accumulated in aquatic ecosystems in recent years and considered the most important threat for aquatic animals (Mance, 2012). Understanding the effect of these toxicants on aquatic animals supports the larger ecotoxicological goal of comprehending the action of ecotoxicans on their population (Bols et al., 2001). Fish are one of the most widely distributed organisms in the aquatic environment, which faced with various discharged wastewater; especially heavy metals (Klaverkamp et al., 1984). Zinc, an essential element, is one of the most common heavy metal pollutants which is widely used in industry (Kori-Siakpere and Ubogu, 2008). In addition, todays with increasing nanotechnology; zinc oxide nanoparticles (ZnO-NPs) is one of the most abundantly used nanomaterial in consumer products and biomedical applications due to its specific properties, e.g. transparency, high isoelectric point, biocompatibility, and photocatalytic efficiency (Darroudi et al., 2013). Therefore, Large-scale production and consumption of this metal will result in discharge of the products containing nanozinc to aquatic ecosystems and agricultural lands (Chatterjee, 2008; Fabrega et al., 2011).
Zn toxicity to fishes is well known and studies revealed that high concentrations of zinc in water resources exerts adverse effect in fish such as accruing structural damage, affecting the growth development and survival, depressive effect on tissue respiration leading to death by hypoxia, changes in ventilatory and heart physiology, and adverse effect on hematological indices of fishes (Crespso et al., 1979; De Schamphelaere and Janssen, 2004; Kori-Siakpere and Ubogu, 2008). Many researchers studied the ecotoxicity of nanomaterials to aquatic ecosystems (Moore, 2006; Handy et al., 2008; Klaine et al., 2008; Handy et al., 2011; Khabbazi et al., 2014a; 2014b). However, the environmental impacts of Zn nanoparticles (NPs) are, as yet, unknown and data on hematological indices from Zn-NPs in Capoeta capoeta gracilis (khramulya) are generally lacking. In addition, the relative hazard of hematology from nano forms of Zn compared to traditional metal salts is unknown.
C. capoeta gracilis, one of the subspecies of the genus Capoeta, is distributed throughout southwest, south and central Asia. This subspecies is a popular taxon in the south Caspian Sea basin of Iran (Abdoli, 2000). Although, this species not exploited commercially; but it has high ecological role in the Caspian Sea basin ecosystems and food webs (Patimar, 2008). This species suffers from habitat loss due to anthropogenic emissions of Zn in rivers and aquatic ecosystems. Therefore, the aim of this study is to determine the effects of dissolved Zn sulfate and Zn-NPs on the hematological indices of this species following waterborne exposure to these materials to test the adverse effect of this material on C. capoeta gracilis. Another goal of the current study is to compare the effects of Zn metal with Zn-NPs, to identify any nano-specific hematological on this species in contrast with Zn metal form.
Materials and Methods
Number of 100 live specimens of C. capoeta gracilis was obtained from Zarrin Gol River in Gorgan Province, Iran. Fish were transferred to the laboratory in Gorgan University of Agricultural and Natural Resource and accumulated randomly in 100 L aquariums for one week to reduce mortality rate due to handling stress.
Toxicity test
Acute toxicity test were performed according to O.E.C.D (Organisation for Economic Co-operation and Development, 1993) and Probit analysis to assess sublethal toxicity of Zn for this species. Furthermore, due to ethical statements, minimum number of 6 samples per replicate and trial (6×4×3, 72 total) with mean weight 15 ± 3.5 gr were exposed for 14 days to sublethal toxicity of 5, 20 and 200 ppm zinc sulfate and zinc nanoparticles and one control group (no toxicant) with three replicate. There were no significant differences between aquariums in water quality and the following characteristics were constant: pH: 7.56 ± 0.45 (TS1); temperature: 19 ± 1°C; hardness: 293 ± 2.35 ppm and dissolved oxygen: 7.80 ± 1 mg L-1 (DO-5510 Oxygen meter). 50% of the aquarium water siphoned for fecal removing every 12 h and Zn/ ZnSo4 concentration re-dosed after each water change based on half of toxicant stock in each treatment tank. The photoperiod was 12 h light and 12 h dark. Zn Nano colloid was purchased from Nonaka Company, Iran (Antimicrobial Product 2 brand, mean particle size of 20-30 nm, Figure 1) and the colloid solved into the water by using ultrasound machine. In addition, zinc sulfate powder was purchased from SIGMA-ALDRICH Company (ZnS, Density 4.1 g/mL).
Figure 1: Particle size of Zn nanoparticles used in this study (The scale is 2.0 nm)
4 samples per trial and replicate (total 48) were used for blood sampling process. After 14 days experiment, blood samples were collected by cutting caudal fin and using capillary tube and stored in ice. EDTA used to prevent blood clotting. Afterwards, blood tubes were centrifuged for 5 minutes for blood plasma fractionation. Hematological parameters were estimated according to routine clinical methods (Wintrobe, 1978). The acid-hematin method of Sahli in hemometer was used to analyze hemoglobin percentage and Naeubaur’s double hemocytometer was used to enumerate the erythrocytes (Mukherjee, 1988). Mean corpuscular volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC) were calculated based on Decie and Lewis (Decie and Lewis, 1991). One-way analyses of variance (ANOVA) were used to analyze hematological parameters. Differences between means were determined using Duncan’s multiple range tests at 5% probability level.
Results
No mortality observed during the accumulation test and control group. Results showed a decreasing trend in hematocrit, RBC, MCHC and hemoglobin with increasing in toxicant concentration while MCV and MCH both increased by concentration. Differences between nano and sulfate group was significant in 5 and 20 ppm concentration for hematocrit and RBC, and all concentrations for hemoglobin and MCHC. In addition, analysis of WBC showed that this parameter increased by toxicant concentration both in nano and sulfate group and was significantly difference between groups (Figure 2). Difference between other hematological parameters in nano Zn and Zn sulfate were represented in Figure 2.
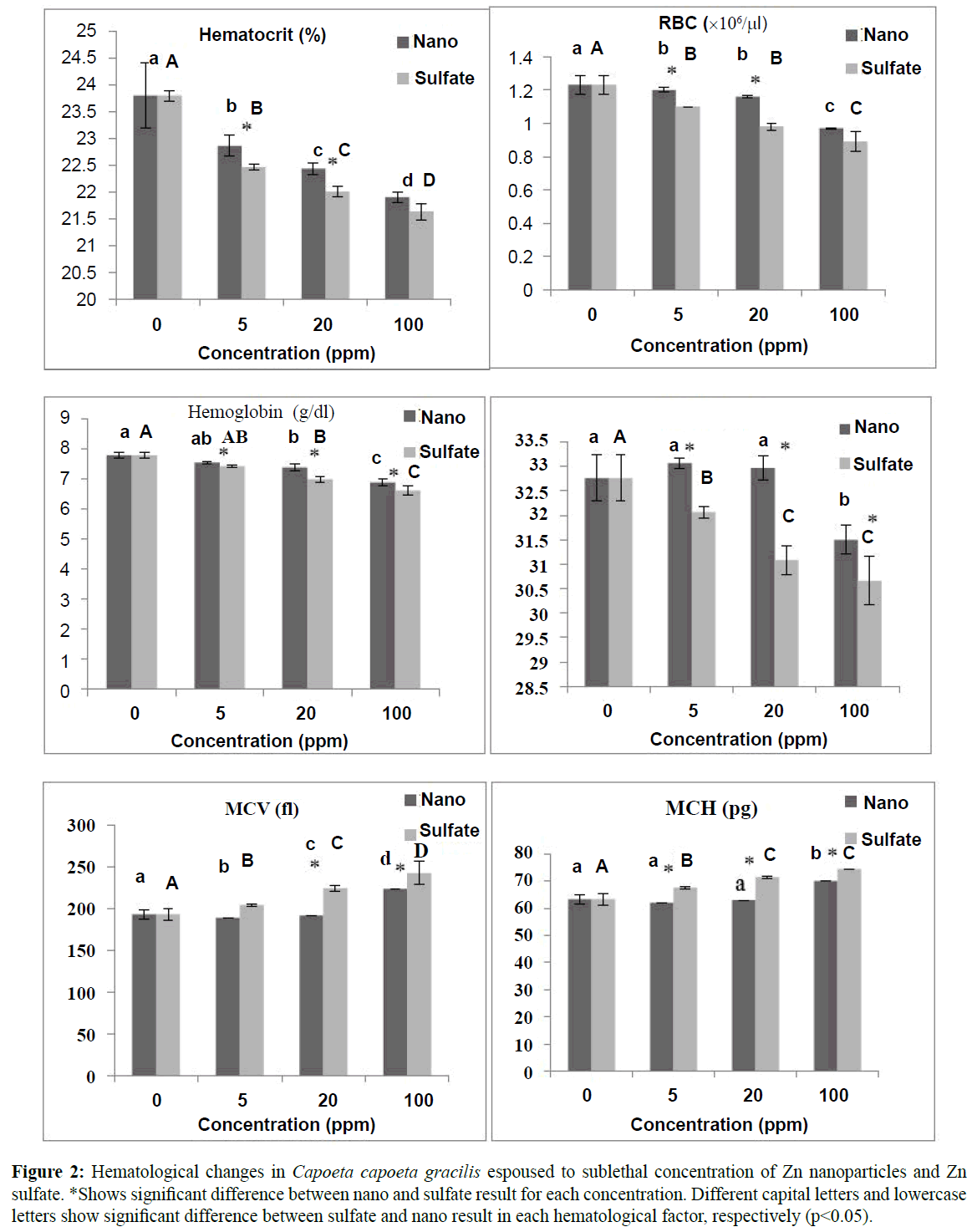
Figure 2: Hematological changes in Capoetacapoetagracilis espoused to sublethal concentration of Zn nanoparticles and Zn sulfate. *Shows significant difference between nano and sulfate result for each concentration. Different capital letters and lowercase lettersshow significant difference between sulfate and nano result in each hematological factor, respectively (p<0.05).
Discussions
Changes in the quantitative and qualitative characteristics of blood cells occur when anomalies in blood components interfere with normal functions (Landis and Yu, 2004). Results of this study showed that both form of Zn in this study, had destructive effect on hematological parameters of C. capoeta gracilis.
All metal exposures induced changes in fish blood indicating stress (Witeska and Kosciuk, 2003). In this study, hematocrit and hemoglobin decreased after exposer to Zn. These reduction due to Zn toxicity were also observed by Annune et al. (1994) and Kori-Siakpere and Ubogu (2008) for Heteroclarias species. Both hemoglobin and hematocrit are associated with RBC variation in fish blood. Haemoconcentration and haemodilution were observed in this study for both Zn form. Reduction of RBC after exposure to heavy metals was reported by Kori-Siakpere and Ubogu (2008) for Heteroclarias sp. and Neumosok and Hughes24 for Colis fasciatus after espouser to sublethal toxicity of zinc. Witeska and Kosciuk (2003) stated that heavy metals might alter the properties of hemoglobin by decreasing their affinity towards oxygen binding capacity rendering the erythrocytes more fragile and permeable. This process could deform cell swelling and damage erythrocytes. In addition, the perturbation RBCs may be attributed to a defense reaction against toxicity through the stimulation of erythropoiesis (Maheswaran, 2008).
In the values obtained in the hematological indices, MCV increased and MCH decreased significantly after exposer to zinc (Figure 2). A similar observation was made for Cyprinus carpio after cadmium exposure (Koyama and Ozaki, 1984) and Heteroclarias sp. after exposure to zinc. Adeyemo (2005) stated that hypoxia or microcytic anaemia after exposure to toxicant could cause red blood cell shrinking and MCV fluctuation. In addition, fluctuation in the MCH clearly indicates that the concentration of hemoglobin in the red blood cells were much lower in the exposed fish than in the control fish, thereby, depicting an anaemic condition (Adeyemo, 2005). Results showed significant decrease in the MCHC especially for sulfate group after exposure to zinc. This is probably an indication of red blood cell swelling and /or to a decrease in hemoglobin synthesis. Buckley et al. (1976) stated that prolonged reduction in hemoglobin content is deleterious to oxygen transport and any blood dyscrasia and degeneration of the erythrocytes could be ascribed as pathological conditions in fishes exposed to toxicants.
Significant increasing in WBC of C. capoeta gracilis after exposure to zinc were also observed by Kori-Siakpere and Ubogu (2008). Many researchers reported reduction in WBC after exposure to heavy metals in fishes (Remyla et al., 2008; Shah and Altindag, 2005). Changes in white blood cell count suggest dysfunction in hematological tissues (spleen and kidney) or certain infectious diseases (Khabbazi et al., 2014b). In fact, increasing or decreasing numbers of white blood cells are a normal reaction to a chemical such as zinc and demonstrating the effect of the immune system under toxic conditions. However, results showed significant decrease in lymphocyte especially in sulfate group (Figure 2). Witeska (2005) stated that cortisol secreted during stress reaction shortens the life span of lymphocytes, promotes their apoptosis and reduces their proliferation. Lymphocyte counts lower than normal (lymphopenia) can be an indicator of immune system deficiency and poisonous substance treatments can also deplete the body’s supply of lymphocytes (Banaee et al., 2008). In addition, Banaee et al. (2008) stated that most infections cause neutrophilia. The degree of elevation often indicates the severity of the infection. Analysis showed that neutrophil increased significantly after long-term espouser especially for sulfate group.
Results showed that there were differences in destructive effect of zinc nanoparticles and zinc sulfate on C. capoeta gracilis hematological indices. Figure 2 shows that toxicity of zinc sulfate decreased RBC more that nano form. This shows that zinc sulfate more destroyed RBC, and also altered their shape and size by effecting the structure and function of cell membrane. Hematocrit and hemoglobin are directly influenced by fluctuation of RBC. Results showed that these two indices were also more decreased in sulfate form. Furthermore, there were significant differences between number of WBCs in blood samples of nano and sulfate group. Results indicated that WBCs were more increased in sulfate group that nano group which indicated zinc sulfate had more tissue damage effect on C. capoeta gracilis. Data on comparison of nanometals versus metal ions are scarce but less severity of nano forms is well known (Handy et al., 2008; Shaw et al., 2012; Al-Bairuty et al., 2013).
Shaw and Handy (2011) declared that nanometals may not be as acutely toxic as dissolved metals. In fact, the mechanisms of uptake of nanometals are likely to be very different to dissolved metals. This process causes differences in severity of adverse effect of between nanometals and dissolved metals. In addition, metal ions have more ability to accumulate in tissue than nanoparticles. This phenomenon results more injury from metal ions. Shaw et al. (2012) studied on the adverse effect of copper nanoparticles and copper sulphate on rainbow trout and declared that the accumulation of Cu in the gills of fish exposed to CuSO4 is 2-fold grater that Cu nanoparticles. They also stated that while the final toxic responses may be of similar types, the mechanisms and severities driving the toxicity are different for the two forms of Cu and adverse effect of nano forms are less than the equivalent metal salts. Moreover, Shaw and Handy (2011) declared that the toxicity degree of nano and dissolved metals may be driven by the surface chemistry of the particles. These ideas are not yet proven by experimental data, but for example, one might expect a particle with an oxidising metal surface to cause oxidative stress on/in the organism. Nanoparticles have less surface area than their ion forms. These characteristic results to different solubility in water and consequently uptake level in aquatic animals. Perhaps, dissolved metals forms aggregate more than nano forms on gills and therefore, cause more stress and infectious in fishes. However, more studies needs to prove this claim.
In conclusion, this study showed the degree of hematological changes in C. capoeta gracilis after exposure to zinc nanoparticles and zinc sulfate. Analysis showed that the severity of these changes were less in Nano group. However the mechanism of these changes in destructive effect is not recognized and needs further studies.
Ethics Statement
All experiments performed on fishes in this study complied with Society of Toxicology (code of ethics January 31, 1985; Revised June 1, 2005; Reviewed and Reaffirmed September 14, 2011; Revised November 5, 2012) and Canadian Council on Animal Care (CCAC, 1998). All analyses and experiments were performed to minimize suffering. Study was conducted with minimal number of fish.
Acknowledgement
Authors are thankful from Gorgon University of Agricultural and Natural Resource for financial support.
9873
References
- Abdoli, A.(2000) The inland water fishes of Iran. Iranian Museum of Nature and Wildlife, Tehran. (in Farsi, English abstract)
- nAdeyemo, O.K.(2008) Haematological and histopathological effects of Cassava Mill Effluent in Clariasgariepinus, African Journal of Biotechnology, 8, 179-183
- nAl-Bairuty, G.A., Shaw, B.J., Handy, R.D., Henry, T.B.(2013) Histopathological effects of waterborne copper nanoparticles and copper sulphate on the organs of rainbow trout (Oncorhynchusmykiss), Aquatic Toxicology, 126, 104-115
- nAnnune, P.A., Lyaniwura, T.T., Ebele S.O., Olademeji, A.A.(1994) Effects of Sublethal Concentrations of Zinc on Haematological Parameters of Water Fishes. Clariasgariepinus (Burchell) and Oreochromisniloticus (Trewawas), Journal of Aquatic Science,9, 1-6
- nBanaee, M., Mirvagefei, A.R., Rafei, G.R., MajaziAmiri, B. (2008) Effect of sub-lethal Diazinon Concentrations on Blood Plasma Biochemistry, International Journal of Environmental Research, 2: 189-198
- nBols, N.C., Brubacher, J.L., Ganassin, R.C., Lee, L.E.G.(2001) Ecotoxicology and innate immunity fish. Developmental & Comparative Immunology,25, 853-873
- nBuckley, J.A., Whitmore, C.M., Matsuda, R.I. (1976) Changes in blood chemistry and blood cell morphology in coho salmon, Oncorhynchuskisutch following exposure to sublethal levels of total residual chlorine in municipal wastewater, Journal of Fisheries Research Board of Canada,33: 776-782
- nChatterjee, R. (2008). The challenge of regulating nanomaterials.Environmental Science & Technology, 42, 339–343
- nCrespso, S., Flos, B., Balasch-Alonso, G. (1979) Zinc in the gills of dogfish (Scychorhinuscanicula) related to experimental aquatic zinc pollution, Comparative Biochemistry and Physiology,63, 261 -266
- nDarroudi, M., Sabouri, Z., KazemiOskuee, R., Kargar, H., Hosseini, H.A. (2013) Neuronal toxicity of biopolymer-template synthesized ZnO nanoparticles, Nanomedicine Journal,1, 88-93
- nDe Schamphelaere, K.A., Janssen, C.R. (2004) Bioavailability and chronic toxicity of zinc to juvenile rainbow trout (Oncorhynchusmykiss): comparison with other fish species and development of a biotic ligand model, Environmental Science & Technology,38, 6201-6209
- nDecie, S.I.V., Lewis, S.M. (1991) Practical Hemotology, 7th Edition. Churchill Livingstone, London/Melbourne/New York
- nFabrega, J., Luoma, S.N., Tyler, C.R., Galloway, T.S., Lead, J.R. (2011) Silver nanoparticles: Behavior and effects in the aquatic environment, Environment International37, 517-531
- nHandy, R.D., von der Kammer, F., Lead, J.R., Hassellöv, M., Owen, R., Crane, M. (2008) The ecotoxicology and chemistry of manufactured nanoparticles, Ecotoxicology,17, 287-314
- nHandy, R.D., Al-Bairuty, G., Al-Jubory, A., Ramsden, C.S., Boyle, D. et al, (2011) Effects of manufactured nanomaterials on fishes: a target organ and body systems physiology approach, Journal of Fish Biology,79, 821-853
- nHandy, R.D., Henry, T.B., Scown, T.M., Johnston, B.D., Tyler, C.R., (2008). Manufactured nanoparticles: their uptake and effects on fish—a mechanistic analysis, Ecotoxicology,17, 396-409
- nKhabbazi, M., Harsij, M., Hedayati, S.A., Gerami, M.H., GhafariFarsani, H. (2014a) Histopathology of rainbow trout gills after exposure to copper, Iranian Journal of Ichthyology, 1, 191-196
- nKhabbazi, M., Harsij, M., Hedayati, S.A., Gholipoor, H., Gerami, M.H. et al. (2014b) Effect of CuO nanoparticles on some hematological indices of rainbow trout Oncorhynchusmykiss and their potential toxicity, Nanomedicine Journal,2, 67-73
- nKlaine, S.J., Alvarez, P.J.J., Batley, G.E., Fernandes, T.F., Handy, R.D. et al. (2008) Nanomaterials in the environment behavior, fate, bioavailability, and effects, Environmental Toxicology and Chemistry,27, 1825-1851
- nKlaverkamp, J.F., MacDonald, W.A., Duncan, D.A., Wagemann, R. (1984). Metallothionein and accumulation to heavy metals in fish: a review. Contaminant Effects on Fisheries, edited by Cairns, VW Hodson, PV and Nriagu, JO, Wiley, New York
- nKori-Siakpere, O., Ubogu, E.O., (2008) Sublethalhaematological effects of zinc on the freshwater fish, Heteroclarias sp.(Osteichthyes: Clariidae), African Journal of Biotechnology7, 2068-2073
- nKoyama, J., Ozaki, H. (1984) Haematological changes in fish exposed to low concentrations of cadmium in the water, Bulletin of the Japanese Society for the Science of Fisheries,50, 199-203
- nLandis, W.G., Yu, M. (2004) Introduction to Environmental Toxicology.Crc Press
- nMaheswaran, R., Devapaul, A., Muralidharan, S., Velmurugan, B., Ignacimuthu, S. (2008). Haematological studies of freshwater fish, Clariasbatrachus (L.) exposed to mercuric chloride, International Journal of Integrative Biology,2, 49-54
- nMoore, M.N., (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? EnvironmentInternational,32, 967-976
- nMukherjee, K.L. (1988) Medical Laboratory Technology. Aprocedure manual for routine diagnostics tests, Vol I., Tata-McGraw- Hill, New Delhi
- nNeumosok, J.G., Hughes, G.M. (1998) The effect of Copper Sulphate on some biochemical parameters rainbow trout, EnvironmentalPollution,49, 77- 85
- nOECD (Organisation for Economic Co-operation and Development), OECD Guidelines for Testing of Chemicals OECD, Organization for Economic. Paris 1993
- nPatimar, R. (2008) Fish species diversity in the lakes of Alma-Gol, Adji-Gol, and Ala-Gol, Golestan province, northern Iran, JournalofIchthyology,48, 911-917
- nRemyla, S.R., Ramesh, M., Sajwan, K.S., Kumar, K.S. (2008) Influence of zinc on cadmium induced haematological and biochemical responses in a freshwater teleost fish Catlacatla, Fish Physiology and Biochemistry, 34, 169-174
- nShah, S.L., Altindag, A. (2005) Alterations in the immunological parameters of Tench (Tincatinca L. 1758) after acute and chronic exposure to lethal and sublethal treatments with mercury, cadmium and lead, Turkish Journal of Veterinary and Animal Science, 29, 1163-1168
- nShaw, B.J., Handy, R.D. (2011) Physiological effects of nanoparticles on fish: a comparison of nanometals versus metal ions, Environmental International, 37, 1083-1097
- nShaw, B.J., Al-Bairuty, G., Handy, R.D. (2012) Effects of waterborne copper nanoparticles and copper sulphate on rainbow trout, (Oncorhynchusmykiss): physiology and accumulation, AquaticToxicology,116, 90-101
- nWintrobe, M.M.(1978)Clinical Hematology. Kipton, London. Zar, J.H., 1974. Biostatistical Analysis.Prentice-Hall, Engelwood Cliffs, NJ
- nWiteska, M.(2005)Stress in fish—hematological and immunological effects of heavy metals, Electronic Journal of Ichthyology,1, 35-41
- nWiteska, M., Kosciuk, B. (2003) Changes in common carp blood after short-term zinc exposure, Environmental Science and Pollution Research, 3, 15-24
- nMance, G. (2012) Pollution threat of heavy metals in aquatic environments. Springer Science and Business Media.








