Ahmed F. Basyony1, Mohammad M. Aboulwafa2*, Mohamed M. Hafez2, Khalid A. Abou Gazia3
1Department of Microbiology and Immunology, Faculty of Pharmacy, Modern Sciences and Arts University
2Microbiology and Immunology Department, Faculty of Pharmacy, Ain Shams University
3Department of Microbiology and Immunology, Animal Reproduction Research Institute, Agriculture Research Center, Egypt
*Corresponding Author:
Mohammad M. Aboulwafa
Department of Microbiology and Immunology
Faculty of Pharmacy, Ain Shams University
Al Khalifa Al Maamoun St., Abbassia, Cairo, Egypt
Tel: (202)24051107
Mobile: (02)0102350371
E-mail: maboulwafa@yahoo.com
Background: Brucellosis is a zoonotic disease that affects wild and domestic animals causing a decrease in reproductive efficiency and abortion and can be transmitted to human. The incidence of human disease is closely tied to the prevalence of infection in animals and considered as an important health problem in Egypt.
Methods and Findings: In this study, blood specimens from 68 patients showed clinical signs and/or history of brucellosis and from different investigated animals (76 buffalo, 145 cattle and 191 sheep) were collected and serodiagnosed for Brucella infection. The sera of these blood specimens were first screened by rose bengal plate test (RBPT) and those giving positive reaction were retested by the standard tube agglutination test (SAT), EDTA modified SAT and rivanol test to determine their titers. The results for clinical specimens showed that 89.7%, 82.35%, 66.18% and 58.82% were positive using RBPT, SAT, EDTA modified SAT and rivanol test, respectively. The respective percentages of brucellosis in buffalo were 44.7%, 43.42%, 43.42% and 43.24%; while the respective percentages of brucellosis in cattle were 46.9%, 43.45%, 39.31% and 37.93%. In addition, serological examination of 191sheep revealed that 60.2%, 56.54%, 53.4% and 51.83% were positive using RBPT, SAT, EDTA modified SAT and rivanol test, respectively.
Conclusion: The results give clear evidence for: (i) the real picture of brucellosis surveillance among human cannot be reflected using single serodiagnostic test, (ii) In comparison to human, serodiagnosis of Brucella among animals are less dependent on test type and such dependency took the order sheep > cattle > buffalo, (iii) serodiagnosis of Brucella among buffalo had nearly no dependency on test type.
Introduction
The genus Brucella is aerobic, facultative intracellular, Gram negative coccobacilli [1]. The main pathogenic species worldwide are B.abortus, B.melitensis and B.suis which cause abortion in their natural hosts resulting in huge economic losses. They also account for most cases of human brucellosis [2]. Brucellosis is a zoonotic disease that is widely distributed throughout the developing world and has been recognized as a global problem of wild and domestic animals causing a decrease in reproductive efficiency and abortion [3]. The incidence of human disease is closely tied to the prevalence of infection in animals with half a million of new human cases reported annually worldwide [4] and considered as an important health problem in Egypt and an important cause of acute febrile illness (AFI) [5-7]. Diseased animals excrete Brucella through the urine, milk, placenta and the products of miscarriages. In this way, the bacteria disseminated and infect other animals and humans [8]. Transmission of the infection to humans occurs following direct contact with infected animals and their secretions during septic abortion or at the time of slaughter. Infection can occur via injured skin, inhalation or inoculation into the conjunctival sac of the eyes. Foodborne infection is more frequently via the ingestion of unpasteurized dairy products [9-11]. Acute signs and symptoms of human brucellosis mainly include undulating fever, sweats, headache, myalgia, anorexia, back pain, fatigue and other clinical manifestations such as splenomegaly, hepatomegaly and spondylitis [12]. Complications of human brucellosis may include infective endocarditis [13], splenic, liver and pulmonary abscesses [14] with splenomegaly or hepatomegaly [15], osteoarticular manifestations, genitourinary complications, neurological findings, mucocutaneous manifestations [16], deep vein thrombosis [17], meningitis [18], nephritis [19] and ocular manifestations [20]. Brucella bacteremia can result in abortion in pregnant women, especially during the early trimesters. Abortion is a frequent complication of brucellosis in animals, where placental localization is believed to be associated with erythritol, a growth stimulant for Brucella [21]. Although not routinely diagnosed, brucellosis is reported in all domestic animals in the Near East region, including Egypt. The highest incidence of human brucellosis is reported in Saudi Arabia, Iran, the Palestinian Authority, Syria, Jordan, and Oman. The most common Brucella species reported in Egypt is B.melitensis [22]. In this article, a number of serodianostic techniques were used for detection of Brucella infection among humans and different animal species. The percentages of disease detection of the applied tests among investigated cases were calculated and provided.
Materials and Methods
Specimen collection
Blood specimens were obtained from 68 patients from Abbasia Fever Hospital (25 patients) and different private laboratories (43 patients) who showed suspected brucellosis, depending upon history and/or clinical signs as well as from different investigated animals, both apparently healthy animals and suggestive infected cases (suffering from abortion), from different farms. These investigated animals comprised 76 buffalo (1 herd), 145 cattle (3 different herds) and 191 sheep (2 different herds). For clinical specimens, the treatment/ therapy history was not considered during specimens collection since the study focused primarily on serodiagnosis of suspected cases and not concerned with the impact of therapy on the incidence/prevalence of brucellosis. While in case of animals, the infected ones after establishment of diagnosis are killed and no treatment/therapy is recommended.
Chemicals
Rose bengal plate test antigen, standard Brucella concentrated antigen for standard tube agglutination test, rivanol test antigen and rivanol reagent were provided by Central Veterinary Lab., Newhow, Weybridge, Surrey KT 15, England. Ethylene diamine tetra acetic acid (EDTA) was purchased from El-Nasr pharmaceutical chemicals Co. (ADWIC), Abuzaabal, Qalyubiyah, Egypt. 0.5% phenol saline solution was used for SAT and EDTA modified SAT.
Preparation of sera
Blood specimens were transferred to sterile dry vacutainer tubes which were left at room temperature for about one hour to facilitate blood clotting before they were transferred to the laboratory. In the laboratory, vacutainer tubes were kept in refrigerator (4°C) overnight to help serum separation and the clear sera that oozed from the clotted blood specimens were aspirated by sterile Pasteur pipettes and put in sterile screw capped tubes to be stored in the deep freezer (-20°C) until being tested. For some blood specimens, centrifugation at 3000 rpm for 10 minutes was applied to obtain clear sera.
Serological tests
The sera of blood specimens were first screened by RBPT and those giving negative results were discarded, whereas sera giving positive reaction were retested by the SAT, EDTA modified SAT and rivanol test to determine their titers. RBPT, SAT and rivanol test were carried out as described by Alton et al. [23] and EDTA modified SAT was done as described by MacMillan and Cockrem [24].
Results
The results for clinical specimens showed that 61 (89.70%), 56 (82.35%), 45 (66.18%) and 40 (58.82%) were positive using RBPT, SAT, EDTA modified SAT and rivanol test, respectively (Table 1). Serum specimens that showed a titer of 1/80 for SAT and EDTA modified SAT were considered as suspicious cases for human brucellosis. The respective percentages of brucellosis in buffalo were 34 (44.70%), 33 (43.42%), 33 (43.42%) and 32 (43.24%); while the respective percentages of brucellosis in cattle were 68 (46.90%), 63 (43.45%), 57 (39.31%) and 55 (37.93%). In addition, serological examination of 191 sheep revealed that 115 (60.20%), 108 (56.54%), 102 (53.40%) and 99 (51.83%) were positive using RBPT, SAT, EDTA modified SAT and rivanol test, respectively (Table 2). Serum specimens that showed a titer of 1/20 for SAT and EDTA modified SAT were considered as suspicious cases for animal brucellosis. The titer results for SAT, EDTA modified SAT and rivanol test for clinical and animal specimens are shown in Table 3 and Table 4, respectively.
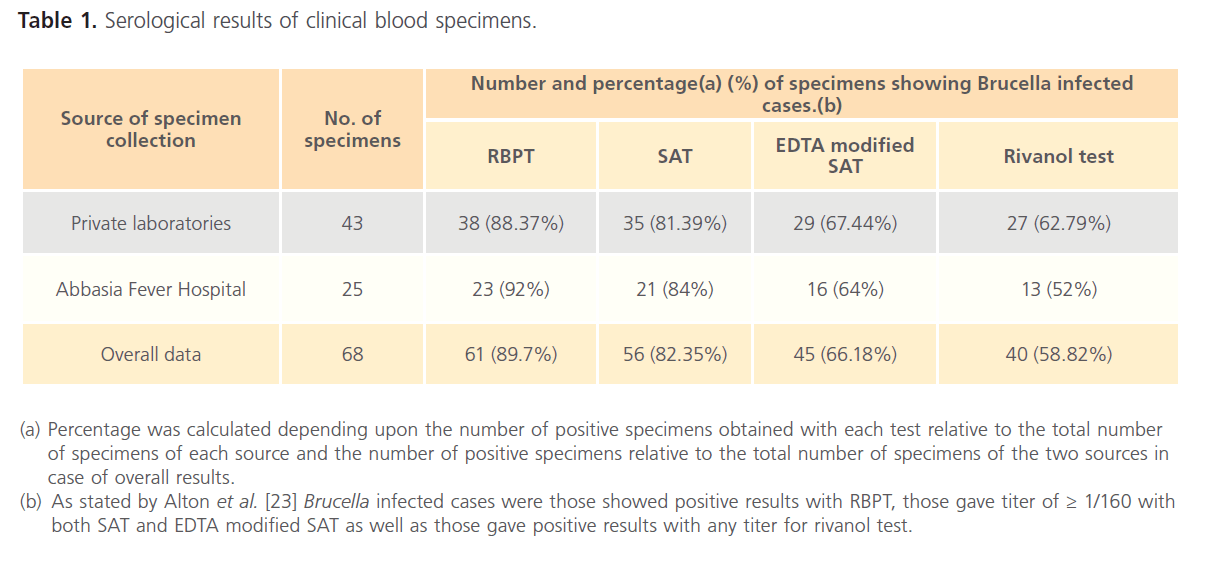
Table 1: Serological results of clinical blood specimens.
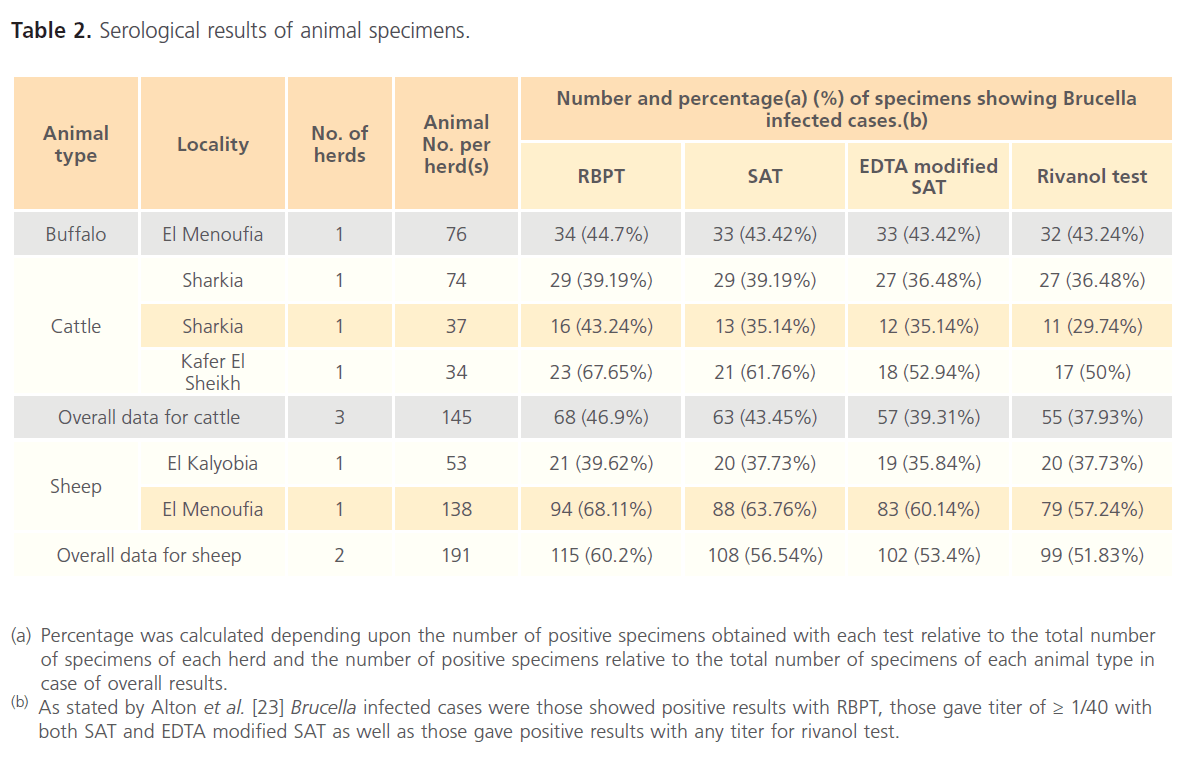
Table 2: Serological results of animal specimens.
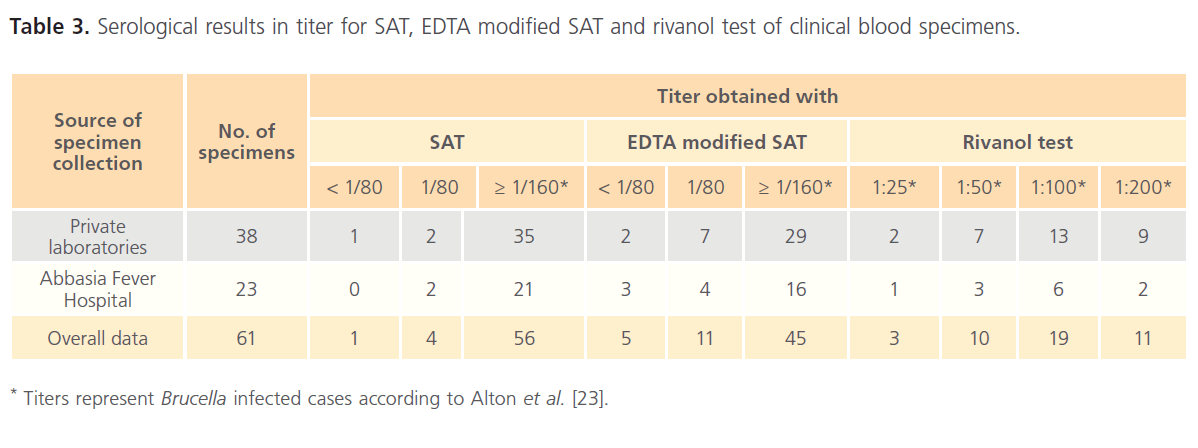
Table 3: Serological results in titer for SAT, EDTA modified SAT and rivanol test of clinical blood specimens.
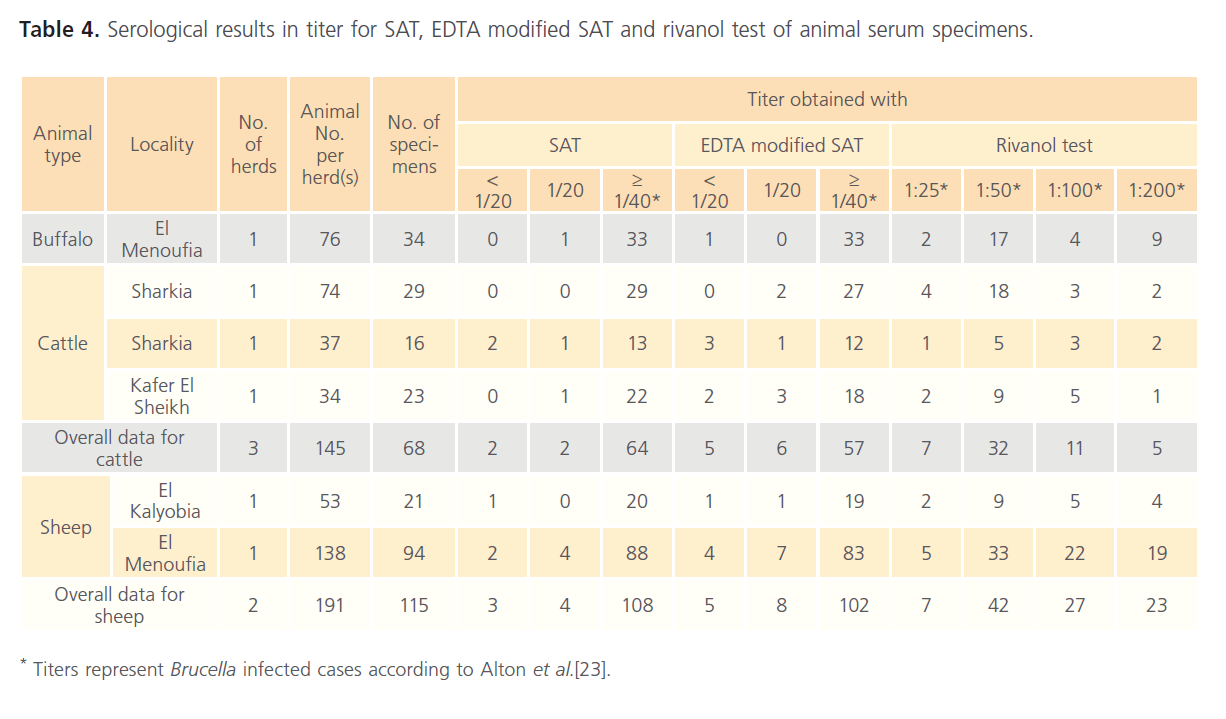
Table 4: Serological results in titer for SAT, EDTA modified SAT and rivanol test of animal serum specimens.
Discussion
The serological diagnosis showed high prevalence of human brucellosis among suspicious human patients. The high prevalence of human brucellosis in this study compared to that reported by Fouad et al. [25] (26%) and Refai [22] (11%) was due to that the patients involved in this study were selected based on clinical evidence and/or personal history for brucellosis. However, 58% seropositive cases were recorded between family members of infected cases in Saudi families [26] and 84.9% prevalence was reported by Nimri [27]. In addition, Kazemi et al. [28] reported 80.76% seropositive individuals among suspicious human patients.
Our results also showed high prevalence of brucellosis between different investigated animals and this was because that all herds used in the study were suffered from history of brucellosis. This high prevalence was agreed with that reported by Chauhan et al. [29] (44%) and Nasir et al. [30] (35.40%) for buffalo and with that reported by Genc et al. [31] (55.2%); Otlu et al. [32] (34.64%) and Sahin et al. [33] (39.5%) for cattle. In addition, the high prevalence of sheep brucellosis in this study was agreed with that reported by Al- Talafhah et al. [34] (56%); Gupta et al. [35] (59%); Nashwa et al. [36] (31.3%); Otlu et al. [37] (40.1%) and Celebi and Ataby [38] (36.7%).
The results revealed that the number of positive reactions with rivanol test were found to be < EDTA modified SAT < SAT < RBPT. The high prevalence rate obtained with RBPT may be attributed to the high sensitivity and low specificity of the test. RBPT is mainly used for screening purposes which is rapid, simple and sensitive test but has low specificity and is usually followed by one of more specific confirmatory assays [30]. Several serological assays have been developed to diagnose brucellosis including SAT which stills the most reliable method [39]. Gall and Nielsen [40] reported that SAT had higher specificity (95.7%) but lower sensitivity (75.9%) than RBPT which had sensitivity and specificity of 81.2% and 86.3%, respectively. Thus, in the present study, sera that gave positive results with RBPT were retested using the more specific test (SAT). On the other hand, false-positive reactions can also be seen in the SAT and they occasionally result from cross-reactions with antibodies to Salmonella spp., Yersinia spp., Vibrio cholera, Francisella tularensis or Escherichia coli O:157 [41] resulting in doubtful reactions (with titer of 1/80 or 1/20 for clinical and animal specimens, respectively). Macmillan and Cockrem [24] stated that agglutination reaction was sufficiently affected by the action of EDTA. It was reported that non specific reactions with Brucella could be reduced by addition of EDTA [42, 43]. Thus, EDTA modified SAT showed less positive reactions than that obtained with SAT; however, doubtful reactions could also be observed for EDTA modified SAT. Rivanol test is useful in detection of chronic cases that mainly contain IgG. The rivanol test detects principally IgG1, and to a lesser extent IgG2, because initial treatment of sera with rivanol solution removes IgM by precipitation, reduces the reactivity of IgG2 and promotes the reactivity of IgG1. This gives the rivanol test low sensitivity but high specificity [44]. Thus, rivanol test showed the least number of positive reactions. In conclusion, the results showed that: (i) the real picture of brucellosis surveillance among human cannot be reflected using single serodiagnostic test, (ii) In comparison to human, serodiagnosis of Brucella among animals is less dependent on test type and such dependency took the order sheep > cattle > buffalo, (iii) serodiagnosis of Brucella among buffalo had nearly no dependency on test type.
158
References
- Freeman BA, Vana LR (1958) Host-parasite relationships in brucellosis. Infection of normal guinea pig macrophages in tissue culture. Journal of Infectious Diseases 102(3): 258-267.
- Corbel MJ (1997) Brucellosis: an overview. Emerging Infectious Diseases 3: 213-221.
- Rijpens NP, Jannes G, Van Asbroeck M, Rossau R, Herman LM (1996) Direct detection of Brucellaspp. in raw milk by PCR and reverse hybridization with 16S- 23S rRNA spacer probes. Applied and Environmental Microbiology 62 (5): 1683-1688.
- Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV (2006) The new global map of human brucellosis. Lancet Infectious Diseases 6: 91-99.
- Montasser MF, Ibrahim FH, Wahab MF (1991) Rapid diagnosis of non prolonged febrile illnesses necessitating fever hospital admission. Journal of the Egyptian Public Health Association 66: 675-692.
- Afifi S, Earhart K, Azab MA, Youssef FG, El-Sakka H “et al.” (2005) Hospital-based surveillance for acute febrile illness in Egypt: a focus on community-acquired bloodstream infections. American Journal of Tropical Medicine and Hygiene 73: 392-399.
- Jennings G, Hajjeh R, Girgis F, Fadeel M, Maksoud M, “et al.” (2007) Brucellosisas a cause of acute febrile illness in Egypt. Transactions of the Royal Society of Tropical Medicine and Hygiene 101: (7) 707 – 713.
- Doganay M, Aygen B (2003) Human brucellosis: an overview. International Journal of Infectious Diseases 7:173-182.
- Wallach JC, Miguel SE, Baldi PC, Guarneru E, Goldbaum FA, “et al.” (1994) Urban outbreak of Brucellamelitensisinfection in an Argentine family: clinical and diagnostic aspects. FEMS Immunology and Medical Microbiology 8: 49-56.
- Matar GM, Khneisser IA, Abdel Noor AM (1996) Rapid Laboratory confirmation of human brucellosis by PCR analysis of a target sequence on the 31 kliodalton. Brucellaantigen DNA. Journal of Clinical Microbiology 34 (2): 477- 478.
- Araj GF (2000) Human brucellosis revisited: A persistant Saga in the Middle East. British Medical Journal (Middle East) 7: 6-15.
- Solera J, Lozano E, Martinez-Alfano E, Espinosa A, Castillejos ML, “et al.” (1999) Brucellar spondylitis: review of 35 cases and literature survey. Clinical Infectious Diseases 29: 1440-1449.
- Cohen N, Golik A, Alon I (1997) Conservative treatment for Brucellaendocarditis. Clinical Cardiology 20: 291-294.
- Vallejo JG, Stevens AM, Dutton RV, Kaplan SL (1996) Hepatosplenic abscesses due Brucellamelitensis: report of a case involving a child and review of the literature. Clinical Infectious Diseases 22: 485-489.
- Colmenero JD, Reguera JM, Martos F (1996) Complications associated with Brucellamelitensisinfection: a study of 530 cases. Medicine (Baltimore) 75: 195-211.
- Franco MP, Mulder M, Gilman RH, Smits HL (2007) Human brucellosis. Lancet Infectious Diseases 7: 775-786.
- Odeh M, Pick N, Oliven A (2000) Deep vein thrombosis associated with acute brucellosis: case report. Angiology 51: 253-256.
- Mousa AR, Koshy TS, Araj GF, Marafie AA, Muhtaseb SA, “et al.” (1986) Brucellameningitis: presentation, diagnosis and treatment; a prospective study of ten cases. Quarterly Journal of Nuclear Medicine 60: 873-85.
- Odeh M, OlivenA (1996) Acute brucellosis associated with massive proteinuria. Nephron 72: 688-689.
- AbdElrazak M (1991) Brucellaoptic neuritis. Archives of Internal Medicine 151: 776-778.
- Corbel MJ, World Health Organization, Food and Agriculture Organization of the United Nations, International Office of Epizootics (2006) Brucellosis in humans and animals. World Health Organization. Geneva, Switzerland. ISBN: 9241547138, 9789241547130.
- Refai M (2002) Incidence and control of brucellosis in the Near East region. Veterinary Microbiology 90: 81- 110.
- Alton GG, Jones LM, Angus RD, Verger JM (1988) Techniques for the Brucellosis Laboratory. Institut National de la RechercheAgronomique (INRA), Paris, France, ISBN: 278000428.
- Macmillan AP, Cockrem DS (1985) Reduction of non-specific reaction to the Brucellaabortusserum agglutination test by addition of EDTA. Research in Veterinary Science 38(3): 288-291.
- Fouad K, Nour El-Din A, Salah A, Murad A (1996) Study of Brucellainfection as an occupational risk among abattoir workers in Alexandria. Bulletin of Alexandria Faculty of Medicine 32: 299-306.
- Alsubaie S, Almuneef M, Alshaalan M, Balkhy H, Albanyan E, “et al.” (2005) Acute brucellosis in Saudi families: Relationship between Brucellaserology and clinical symptoms. International Journal of Infectious Diseases 9: 218-224.
- Nimri LF (2003) Diagnosis of recent and relapsed cases of human brucellosis by PCR assay. BMC infectious diseases 3: 5.
- Kazemi B, Namin YAS, Dowlatshahi M, Bandepour B, Kafilzadeh F, “et al.” (2008) Detection of Brucellaby peripheral blood PCR and comparison with culture and serological methods in suspected cases. Iranian Journal of Public Health 37(4): 96-102.
- Chauhan HC, Chandel BS, Shah NM (2000) Seroprevalence of brucellosis in buffaloes in Gujrat. Indian Veterinary Journal 77: 1105- 1106.
- Nasir AA, Parrveen Z, Shah MA, Rashid M, (2004) Prevalence of brucellosis in animals at government and private farms in Punjab. Pakistan Veterinary Journal 24: 144-146.
- Genc O, Otlu S, Sahin M, Aydin F, Gokce HI (2005) Seroprevalence of brucellosis and leptospirosis in aborted dairy cows. Turkish Journal of Veterinary and Animal Sciences 29: 359-366.
- Otlu S, Sahin M, Atabay HI, Unver A (2008) Serological investigations of brucellosis in cattle, farmers and veterinarians in the Kars District of Turkey. ActaVeterinaria Brno 77: 117-21.
- Sahin M, Genc O, Unver A, Otlu S (2008) Investigation of bovine brucellosis in the Northeastern Turkey. Tropical Animal Health and Production 40: 281-286.
- Al-Talafhah AH, Lap SQ, Al-Tarazi Y (2003) Epidemiology of ovine brucellosis in Awassi sheep in Northern Jordan. Preventive Veterinary Medicine 60: 297-306.
- Gupta VK, Deepak KV, Rout PK, Singh SV, Vihan VS (2006) Polymerase chain reaction (PCR) for detection of Brucellamelitensisin goat milk. Small Ruminant Research 65:79-84.
- Nashwa MH, Hoda MZ, Sami SA (2007) Identification and differentiation of BrucellamelitensisRev.1 vaccines and B. melitensisbiovar 3 field isolates in Egypt by serological and PCR-RFLP techniques. Journal of Applied Sciences Research 3: 841-847.
- Otlu S, Sahin M, Unver A, Celebi O (2007) Detection of Brucellamelitensisand Chlamydophilaabortusantibodies in aborting sheep in the Kars province of Turkey. The Bulletin of the Veterinary Institute in Pulawy51:493-495.
- Celebi O, Atabay HI (2009) Seroepidemiological investigation of brucellosis in sheep abortions in Kars, Turkey. Tropical Animal Health and Production 41: 115-119.
- Sirmatel F, Turker M, Bozkurt AI (2002) Evaluation of the methods used for the serologic diagnosis of brucellosis. Mikrobiyolojibulteni36: 161–167.
- Gall D, Nielsen K (2004) Serological diagnosis of bovine brucellosis: a review of test performance and cost comparison. Scientific and Technical Review of the Office International des Epizooties 23: 989- 1002.
- Ertek M, Yazgi H, Ozkurt Z, Ayyildiz A, Parlak M (2006) Comparison of the diagnostic value of the standard tube agglutination test and the ELISA IgG and IgM in patients with brucellosis. Turkish Journal of Medical Sciences 36(3): 159-63.
- Trap D, Garin B, Moutou F, Gaumont R (1985) Bovine brucellosis: elimination of non-specific sero-agglutination by using EDTA and agglutination at 56?C. Revue de MedecineVeterinaire136(5): 399- 409.
- Otto M, Radostits C, Gay C, Douglas C, Blood K, “et al.” (2000) Veterinary Medicine, 9thEd. W.B. Saunders Co. London, UK. ISBN: 0702026042.
- Mikolon AB, Gardner IA, Hietala SK, Hernandez-de-Anda J, ChamizoPestana E, “et al.” (1998) Evaluation of North American antibody detection tests for diagnosis of brucellosis in goats. Journal of Clinical Microbiology 36(6): 1716-1722.









