Keywords
CPAP, Acute Respiratory Distress Syndrome, CPB
Introduction
During cardiac surgery cardiopulmonary bypass (CPB) serves four basic functions: respiration, circulation, temperature management and provision of a blood less field. CPB allows the surgeon to operate on a non-beating heart at hypothermic temperatures facilitating surgery in conditions where surgery was considered to be impossible before. The venous return is diverted from the heart by cannulation in vena cava or right atrium and aortic outflow is provided through aortic cannulation distal to a cross clamp and thus the circulation through heart and lungs are altogether bypassed. The oxygen supply to lung occurs by the bronchial artery [1]. This nonphysiological condition ultimately culminates to an array of problems in early postoperative period and pulmonary dysfunction was one of the earliest recognized complications of cardiac surgery using CPB [2]. Postoperative pulmonary dysfunction after CPB may include simple atelectasis, pleural effusions, pneumonia, cardiogenic pulmonary edema, pulmonary embolism, and various degrees of acute lung injury ranging from the mild to the most severe (i.e., acute respiratory distress syndrome [ARDS]) [3].
Numerous studies have been undertaken to recognize, prevent and reduce the extent of pulmonary dysfunction at the earliest possible period. Of these studies, ventilatory strategy during CPB has gained particular interest [3]. In this study, the authors compared no ventilation to low volume normal frequency ventilation and continuous positive airway pressure maintained during CPB to observe effect on postoperative pulmonary function.
Methods
Institutional ethics committee approval was obtained. The study was structured as a prospective, randomised and double blind study. Patients were selected in preanesthesia check up clinic on the basis of inclusion and exclusion criteria, explained regarding the study and informed written consent was obtained. Inclusion criteria were as the following: 18 to 65 years of age, both genders, elective cardiac surgery under cardiopulmonary bypass and patient’s voluntary agreement for participation. Exclusion criteria were: patient refusal, regular smoking, redo surgery, concomitant systemic comorbidity including myocardial infarction within six weeks prior to surgery, pre-operative renal failure (serum creatinine>1.3 mg/dl) or hepatic dysfunction (serum aspartate/ alanine amino transferase>40 U/l), any kind of pulmonary disease and pre-operative use of steroids and ejection fraction below 30%. 45 patients were included to this study and allocated to three groups by a computer generated randomisation chart: group V received low volume ventilation, group NV received no ventilation and group C received CPAP during CPB.
Anesthesia management were done according to institution standardised protocol. All patients were induced following narcotic based coinduction technique with fentanyl 5 μg/ kg, midazolam 0.1 mg/kg and sleeping dose of thiopentone sodium. Rocuronium was used to assist endotracheal intubation. Maintenance was done with isoflurane, midazolam, fentanyl and vecuronium. Monitoring included, invasive arterial blood pressure, ecg, etCO2, central venous pressure, pulse oxymetry, temperature and urine output for all cases. Heparin 4 mg/kg was used to achieve adequate anticoagulation (Activated clotting time>480 seconds) prior to bypass. Pump was primed with a crystalloid based solution with heparin and mannitol. After cannulation of major vessels cardiac arrest was induced by ice slush and following application of cross clamp a potassium based cardioplegia was infused. All patients underwent non-pulsatile hypothermic (30-32ºC) CPB with a membrane oxygenator and arterial line filter at pump flow rates of 2-2.4 L/min/m2 body surface area to maintain mean arterial pressure of 50-80 mm Hg. Arterial blood gas analysis was done every 15-30 minutes to maintain arterial carbon dioxide partial pressure of 35-40 mm Hg unadjusted for temperature (alpha stat), oxygen partial pressures of 150-250 mm Hg and hematocrit>21%. Following application of aortic cross clamp, different ventilatory strategy was applied in different groups. In group NV- ventilation stopped, in group V- ventilation continued with tidal volume 2 ml/kg, FiO2=50%, respiratory rate 14/ minute, inspiratory expiratory ratio 1:2 and PEEP 5 cm H2O, and in group C- CPAP of 10 cm H2O applied. After release of aortic cross clamp normal ventilation was resumed. Postoperatively arterial blood gas analysis values, inspiratory capacity and other standard parameters were recorded. The primary outcome measure was extubation time. The secondary parameters included arterial blood gas value including PaO2, PaCO2; inspiratory capacity, ICU stay and hospital stay.
For sample size calculation data was collected from a previous study. It was estimated that 15 patients is required per group to compare extubation time in ventilated patients with non ventilated patients with 80% power and 5% probability of Type I error. Data was summarized as mean and standard deviation for numerical variables and counts and percentages for categorical variables. The median and interquartile range was used for numerical variables that show a skewed distribution. The independent samples t test was employed for intergroup comparison of numerical variables, if normally distributed, or the Mann-Whitney U test if otherwise. Categorical variables were compared between groups by Fisher’s exact test. All analyses were two-tailed and p value less than 0.05 was considered statistically significant.
Results
45 patients were included in this study and randomised equally to three groups. Age and gender distribution, body weight, aortic cross clamp time and cardiopulmonary bypass time were comparable between the groups (Table 1). Types of surgery undertaken in this study are depicted in Table 2.
| |
Group V
(n=15) |
Group NV
(n=15) |
Group C
(n=15) |
P Value |
| Age (Year) |
29.83±10.45 |
35.23±13.28 |
35.25±11.0 |
0.18 |
| Gender (M:F) |
9:9 |
7:10 |
8:7 |
0.73 |
| Body weight (Kg) |
43.72±11.06 |
45.52±8.96 |
52.62±9.35 |
0.6 |
| Axcl time (min) |
60.66±28.03 |
52.00±23.71 |
78.50±21.35 |
0.33 |
| CPB Time (min) |
84.33±41.95 |
80.70±29.13 |
96.43±34.04 |
0.76 |
Axcl= Aortic cross clamp, CPB= Cardiopulmonary bypass]
Table 1: Comparison of age, gender, body weight and surgery parameters
| |
VSD |
AVR |
ASD |
MVR |
Single atrium |
DVR |
RSOV |
TOF |
OMC |
TOTAL |
| Gr V |
2 |
1 |
2 |
6 |
1 |
2 |
1 |
0 |
0 |
15 |
| Gr NV |
2 |
0 |
1 |
9 |
1 |
0 |
0 |
1 |
1 |
15 |
| Gr C |
2 |
2 |
2 |
9 |
0 |
0 |
0 |
0 |
0 |
15 |
[VSD= Ventricular septal defect, AVR= Aortic valve replacement, ASD= Atrial Septal Defect, MVR= Mitral Valve Replacement, DVR= Double (Mitral+ Aortic ) Valve Replacement, RSOV= Ruptured Sinus of Valsalva , TOF= Tetralogy of Fallot, Total Correction, OMC= Open Mitral Commissurotomy).
Table 2: Types of surgery
Arterial oxygen tension was compared between the groups after intubatuion (Baseline), after institution of CPB, after aortic cross clamp off and after the discontinuation of CPB. Figure 1 revealed significantly better PaO2 values after removal of cross clamp in low volume ventilation group.
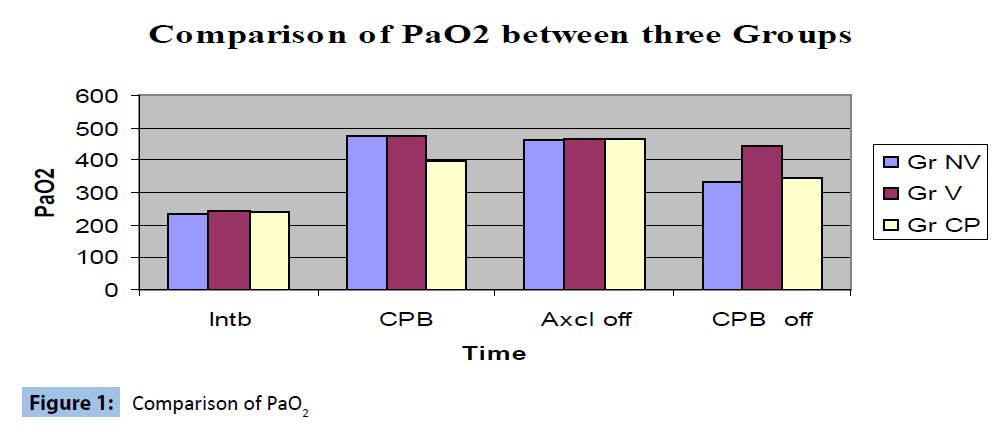
Figure 1: Comparison of PaO2
Comparison of PaCO2 levels between the groups at the same times (groups after intubatuion (Baseline), after institution of CPB, after aortic cross clamp off and after the discontinuation of CPB) revealed low PaCO2 values in CPAP group but this change was not significant (Figure 2).
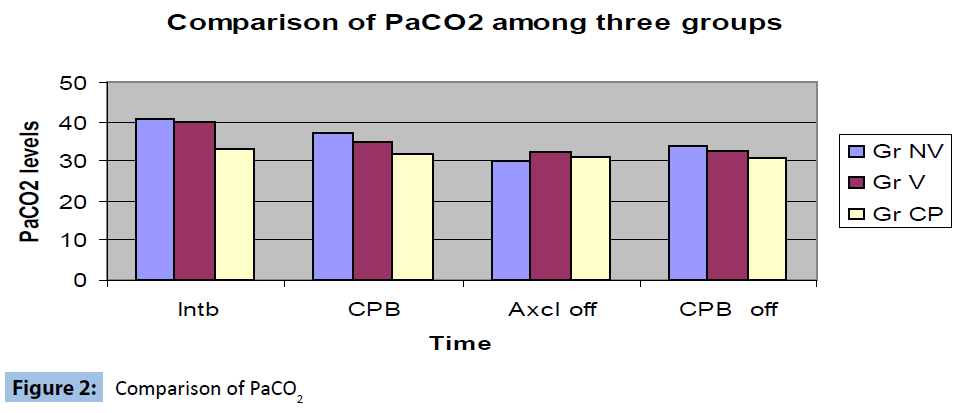
Figure 2: Comparison of PaCO2
Inspiratory capacity (IC) measured with bedside spirometry was compared between the groups and revealed significant increase in IC in the low volume ventilation group (Figure 3).
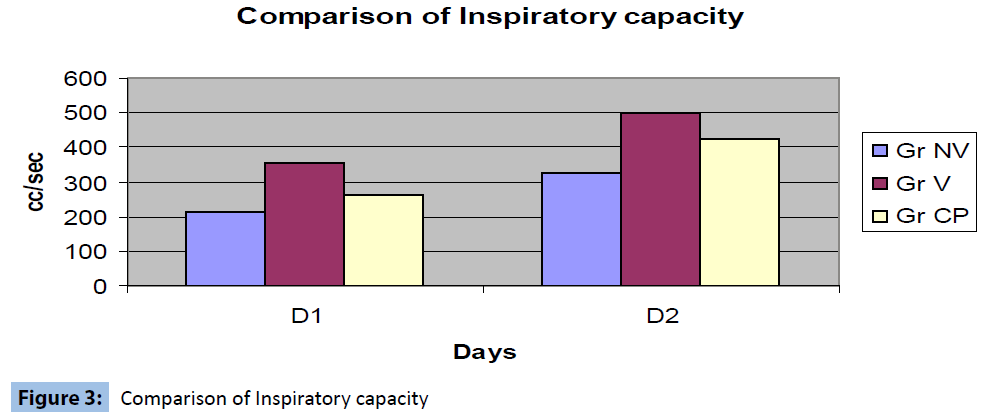
Figure 3: Comparison of Inspiratory capacity
Ventilation time, as measured by time of extubation was compared and the low volume ventilation group showed significant low ventilation time as shown in Table 2. Postoperative intensive care unit stay was significantly lower in the low ventilation group. Total hospital stay was similar between the groups (Table 2).
Discussion
The pulmonary dysfunction after CPB has is fairly common and associated with significant mortality and morbidity [3-7]. Studies have reported variable incidence of postoperative pulmonary dysfunction (PPD) between 8 to 79% [3,8] in patients submitted to open heart surgery with CPB. The basic pathophysiology behind PPD after general anesthesia is related to alteration in lung mechanics including upward shift of the diaphragm, relaxation of the chest wall, altered chest wall compliance, and a shift in blood volume to the abdomen from the thorax [9-12] and subsequent ventilation perfusion matching, whereas after cardiac surgery with CPB the lungs suffer from some additional insult. During CPB after application of cross clamp the lungs are almost excluded from the circulation except the bronchial arteries. This ischemic insult and subsequent reperfusion injury, along with the generalised inflammatory response after the CPB, alters pulmonary capillary permeability. This change and changes in mechanical properties (i.e., elastance or compliance and resistance) of the pulmonary apparatus (particularly the lung as opposed to the chest wall) in combination gives rise to majority of PPDs after cardiac surgery [3]. These conditions are aided by insufficient alveolar distension to activate the production of surfactant, abnormal pulmonary mechanics, retention of secretions and atelectasis [3]. CPB results in sequestration of blood in the microcirculation, pulmonary ischemia, injury to the pulmonary capillary walls and results in release of inflammatory mediators [13] increased capillary permeability [14], interstitial edema [15], and increase in intrapulmonary shunt [16] and formation of microthrombi which altogether cause closure of small airways and ventilation perfusion mismatch in the postoperative period. Therefore efforts to protect the lung from CPB induced insult should incorporate strategies to reduce pulmonary ischemia after application of cross clamp and prevent lung collapse during CPB.
Previous studies on decreasing postoperative atelectasis after cardiac surgery mostly centred on recruitment manoeuvre and continuous positive airway pressure (CPAP). Serita et al. [17] applied recruitment manoeuvre according to pulmonary compliance and found significant improvement of lung function after recruitment. Minkovich et al. [18] conducted a randomised controlled trial to examine the efficacy of consecutive vital capacity manoeuvres (C-VCMs) to improve oxygenation in patients after cardiac surgery. They found lung inflation at pressure of 35 cm H2O sustained for 15 seconds before separation from CPB and at 30 cm H2O for 5 seconds after admission to the intensive care unit (ICU) significantly improves lung function and alveolar oxygenation. Claxton et al. [19] examined the effect of pressure-controlled stepwise increase in positive end-expiratory pressure up to 15 cm H2O and tidal volumes of up to 18 ml/ kg until a peak inspiratory pressure of 40 cm H2O was reached for 10 cycles and maintenance of 5 cm H2O PEEP until extubation and found significant improvement.
Studies on CPAP during CPB have yielded rather inconclusive results. Loeckinger et al. [20] examined the effect of CPAP of 10 cm H2O and found significant improvement in oxygenation. Alavi et al. [21] also found similar results. In 2012, Schreiber et al. [22] conducted a meta analysis on the effect of different lung-protective strategies in patients during cardiopulmonary bypass and found CPAP is associated with significant increase in oxygenation parameters immediately after weaning from CPB, but this effect was not sustainable and did not improve patient outcome. All those studies pointed in favour of CPAP, where as Gilbert et al. [23] found low levels of CPAP applied during CPB did not significantly change either mechanical properties or oxygenation. Figueiredo et al. [24] examined 30 adult patients posted for myocardial revascularisation under CPB and found CPAP at 10 cm H2O administered during CPB, although had lightly improved PaO2/FiO2 at 30 minutes post-CPB, had no significant sustained effect on postoperative pulmonary gas exchange and concluded that application of 10 cm H2O CPAP does not improve postoperative pulmonary gas exchange in patients posted for myocardial revascularisation.
Low volume ventilation during CPB has been much less studied strategy because mobility of the surgical field interferes with the surgery. Beer et al. [25] found reduced postoperative serum chemokine concentration in patients with low tidal volume ventilation during cardiopulmonary bypass. Another study [26] found reduced release of matrix metalloproteinases and improved oxygenation in patients receiving low volume ventilation during CPB. Fernando et al. [27] reviewed protection strategies during cardiopulmonary bypass: ventilation, anesthetics and oxygen and concluded, “The application of lung-protective ventilation with lower tidal volumes and higher positive end-expiratory pressure reduces inflammation, thereby reducing postoperative pulmonary complications.”
The authors conglomerated the protective ventilatory strategies into one study. The recruitment manoeuvre is a well accepted method to prevent atelectasis and have been proved to be effective in other scenarios like ARDS (Table 3). The authors therefore explored the other two controversial and less investigated areas to find out the ideal ventilatory strategy during CPB. The authors confined the tidal volume for low volume ventilation at 2 ml/kg as it did not cause movement of surgical field. The rationality behind use of 50% oxygen was that 100% oxygen if remains in the alveolus will be absorbed and cause collapse of the alveoli. The authors found significant improvement of inspiratory capacity in the low volume ventilation group on day 1 and day 2. The PaO2 values were significantly improved in low volume ventilation group after removal of cross clamp and indicated better oxygenation. There was no significant difference in the PaCO2 values between the groups.
| |
Group V |
Group NV |
Group CP |
P Value |
| Extubation(Hours) |
12.44 ± 6.11 |
16.47 ± 5.88 |
13.46 ± 1.38 |
Gr V vs NV
P = 0.057 |
| ICU Stay (Days) |
2.00 ± 0.00 |
2.4 ± 0.6 |
2.00 ± 0.00 |
Gr V vs NV
P = 0.003
Gr CP vs V
P = 0.02 |
| Discharge (Days) |
7.00 ± 0.00 |
7.8 ± 0.7 |
7.12 ± 0.3 |
NS |
| |
Group V |
Group NV |
Group CP |
P Value |
| Extubation(Hours) |
12.44 ± 6.11 |
16.47 ± 5.88 |
13.46 ± 1.38 |
Gr V vs NV
P = 0.057 |
| ICU Stay (Days) |
2.00 ± 0.00 |
2.4 ± 0.6 |
2.00 ± 0.00 |
Gr V vs NV
P = 0.003
Gr CP vs V
P = 0.02 |
| Discharge (Days) |
7.00 ± 0.00 |
7.8 ± 0.7 |
7.12 ± 0.3 |
NS |
Table 3. Comparison of hours of extubation, ICU stay and discharge
Alavi et al. [21] has conducted a similar study on on-pump coronary artery bypass grafting surgery patients and found CPAP and intermittent mandatory ventilation during CPB was associated with better postoperative ABG measurements and (A-a) DO2. The authors experienced better PaO2 profile with low volume ventilation group. Respiratory mechanics were not addressed in their study.
The limitation of the study is long term outcome has not been incorporated into this study. The oxygenation parameters including arterial oxygen content, oxygen consumption, extraction ratio and shunt fraction were planned to be included in this study but could not be done due to nonavailability of resources. Further study may be done in this context with larger sample size and including the oxygenation parameters and follow up details to reach a stronger recommendation.
Conclusion
In conclusion, low volume ventilation during CPB is associated with better oxygenation and pulmonary mechanics after cardiac surgery than CPAP or no ventilation. Low volume ventilation does not cause significant movement in the lung field. CPAP with 10 cm H2O does not significantly improve postoperative pulmonary function than no ventilation.
3700
References
- Durukan,A.B.,Gurbuz,H.A., Salman, N., Unal,E.U., Ucar,H.I. &Yorgancioglu,C.E. Ventilation during cardiopulmonary bypass did not attenuate inflammatory response or affect postoperative outcomes. Cardiovasc J Afr 2013;24(6):224-30.
- Rea, H.H., Harris,E.A., Seelye,E.R., Whitlock,R.M. &Withy,S.J. The effects of cardiopulmonary bypass upon pulmonary gas exchange. J ThoracCardiovascSurg 1978;75(1):104-20.
- Wynne, R. &Botti, M. Postoperative pulmonary dysfunction in adults after cardiac surgery with cardiopulmonary bypass: clinical significance and implications for practice. Am J Crit Care 2004;13(5):384-93.
- Andrejaitiene, J., Sirvinskas, E. &Bolys, R. The influence of cardiopulmonary bypass on respiratory dysfunction in early postoperative period. Medicina 2004;40 (1) :7-12.
- Schramel, R., Schmidt, R., Davis, F., Palmisano, D. &Creech O. Pulmonary lesions produced by prolonged perfusion. Surgery 1963;54:224-231.
- Baer,D.M. & Osborn,J.J. The postperfusion pulmonary congestion syndrome. Am J ClinPathol 1960;34:442-445.
- Asada, S. &Yamaguchi, M. Fine structural change in the lung following cardiopulmonary bypass: its relationship to early postoperative course. Chest 1971;59:478-483.
- Johnson,L.G. & McMahan,M.J. Postoperative factors contributing to prolonged length of stay in cardiac surgery patients. DimensCrit Care Nurs 1997;16:243-250.
- Hedenstierna, G., Strandberg, A. &Brismar, B. Functional residual capacity, thoracoabdominal dimensions, and central blood volume during general anesthesia with muscle paralysis and mechanical ventilation. Anesthesiology1985;62:247-254.
- Froese,A.B. & Bryan,A.C. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974;41:242-255.
- Klingstedt, C., Hedenstierna, G., Baehrendtz, S., et al. Ventilation-perfusion relationships and atelectasis formation in the supine and lateral positions during conventional mechanical and differential ventilation. ActaAnaesthesiolScand 1990;34:421-429.
- Brismar, B., Hedenstierna, G., Lundquist, H., Strandberg, A., Svensson, L&Tokics, L. Pulmonary densities during anesthesia with muscular relaxation: a proposal of atelectasis. Anesthesiology 1985;62:422-428.
- Utley JR. Pathophysiology of cardiopulmonary bypass: a current review. Aust J Card ThoracSurg 1992;1:46-52.
- Martin, W., Carter, R., Tweddel, A., et al. Respiratory dysfunction and white cell activation following cardiopulmonary bypass: comparison of membrane and bubble oxygenators. Eur J CardiothoracSurg 1996;10:774-783.
- Royston, D., Minty,B.D., Higenbottam,T.W., Wallwork, J. & Jones,G.J. The effect of surgery with cardiopulmonary bypass on alveolar-capillary barrier function in human beings. Ann ThoracSurg 1985;40:139-143.
- Reeve,W.G., Ingram,S.M. & Smith D.C. Respiratory function after cardiopulmonary bypass: a comparison of bubble and membrane oxygenators. J CardiothoracVascAnesth 1994;8:502-508.
- Serita, R., Morisaki, H. & Takeda J. An individualized recruitment maneuver for mechanically ventilated patients after cardiac surgery. J Anesth 2009; 23(1): 87-92.
- Minkovich, L., Djaiani, G., Katznelson, R., Day, F., Fedorko, L., Tan, J., Carroll, J., Cheng, D. &Karski, J. Effects of alveolar recruitment on arterial oxygenation in patients after cardiac surgery: a prospective, randomized, controlled clinical trial. J CardiothoracVascAnesth. 2007;21(3):375-8.
- Claxton, B.A., Morgan, P., McKeague, H., Mulpur, A. &Berridge, J. Alveolar recruitment strategy improves arterial oxygenation after cardiopulmonary bypass. Anaesthesia. 2003;58(2):111-6.
- Loeckinger, A., Kleinsasser, A., Lindner,K.H., Margreiter, J., Keller, C. &Hoermann, C. Continuous positive airway pressure at 10 cm H(2)O during cardiopulmonary bypass improves postoperative gas exchange. AnesthAnalg 2000;91(3):522-7.
- Alavi, M.,Pakrooh, B., Mirmesdagh, Y., Bakhshandeh, H., Babaee, T., Hosseini, S. &Kargar, F. The Effects of Positive Airway Pressure Ventilation during Cardiopulmonary Bypass on Pulmonary Function Following Open Heart Surgery. Res Cardiovasc Med 2013;2(2):79-84.
- Schreiber,J.U., Lancé,M.D., De Korte, M., Artmann, T., Aleksic, I. &Kranke, P. The effect of different lung-protective strategies in patients during cardiopulmonary bypass: a meta-analysis and semiquantitative review of randomized trials. J Cardiothorac Vasc Anesth 2012;26(3):448 -54.
- Gilbert,T.B., Barnas,G.M. &Sequeira,A.J. Impact of pleurotomy, continuous positive airway pressure, and fluid balance during cardiopulmonary bypass onlung mechanics and oxygenation. J CardiothoracVascAnesth 1996;10(7):844-9.
- Figueiredo, L.C., Araújo, S., Abdala,R.C., Abdala, A. &Guedes, C.A. CPAP at 10 cm H2O during cardiopulmonary bypass does not improve postoperative gas exchange. Rev Bras Cir Cardiovasc 2008;23(2):209-15.
- Beer, L., Szerafin, T., Mitterbauer, A., Debreceni, T., Maros, T., Dworschak, M., Roth,G.A. &Ankersmit, H.J. Low Tidal Volume Ventilation during Cardiopulmonary Bypass Reduces Postoperative Chemokine Serum Concentrations. Thorac Cardiovasc Surg 2014;62(8):677-82.
- Beer, L., Warszawska,J.M., Schenk, P., Debreceni, T., Dworschak, M., Roth,G.A., Szerafin, T. &Ankersmit,H,J. Intraoperative ventilation strategy during cardiopulmonary bypass attenuates the release of matrix metalloproteinases and improves oxygenation. J Surg Res 2014.
- Ferrando, C., Soro, M. &Belda,F.J. Protection strategies during cardiopulmonary bypass: ventilation, anesthetics and oxygen. Curr Opin Anaesthesiol 2015;28(1):73-80.









