Lubna Mushtaque Vohra* and Tariq Siddiqui
Ziauddin University Hospital, Pakistan
*Corresponding Author:
Lubna Mushtaque Vohra
Assistant Professor, Ziauddin University Hospital, Pakistan
Tel: 923215303685
E-mail: lubna_mushtaque@hotmail.com
Received Date: 26 December 2016; Accepted Date: 20 January 2017; Published Date: 24 January 2017
Citation: Vohra LM, Siddiqui T. Complete Response of Radiation related Angiosarcoma of the Breast with CMF Chemotherapy. Arch Can Res. 2017, 5:1. doi: 10.21767/2254-6081.1000125
Keywords
Angiosarcoma; Breast; Radiation; Chemotherapy
Introduction
One of the side effects of treating breast cancer with radiation to the chest wall or the conserved breast is the development of angiosarcoma [1,2].
This is a rare event and often occurs many years beyond the successful therapy of breast cancer itself [3]. The treatment of radiation related angiosarcoma (RAAS) of the breast in such settings is carried out by surgery, radiation, and chemotherapy. Nevertheless, the fact that the angiosarcoma itself is brought about by radiation therapy may result in a debate amongst clinicians as to the best modality of therapy in any given case. Chemotherapy regimens include many agents including the taxanes which have an antiangiogenic as well as a cytotoxic effect [4].
We would like to propose that CMF chemotherapy in the classical mode should be considered as an antiangiogenic and chemotherapy option in such cases.
Case Report
The lady is a 50-year-old of Pakhtoon ethnicity diagnosed having invasive ductal carcinoma of breast, grade III, T2N1Mo disease with ER/PR and Her 2 neu negative in 2008. She underwent breast conserving surgery with axillary clearance followed by adjuvant chemotherapy and radiation to breast and axilla, she was doing well subsequently. However, in 2014 she noticed a small lump in the conserved breast in lower outer quadrant; She underwent a biopsy and was felt to have a recurrent breast cancer. She received two cycles of docetaxel and carboplatinum. The tumor continued to grow and had mastectomy elsewhere. This resulted in a poor outcome with positive tumor margins and bleeding which later became an open wound. At this time she sought an opinion from us. A reassessment of the original biopsy and the second biopsy with an additional pathology opinion showed the entire biopsies specimen to be a high grade angiosarcoma and not breast cancer. The immunohistochemical markers were consistent with the diagnosis of angiosarcoma (Figures 1 and 2).
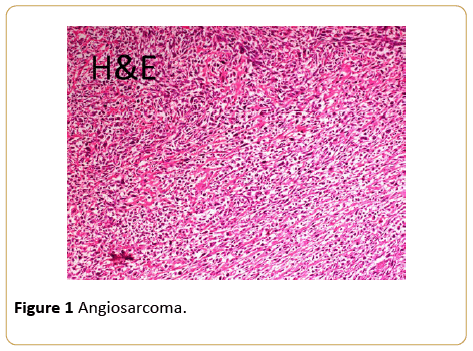
Figure 1: Angiosarcoma.
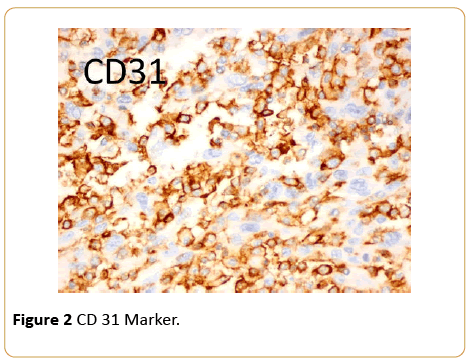
Figure 2: CD 31 Marker.
She was felt to be inoperable given the very large area involves clinically and the first option was felt to be chemotherapy. It was decided to start classical CMF. Midway through the second cycle of CMF the open wound had closed. By the end of the third cycle clinical traces of the disease had disappeared and she was considered fit for surgery. Excision of the tumor area was done easily with a quick and easy recovery. Histopathology showed only scarring, no tumor was seen. It was felt to be a complete and a successful excision.
Discussion and Conclusion
Angiosarcoma of the breast is a rare condition and may develop de novo or may be related to radiation treatment delivered to the chest region in breast cancer [1]. The early description of the Treves syndrome was angiosarcoma developing in patients who underwent a mastectomy which was followed by the development of angiosarcoma [2]. In the breast conserved setting angiosarcomas of the breast can also develop but the average time to such an event is approximately 72 months [3]. In our own case the period was approximately 62 months.
Treating RAAS follows the usual lines of integrating surgery, irradiation and in some cases chemotherapy. Systemic therapies used in these settings have included doxorubicin, liposomal doxorubicin, Ifosfamide, Gemcitabine, Docetaxel and even pazapanib [4]. In the PhaseII Angiotax weekly paclitaxel was employed in 30 cases of which 3 had breast angiosarcoma. In these 3 cases partial responses were seen after paclitaxel which let to successful surgery [5]. In one case report a 30-year remission in metastatic angiosarcoma is recorded following the use of methotrexate [6]. We could find any report relating to the use of Bevicizumab although the vascular endothelial growth factor may be overexpressed in breast angiosarcoma [7]. Finally, metronomic chemotherapy in the form of trofosfamide, pioglitazine, rofecoxib has been used as well [8].
In a recent analysis Depla et al. published a systemic review on treatment and prognostic factors of RAAS after primary breast cancer. In 74 articles encompassing 222 patients only 6% received chemotherapy with surgery with another 9% in which either radiation or chemotherapy alone were employed [9]. The treatment of RAAS with chemotherapy has few publications. We have found no report where CMF in a classical mode has been used in RAAS. CMF was appealing to us for a number of reasons. In this case the prior use of adjuvant FAC and the unaffordability of more expensive options such as liposomal doxorubicin were evident. Docetaxel was used with no effect. The high toxicities of Ifosfamide based regimens were not considered as was gemcitabine. There is a metromomic part to the use of CMF which is the two weekly oral cyclophosphamide treatments. Metronomic chemotherapy is highly antiangiogenic and it is easily affordable in the poorer economic settings. Often cyclophosphamide is its backbone [10]. The response of this patient was quick as within 6 weeks the wound closed completely. After 2 cycles she has undergone surgery with no angiosarcoma seen in the resected specimen and is undergoing re irradiation to the anterior chest wall.
An overall review of chemotherapies used in RAAS indicates no predictable pattern of treatments employed given that RAAS is a rare disease and no studies have been forthcoming prospectively.
Finally, the biology of angiosarcomas in breast conservation and radiation may be different the chest wall chest wall related angiosarcomas or the so called Treves syndrome. CMF may also need to be considered a chemotherapy choice in RAAS based on our experience.
18134
References
- Aydogdu M, Trams G (1996) Angiosarcoma of the breast after conservatively operated breast carcinoma--a sequela of adjuvant radiotherapy? GeburtshilfeFrauenheilkd 56:60-62.
- Stewart FW, Treves N (1981) Classics in oncology: lymphangiosarcoma in postmastectomy lymphedema: a report of six cases in elephantiasis chirurgica. CA Cancer J Clin 31:284-299.
- Anzalone CL, Cohen PR, Diwan AH, Prieto VG (2013) Radiation-induced angiosarcoma. Dermatol Online J 19:2.
- Dickson MA (2014)Systemic treatment options for radiation-associated sarcomas. Curr Treat Options Oncol.
- Penel N, Bui BN, Bay JO (2008) Phase II trial of weekly paclitaxel for unresectableangiosarcoma: The ANGIOTAX Study. J ClinOncol 26:5269-5274.
- Silverman LR, Deligdisch L, Mandeli J, Greenspan EM (1994) Chemotherapy for angiosarcoma of the breast: case report of 30-year survival and analysis of the literature. Cancer Invest 12:145-155.
- Brar R, West R, Witten D, Raman B, Jacobs C, et al. (2009) Breast angiosarcoma: Case series and expression of vascular endothelial growth factor. Case Rep Oncol 2:242-250.
- Depla AL, Scharloo-Karels CH, De jong MA (2014) Treatment and prognostic factors of radiation-associated angiosarcoma (RAAS) after primary breast cancer: a systematic review. Eur J Cancer 50:1779-1788.
- Albertsson P, Lennernas B, Norrby K (2006) On metronomic chemotherapy: modulation of angiogenesis mediated by VEGE-A . ActaOncol 45:144-155.







