Keywords
Abdominal computed tomography; Caudateright lobe ratio; Liver cirrhosis
Introduction
Liver cirrhosis is the end stage of a range of chronic diffuse liver diseases and is forever progressive leading to hepatic dysfunction, portal hypertension and hepatocellular carcinoma [1]. Liver disease has a global distribution [2], and a major public health challenge globally [3]. The global prevalence of cirrhosis from autopsy studies ranges from 4.5% to 9.5% of the general population [4-6]. In the United States, cirrhosis is the twelfth leading cause of hospitalization and death [7]. Cirrhosis is a major cause of morbidity and mortality in developing countries. In most of Africa, and indeed Nigeria, there is paucity of reliable statistics regarding liver-related morbidity and mortality. Besides, patients in this part of the world tend to present late to the hospital especially when stunned with the symptoms of liver failure [8].
The most common causes of hepatic cirrhosis are alcoholic fatty liver disease (AFLD), non-alcoholic fatty liver disease (NAFLD) and viral hepatitis [9]. Less frequent causes of cirrhosis include haemochromatosis, alpha1-antitrypsin deficiency, Wilson's disease, biliary cirrhosis and cardiac cirrhosis. Chronic inflammation leads to potentially reversible liver fibrosis and ends in irreversible cirrhosis with the crosslinking of collagen fibres and the formation of regenerative nodules.
Liver biopsy is currently the gold standard for the diagnosis of cirrhosis [10]. However, most patients will prefer to have a non-invasive test rather than a biopsy, because of the potential for procedural pain and complications such as bleeding, pneumothorax, biliary puncture and death [11]. Biopsy has a significant sampling error of between 14.5-25% when determining presence or absence of cirrhosis score variability [12]. Biopsy also suffers from poor reproducibility and variability in a sampling scoring by pathologists.
Computed tomography has proven to be a useful noninvasive technique for the diagnosis of liver cirrhosis. Computed tomography has an effective role in the evaluation of the liver and the different disorders of this organ in the setting of chronic liver cirrhosis. It has the ability of assessing adjacent structures that are affected in the course of the illness, including the portal and hepatic vessels, the gall bladder, the spleen, and other intra-abdominal organs [13]. These features make it a preferred method of assessing the liver [14].
The regional morphologic changes that occur in the liver as cirrhosis develops are well known [15,16]. Specifically, the volume loss in Couinaud segments IV–VIII is typically compensated in part by relative hypertrophy of segments I–III. Morphological features of cirrhosis on CT include atrophy of the right lobe and medial left lobe (Couinaud segments IV-VIII) with hypertrophy of the left lateral section and caudate (segments I-III), liver surface nodularity (LSN), hepatic fissure expansion including expansion of the gallbladder fossa and the hilarperiportal space, narrowing of the hepatic veins, and development of a right hepatic posterior notch [17-20]. Many of these characteristics can be subjectively identified on imaging, and some can be measured. One measurement used to capture regional hepatic changes in liver disease is the caudate to right lobe (CRL) ratio [21,22]. Other ratios include left lobe-to-right lobe ratio and portahepatis index [23]. The normal caudate to right lobe (C/RL) ratio is less than 0.6, while 0.6 to 0.65 is considered borderline. This is a globally accepted reference value and any value greater than 0.65 is considered to be a case of liver cirrhosis. Using the C/RL ratio, the imaging scientist may be the first to diagnose cirrhosis, especially in its latent stage (when there is no clinical or laboratory indication of liver disease). Some schools of thought have opined that when a patient presents with abnormal liver function test this ratio may be useful in determining the nature of the hepatic disease [24]. The aim of this study is to determine the significance of C/RL ratio in diagnosing liver cirrhosis and if level of accuracy is confirmed, may serve to eliminate the need for liver biopsy in liver cirrhosis.
Materials and Methods
This is a prospective cross-sectional study, which involve the evaluation of the transverse caudate lobe-to-right lobe ratio in patients diagnosed with liver cirrhosis and patients with apparently normal liver who were referred by their physicians for abdominal CT imaging at Union Diagnostics and Clinical Services, Benin City, Nigeria. All the abdominal computed tomography (CT) examinations were performed using a 16- slice Siemens® CT scanner manufactured in Germany. The participants were placed in the supine position in the CT gantry and scanned from the level of about 2 cm above the diaphragm to the iliac region with standard protocols and techniques (slice thickness: 5mm, slice interval: 5 mm, helical scan mode, matrix: 1024*1024, 120 KV, 200 mAs, rotation time: 0.6 seconds, 60 ml of contrapaque ® was injected intravenously for the contrast-enhanced series while the scan was going on using a power injector). The images acquired were analyzed by the researchers and two consultant radiologists. An ethical approval for this study was obtained from the Human Research and Ethics Committee of Faculty of Health Sciences, NnamdiAzikiwe University, Anambra State, Nigeria and management permission to use their facility was also obtained from the study center. The procedure for this study was explained to the participants and their consent was properly sought. All information related to the patients’ identity were treated with high level of confidentiality and used for the purpose of this study only.
Anthropometric measurements including height and weight were obtained and recorded. The weight of the patient was measured using a weighing scale while the height was obtained using a measuring rod with a scale. The BMI was derived from the measured weight and height of the patients using the equation: BMI=Weight (kg)/Height (M)2. The measurement of the liver was done on the CT monitor using the method described by Harbin et al. and illustrated in Figure 1. Three different measurements of the liver were obtained and the average calculated. Serial and recorded tomographs were examined until the main portal vein was identified. A line was drawn parallel to the midsagittal plane through the right lateral wall of the bifurcation of the main portal vein (Figure 1). A second line (line 2) was drawn parallel to line 1 through the most medial margin of the caudate lobe. A third line (line 3) was drawn perpendicular to lines 1 and 2 midway between the main portal vein and the inferior vena cava and extended to the right lateral margin of the liver. The distances (along line 3) between lines 1 and 2(A) was the transverse diameter of caudate lobe and the distance along line 3 between the right margin and line 1(X) was the transverse diameter of the right lobe. The ratio of A to X was calculated as the caudate-to- right lobe ratio. All the data used in this study were collected using structured data proforma and questionnaire. The obtained data were processed and analyzed using Statistical Package for Social Sciences (SPSS) version 21.0 (SPSS Inc. Chicago IL, USA). Data analysis was carried out using Pearson and Spearman Correlation analysis, the chi-square test for categorical data and the student t-test and ANOVA where applicable. At 95% interval, two-tailed p-values less than or equal to 0.05 was considered statistically significant.
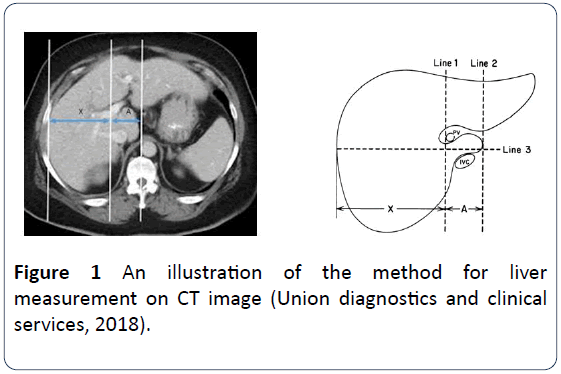
Figure 1: An illustration of the method for liver measurement on CT image (Union diagnostics and clinical services, 2018).
Results
Out of 214 subjects included in this study, those diagnosed with liver cirrhosis with various liver diseases as retrieved from their clinical records were 50% (n=107), while the remaining 50% (n=107) were those with apparently normal liver, which served as the control group. The mean age of the liver cirrhosis patients was) while those of the controls was 46.97 ± 19.36 years (range 19-80 years). There was a statistically significant mean difference between the two study groups (p=0.000). The mean of body mass index (BMI) for liver cirrhosis and the control group subjects was 24.93 ± 4.03kg/m2 and 24.18 ± 2.11 kg/m2 respectively (Table 1). Out of 107 subjects diagnosed with liver cirrhosis, 59.3% (n=63) were males while 40.7% (n=44) were females. In the controls group, males accounted for 54% (n=59) as against 46% (n=48) females. There was no significant difference in the sex distribution of the study subjects (X2=0.564; p=0.484). In the liver diseased group, out of 107 subjects, civic servants were highest 80.7% (n=80) and none were student, while in the control group, both civil servants and self-employed subjects were highest, which is 41.3% (n=44) each respectively. There was statistically significant relationship between the occupations of the two groups (X2=55.986, p=0.000) (Table 2). Among the liver cirrhosis subjects, 59.3% (n=89) had a history of yellowness of eyes while 61.3% (n=92) had history of alcohol use. Out of 61.3% (n=92) identified with history of alcohol consumption, 22% (n=33) had history of alcohol intake for twenty years as highest, followed by those that history of alcohol use for thirty years 19.3% (n=29) and the least were those that had history of alcohol use for fifty years, which is 4% (n=6) (Table 3).
Table 1 Mean of age, weight, height and BMI of study subjects.
| Anthropometric |
Liver cirrhosis Group |
Control group |
P-value |
| Measurement |
Mean±Std |
Range |
Mean±Std |
Range |
| Age (years) |
60.14 ± 13.00 |
39.00-85.00 |
46.97 ± 19.36 |
19.00-80.00 |
0.000* |
| Weight (Kg) |
60.33 ± 5.48 |
50.00-75.00 |
61.98 ± 4.73 |
50.00-75.00 |
0.006* |
| Height (M) |
1.55 ± 0.06 |
1.40-1.70 |
1.59 ± 0.05 |
1.40-1.70 |
0.000* |
| BMI (Kg/M2) |
24.93 ± 4.03 |
13.46-39.38 |
24.18 ± 2.11 |
18.75-33.33 |
0.045* |
Table 2 Socio-demographic characteristics of the subjects.
| Characteristics |
Liver cirrhosis frequency (%) |
Control frequency (%) |
| Sex |
| Male |
63 (59.3) |
59 (55.3) |
| Female |
44 (40.7) |
48 (44.7) |
| Total |
107 (100) |
107 (100) |
| Age (years) |
| < 40 Years |
5 (4.0) |
46 (43.3) |
| 40-49 Years |
22 (20.7) |
20 (18.7) |
| 50-59 Years |
29 (27.3) |
9 (8.0) |
| 60 And Above Years |
51 (48.0) |
31 (29.3) |
| Total |
107 (100) |
107 (100) |
| Occupation |
| Civil Servant |
86 (80.7) |
44 (41.3%) |
| Self Employed |
21 (19.3) |
44 (41.3) |
| Student |
0 (0) |
19 (17.4) |
| Total |
107 (100) |
107 (100) |
Table 3 Medical and social history of study subjects.
| Clinical features |
Liver cirrhosis frequency (%) |
Control frequency (%) |
χ2 |
P-value |
| Yellowness of Eyes |
89 (59.3) |
0 (0) |
|
|
| How long |
| Between one month to one year |
76 (50.7) |
0 (0) |
|
|
| Between one to two years |
13 (8.7) |
0 (0) |
104.942 |
0.000* |
| Above two years |
3 (2.0) |
0 (0) |
|
|
| Diagnosed of Liver disease before |
3 (2.0) |
0 (0) |
|
|
| Do you drink alcohol? |
92 (61.3) |
0 (0) |
|
|
| How long have you been drinking? (years) |
| 0-10 |
9 (6.0) |
0 (0) |
|
|
| Nov-20 |
33 (22.0) |
0 (0) |
|
|
| 21-30 |
29 (19.3) |
0 (0) |
5.552 |
0.981 |
| 31-40 |
15 (10.0) |
0 (0) |
|
|
| 41-50 |
6 (4.0) |
0 (0) |
|
|
NB: The emboldened asterisked numbers are statistically significant
The mean of liver span, right lobe, caudate lobe and C/RL ratio for the liver cirrhosis group are 15.35 ± 0.80 cm, 7.23 ± 1.03 cm, 5.25 ± 0.91 cm and 0.72 ± 0.06 cm respectively, while the mean of liver span, right lobe, caudate lobe and C/RL ratio in the control group are 15.74 ± 1.0 cm, 8.51 ± 0.7 cm, 3.78 ± 0.5 cm and 0.44 ± 0.00 cm respectively. There were statistically significant mean differences in the measured liver dimensions across the two groups (p=0.001, 0.000, 0.000 and 0.000) respectively (Table 4). Comparison of the hepatic dimensions based on sex is shown in Tables 5 and 6. The liver span was significantly higher in normal males and females than in the corresponding cirrhotic subjects (p=0.001, 0.007 respectively). The caudate lobe was significantly higher in cirrhotic male and female subjects compared to the controls (p=0.001), the right lobe of the males and females were significantly smaller in the cirrhotic subjects than in the corresponding normal subjects (p=0.018, 0.001 respectively) (Table 5).
Table 4 Measured liver dimensions in the study subjects.
| CT measurement of the Liver |
Liver cirrhosis Group |
Control group |
P-value |
| Mean ± Std |
Range of liver |
Mean ± Std |
Range of liver |
| Liver Span (cm) |
15.35 ± 0.80 |
13.44-17.41 |
15.74 ± 1.05 |
12.77-19.10 |
0.001* |
| Right Lobe (cm) |
7.23 ± 1.03 |
5.12-11.12 |
8.51 ± 0.72 |
7.18-10.71 |
0.000* |
| Caudate Lobe (cm) |
5.25 ± 0.91 |
3.52-8.03 |
3.78 ± 0.56 |
3.06-5.29 |
0.000* |
| C/RL Ratio |
0.72 ± 0.06 |
0.66-0.89 |
0.44 ± 0.05 |
0.36-0.61 |
0.000* |
NB: The emboldened asterisked numbers are statistically significant
Table 5 Comparison of liver dimensions between subjects of the same sex in each study group.
| Males |
Females |
| CT. measurement of the liver |
Liver cirrhosis |
Control |
P-value |
Liver cirrhosis |
Control |
P-value |
| Mean ± Std |
Mean ± Std |
Mean ± Std |
Mean ± Std |
| Liver span (cm) |
15.51 ± 0.69 |
15.70 ± 1.08 |
0.189 |
15.14 ± 0.90 |
15.80 ± 1.02 |
0.000* |
| Right lobe (cm) |
7.25 ± 0.81 |
8.51 ± 0.77 |
0.000* |
7.23 ± 1.30 |
8.53 ± 0.66 |
0.000* |
| Caudate lobe (cm) |
5.25 ± 0.80 |
3.79 ± 0.57 |
0.000* |
5.23 ± 1.05 |
3.76 ± 0.56 |
0.000* |
| C/RL-ratio |
0.72 ± 0.06 |
0.40 ± 0.06 |
0.000* |
0.72 ± 0.06 |
0.44 ± 0.05 |
0.000* |
NB: The emboldened asterisked numbers are statistically significant
Table 6 Comparison of liver dimensions between subjects of opposite sex in each study group.
| CT measurement of the liver |
Liver cirrhosis |
Control |
| Males |
Females |
p-value |
Males |
Females |
p-value |
| Mean ± Std |
Mean ± Std |
Mean ± Std |
Mean ± Std |
| Liver Span (cm) |
15.51 ± 0.68 |
15.12 ± 0.89 |
0.005* |
15.69 ± 1.08 |
15.80 ± 1.01 |
0.51 |
| Right Lobe (cm) |
7.25 ± 0.81 |
7.21 ± 1.30 |
0.837 |
8.50 ± 0.77 |
8.54 ± 0.65 |
0.701 |
| Caudate Lobe (cm) |
5.25 ± 0.80 |
5.25 ± 1.05 |
0.988 |
3.78 ± 0.57 |
3.76 ± 0.55 |
0.794 |
| C/RL ratio |
0.72 ± 0.06 |
0.72 ± 0.05 |
0.619 |
0.44 ± 0.06 |
0.44 ± 0.05 |
0.474 |
Table 6 compares the liver indices in males and females in each study group. The averages of the liver span, right lobe, caudate lobe and CRL ratio did not differ significantly between male and female cirrhotic subjects (p=0.600, 0.971, 0.809 and 0.682 respectively). Similarly, in the controls, the liver span, caudate lobe, right lobe and CRL ratio did not differ significantly between males and females (p=0.191, 0.358, 0.314 and 0.899 respectively). significantly between males and females (p=0.191, 0.358, 0.314 and 0.899 respectively). significantly between males and females (p=0.191, 0.358, 0.314 and 0.899 respectively). significantly between males and females (p=0.191, 0.358, 0.314 and 0.899 respectively). significantly between males and females (p=0.191, 0.358, 0.314 and 0.899 respectively). significantly between males and females (p=0.191, 0.358, 0.314 and 0.899 respectively).
Discussion
Liver cirrhosis which is among the chronic liver diseases usually occurs from the repeated trauma or insults to the hepatic parenchyma, which normally results to fibrosis and cirrhosis, which is irreversible and the end stage of chronic damage to the liver [25]. It is often a torpid disease; in which majority of the patients remain asymptomatic until the occurrence of decompensation characterized by ascites, hydronephrosis, liver pleural effusion, spontaneous bacterial peritonitis, hepatic encephalopathy or variceal bleeding from portal hypertension [26].
In this study, majority of the patients diagnosed with liver cirrhosis were those within the fifth decade and above with overall mean age of 60.14 ± 13.00, which accounted for over 60% of the total patients with liver cirrhosis while in the control group, younger adults were highest with an overall mean age of 46.97 ± 19.36 years. These findings are not in keeping with the findings of related studies conducted by Lesi et al. [27] in Lagos State, Nigeria, Ndubula et al. [25] and Balla et al. [28] in Sudan. In Lesi et al. [27] study, they reported an average age of 44.1 ± 14 years for the study subjects and 43 ± 11.2 years for the controls. In Ndububa et al. [25] study, which evaluated 145 patients with chronic liver disease, had a mean age 46.8 ± 15.7 years, which was also comparable to the index study. Balla et al. [28] study conducted in Sudan reported an average age of 43.9 years and 38 years respectively for the patients and controls. The differences in our findings could be attributed to the differences in the sample size used for the various studies and the geographical variations of the studies. The mean age of presentation of the patients with liver cirrhosis from the aforementioned studies is usually in the fourth decade. This has been ascribed to the fact that liver cirrhosis and chronic liver disease are treacherous progressive disease, which may not be clinically evident till about seventy five percent of the hepatic cells are affected [29]. Even though, the hepatic insult occurred quite early, the slow progression of the disease may make clinical manifestations show later in life.
In this study, greater numbers of those diagnosed with liver cirrhosis were males when compared with their female counters. This finding is in agreement with findings of similar studies conducted by Balla et al. [28], Giorgio et al. [30], Lesi et al. [27], Ndububa et al. [25] and Adrian et al. In Balla et al. [28] study, males were 62% and females 38%. Giorgio et al, reported males to be 64.9% and females 35.1%. In Lesi et al study, they reported 67% males against 33% females. Ndububa et al. [25] documented 70.3% males and 29.6% females and Adrian et al. study conducted in Switzerland, reported 73.7% males and 26.3% females. This could be attributed to the fact that males are more predisposed to the factors responsible for the development of liver cirrhosis, which include heavy alcohol consumption when compared with their female counterparts [31]. In addition, a study carried by Schimizu et al. [32] in Japan, showed that estradiol is a potent endogenous antioxidant, which suppresses hepatic fibrosis and the greater progression of hepatic fibrosis and hepatocellular carcinoma in men may be due, at least in part, to the lower production of estradiol. Furthermore, it has been reported that after alcohol consumption, estrogen stimulation of Kupffer cells increases their sensitivity to endotoxins and may lead to higher levels of chemical mediators [33]. These findings suggest that chronic alcohol may induce more rapid and more severe liver injury in females than in males, [34]. Although, females are more susceptible to damages associated with alcohol, because they have a smaller volume of distribution and lower gastric alcohol dehydrogenase activity [35].
In this study, the major clinical features at presentation of liver cirrhosis subjects to the clinics included ascites (34.0%), jaundice (59.3%), Renal Mass (24.0%),Gall Bladder Stone (22.7%) and shrunken liver (50.7%). These findings are similar to the findings documented in a related conducted by Lesi et al. [27]. In their study, ascites (66%), hepatomegaly (51%) and jaundice (47%) of the patients respectively. The relatively high number of patients with hepatomegaly in their study may have been due to the fact that the study was inclusive of patients with hepatocellular carcinoma which presents with enlarged macronodular liver. These categories of patients were excluded from our study.
This study, there is a hypertrophy of the caudate lobe in liver cirrhosis. This is in keeping with finding of the study conducted by Lelio et al. [36] in Italy. In their study, which recruited 75 patients with suspected liver cirrhosis or chronic liver disease and 50 normal subjects in Italy, found caudate lobe hypertrophy to be a highly specific sign for liver cirrhosis or others chronic liver disease, noting that it was absent in all the normal subjects examined.
In the index study, the mean value of the caudate lobe in the liver cirrhosis subjects was significantly higher than that of the normal subjects (5.25 ± 0.91 vs 3.78 ± 0.56 cm). This could be attributed to the fact that the venous drainage of the caudate lobe is sustained by emissary veins that pass directly from the caudate lobe to the vena cava, thus fibrosis and subsequent narrowing resulting in obstruction of the hepatic veins in cirrhosis causes greater blood flow through the caudate lobe, thereby resulting in hypertrophy [15].
Previous studies have shown different sensitivities and specificities of the caudate to right lobe ratio in diagnosing liver cirrhosis [28,37]. In a study conducted by Balla et al. [28] in Sudan on 50 patients with liver cirrhosis and other chronic liver disease from schistosomiasis and 20 controls revealed that the caudate-to-right lobe ratio was notably higher in patients with these liver diseases with ratios as high as 0.70 in patients with decompensation. Likewise, Harbin et al. [21] study conducted in United States, reported a sensitivity of 84% and a specificity of 100% in diagnosis of cirrhosis based on the criterion of a caudate-to-right lobe ratio greater than or equal to 0.65 (borderline, 0.60-0.65), while Giorgio et al[30]in Italy, reported a sensitivity of 43% and specificity of 100%. Awaya et al. [11] in Japan, examined 121 patients with pathologically proven chronic liver disease and 115 controls using MRI, reported a sensitivity and specificity of 71.7% and 77.4% respectively.
Report has shown that Computed tomography scan have been reported to be more valuable when compared with ultrasound scans for the evaluation of deep soft tissue structures, and it is the imaging modality employed in this study to position beam attenuation on cirrhotic liver [38]. It is however important to note that in this study 91 (84.7%) of the liver cirrhosis cases had caudate to right lobe ratio greater than 0.66. while 102 (95.3%) of the controls had a caudate to right lobe ratio less than 0.55. Findings from this study have shown that the caudate to right lobe ratio is an objective and reproducible non-invasive assessment of the liver in patients with suspected or clinical features of liver cirrhosis and other related chronic liver disease.
Conclusion
Liver cirrhosis was commonly seen in older adults and also male preponderance was noted in this study. The caudate lobe size as well as caudate-to-right lobe ratio is significantly increased in liver cirrhosis subjects as compared with the controls. Thus, fulfilling the aim of this study, and determining the significance of caudate-to-the right lobe ratio in the diagnosis of liver cirrhosis in a Nigerian population. Therefore, from this study, caudate-to-right lobe ratio shows a very strong reliable parameter with a high reliability for diagnosing liver cirrhosis and other chronic liver diseases, even in its early asymptomatic stage. From all these findings we concluded that; the caudate-to-right lobe ratio in subjects with established liver cirrhosis was significantly higher when compared with that of normal subjects and there was a significant difference in the caudate-to-right lobe ratio of subjects with suspected/ established liver cirrhosis when compared with subjects with apparently normal liver.
24702
References
- Ozaki K, Matsui O, Kobayashi S, Minami T, Kitao A, et al. (2016) Morphometric changes in liver cirrhosis: aetiological differences correlated with progression. Br J Radiol 89: 20150896.
- Mnddrey WC (2001) Update in Hepatology. Annal Internal Med 134: 216-223.
- National Institutes of Health (2002) National Institutes of Health Consensus Development Conference statement: management of hepatitis C: 2002-June 10-12, 2002. Hepatology 36: S3-S20.
- Melato M, Sasso F, Zanconati F (1993) Liver cirrhosis and liver cancer. A study of their relationship in 2563 autopsies. Zentralbl Pathol 139: 25-30.
- Graudal N, Leth P, Marbjerg L, Galloe AM (1991) Characteristics of cirrhosis undiagnosed during life: a comparative analysis of 73 undiagnosed cases and 149 diagnosed cases of cirrhosis, detected in 4929 consecutiveautopsies. J Internal Med 230: 165-171.
- Lim YS, Kim WR (2008) The global impact of hepatic fibrosis and end-stage liver disease. Clinical Liver Disease 12: 733-746.
- Davies GL, Roberts WL (2010) The health care burden imposed by liver disease in aging Baby Boomers. Corr Gastroenterology Rep 12: 1-6.
- Nwokediuko SC, Osuala PC, Uduma UV, Alaneme AK, Onwuka CC, et al. (2013) Patterns of liver disease admission in a Nigerian tertiary hospital. Nigerian J Clin Prac 16: 339-342.
- Desmet VJ, Roskams N (2004) Cirrhosis reversal, a duel between dogma and myth: Eur J Radiol 40: 860-867.
- Awaya H, Mitchell DG, Kamishima T, Holland G, Ito K, et al. (1999) Spectrum of imaging findings of the liver in end-stage cirrhosis. Am J Radiol 173: 1031-1036.
- Regev A, Bertho M, Jeffers LJ, Milikovski C, Molina EG, et al. (2002) S ampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterul 97: 2614-2618.
- Doyle DJ, Malley ME, Jang HJ (2007) Value of unenhanced phase for detection of hepatoellular carcinoma when performing multiphase CT in patients with cirrhosis. J Comp Assis Tomograph 31: 86-92.
- Chalasani B, Horlander H (1999) CT screening for hepatocellular carcinoma in patients with advanced cirrhosis: Am J Gastroenterol 94: 2988-2993.
- Dodd GD, Baron RL, Oliver JH (1999) Spectrum of imaging findings of the liver in end-stage cirrhosis: Part I, gross morphology and diffuse abnormalities. Am J Roentgenol 173: 1031.
- Torres WE, Whitmire LF, Gedgaudasmcclees K (1986) Computed -tomography of the hepatic morphological changes in cirrhosis of the liver. J Comp Assis Tomograph 10: 47.
- Horowitz JM, Venkatesh SK, Ehman RL (2017) Evaluation of hepatic fibrosis: a review from the Society of Abdominal Radiology Disease focus panel. Abdominal Radiol 42: 2037‐ 2053.
- Ito K, Mitchell DG, Kim MJ, Awaya H, Koike S, et al. (2003) Right posterior hepatic notch sign: a simple diagnostic MR finding of cirrhosis. J Magnetic Res Imag 18: 561‐ 566.
- Ito K, Mitchell DG, Gabata T, Hussain SM (1999) Expanded gallbladder fossa: simple MR imaging sign of cirrhosis. Radiol 211: 723‐726.
- Tan KC (2008) The right posterior hepatic notch sign. Radiology 248: 317-318.
- Harbin WP, Robert NJ, Ferrucci JT (1980) Diagnosis of cirrhosis based on regional changes in hepatic morphology: a radiological and pathological analysis. Radiology 135: 273‐283.
- Giorgio A, Amoroso P, Lettieri G (1986) Cirrhosis: value of caudate to right lobe ratio in diagnosis with US. Radiology 161: 443‐445.
- Furuse J, Maru Y, Yoshino M (2000) Assessment of arterial tumor vascularity in small hepatocellular carcinoma; comparison between US and CT. Eur J Radiol 36: 20-27.
- de Ledegen V, Person B, Legoux JL, Sidaner A, Desaint B, et al. (2000) Prevention of Biliary Stent Occlusion by Ursodeoxycholic Acid plus Norfloxacin. Dig Dis Sci 45: 114-145.
- Ndububa DA, Ojo OS, Adetiloye VA, Aladegbaiye AO, Adebayo RA, et al. (2010) The contribution of alcohol to chronic liver disease in patients from South-west Nigeria. Nigerian J Clin Prac 13: 360-364.
- Echejoh GO, Tanko MN, Manasseh AN, Silas OA, Ogala-Echejoh SE, et al. (2008) Liver cirrhosis in Jos, North-Central Nigeria. J Med 3: 26-29.
- Lesi OA, Kehinde MO, Anomneze EE (2004) Chronic liver disease in Lagos: a clinicopathological study. Nigerian Postgrad Med J 11: 91-96.
- Balla EA, Abdo MA, Ayad CE (2013) Evaluation of Caudate and Right hepatic lobes ratio in patients with Schistosomiasismansoni using ultrasound in Al-Fao area. Ind J Sci 4: 11-16.
- Bosetti C, Levi F, Luccini F, Zatonski WA, Negri E, et al. (2007) Worldwide mortality from cirrhosis: an update to 2002. World J Hepatol 46: 827-839.
- Giorgio A, Amoroso P, Lettieri G, Fico P, De Stephano G, et al. (1986) Cirrhosis: value of caudate to right lobe ratio in diagnosis with Ultrasound. Radiology 161: 443-445.
- Frieden TR, Ozick L, McCord C, Nainan OV, Workman S, et al. (2003) Chronic Liver diseases in Central harlemi the role of abachol and viral hepatitis. Hepatology 29: 3.
- Schimizu I, Kohno N, Tamaki K, Shono M, Huang HW, et al. (2007) Female hepatology: Favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J Gastroenterol 13: 4295-4305.
- Ikejima K, Enomoto N, Limuro Y, Ikejima A, Fang D, et al. (1998) Estrogen increases sensitivity of hepatic Kupffer cells to endotoxin. Am J Physiol 274: G669-G676.
- Poynard T, Mathurin P, Lai CL, Guyader D, Poupon R, et al. (2003) A comparison of fibrosis progression in chronic liver diseases. J Hepatol 38: 257-227.
- Lieber CS (1997) Ethanol metabolism, cirrhosis and alcoholism. Clinical Chimica Acta 257: 59-84.
- Di Lelio A, Cestari C, Lomazzi A, Beretta L (1989) Cirrhosis: diagnosis with sonographic study of the liver surface. Radiololgy 172: 389-392.
- Hess CF, Schmiedl U, Koelbel G, Knecht R, Kurtz B (1989) Diagnosis of liver cirrhosis with ultrasound scan: receiver-operating characteristic analysis of multidimensional caudate lobe indexes. Radiology 171: 349-351.
- Heidelbaugh JJ, Sherbondy M (2006) Cirrhosis and chronic liver failure: part 1. Diagnosis and evaluation. Am Physician Assoc 74: 756-762.






