Keywords
COVID-19; Vaccine; Vaccination; Immune response
Abbreviations: RNA: Ribonucleic Acid; DNA: Deoxyribonucleic Acid; IgG: Immunoglobulin G; ELISpot: Enzyme-linked Immunospot; SFC: Spot Forming Cells
Introduction
The coronavirus disease 2019 (COVID-19) outbreak was first reported in Wuhan, China, in late 2019. The respiratory viral pathogen severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) has infected at least 170.6 million individuals and killed more than 3.5 million people globally till May 30, 2021, and counting [1,2]. Physical-distancing and other transmission-mitigation strategies implemented in most countries during the current pandemic to prevent most citizens from being infected [3- 6] although, the mammoth scale of the outbreak of the COVID-19 pandemic has overwhelmed healthcare systems globally. Consequently, many countries worldwide have prioritized vaccine development for the containment of COVID-19. So far, there are 60 vaccine candidates in clinical trials, 13 of which are in the phase 3 stage. Although several leading COVID-19 vaccines have been shown to confer protection against SARS-CoV2 infection [7-9], the emergence of novel SARSCoV-2 variants [10,11], and the continuous decrease in the titres of antibodies in vaccinated individuals [12], raises public health concerns regarding the efficacy and duration of protection induced by the administration of such first-generation vaccines, which were developed rapidly for emergency use. Currently, many COVID-19 vaccines developed for emergency use have their own advantages and disadvantages. For instance, mRNA vaccines such as BNT162b2 and mRNA-1273 have been shown to induce >90% protection in the early stages of SARSCoV-2 infection. However, the incidence of adverse reactions has raised concerns regarding the safety of such vaccines. Furthermore, stringent cold chain requirements for mRNA vaccines pose a significant logistical challenge [7,9,13]. Inactivated vaccines and recombinant protein-based vaccines, which are also part of the leading vaccine candidates, have exhibited lower incidences of adverse reactions. However, compared to mRNA vaccines, they exhibit inferior immunogenicity, even with the use of adjuvants [14–17]. T cell responses induced by the inactivated vaccine BBIBP-CorV [15] and recombinant vaccine ZF2001 [18] are relatively low. On the other hand, adenovector based vaccines, such as Ad5- vectored vaccine (CanSino), induce strong T cell responses, but only less effective neutralizing antibody (NAb) responses than other approaches in humans [19]. These differences are likely to become more pronounced after the approval of such vaccine candidates for use in large populations.
the incidence of adverse reactions has raised concerns regarding the safety of such vaccines. Furthermore, stringent cold chain requirements for mRNA vaccines pose a significant logistical challenge [7,9,13]. Inactivated vaccines and recombinant protein-based vaccines, which are also part of the leading vaccine candidates, have exhibited lower incidences of adverse reactions. However, compared to mRNA vaccines, they exhibit inferior immunogenicity, even with the use of adjuvants [14–17]. T cell responses induced by the inactivated vaccine BBIBP-CorV [15] and recombinant vaccine ZF2001 [18] are relatively low. On the other hand, adenovector based vaccines, such as Ad5- vectored vaccine (CanSino), induce strong T cell responses, but only less effective neutralizing antibody (NAb) responses than other approaches in humans [19]. These differences are likely to become more pronounced after the approval of such vaccine candidates for use in large populations.
Categories of covid-19 vaccines and immune response
There are more vaccine candidates simultaneously in the pipeline for COVID-19 than ever before for an infectious disease. All of them are trying to achieve the same thing – immunity to the virus, and some might also be able to stop transmission. They do so by stimulating an immune response to an antigen, a molecule found on the virus. In the case of COVID-19, the antigen is typically the characteristic spike protein found on the surface of the virus, which it normally uses to help it invade human cells. There are four categories of vaccines: INACTIVED VIRUS, PROTEIN SUBUNIT, VIRAL VECTOR and NUCLEIC ACID (RNA AND DNA). Some of them try to smuggle the antigen into the body, others use the body’s own cells to make the viral antigen [23,24].
As we can see in Figure 1, Vaccines safely deliver an immunogen (antigen able to elicit an immune response) to the immune system in order to train it to recognize the pathogen when it is encountered naturally by activating, CD4+ helper T cells that in turn stimulate: (i) B-cells to produce neutralizing antibodies specific to the virus (ii) CD8+ cytotoxic T cells to recognize and kill cells infected by the virus [25].

Figure 1 An immune response is induced by vaccines [25].
Inactivated virus vaccines: Many conventional vaccines use whole viruses to trigger an immune response. There are two main approaches. Live attenuated vaccines use a weakened form of the virus that can still replicate without causing illness. Inactivated vaccines use viruses whose genetic material has been destroyed so they cannot replicate, but can still trigger an immune response. Both types use well- established technology and pathways for regulatory approval, but live attenuated ones may risk causing disease in people with weak immune systems and often require careful cold storage, making their use more challenging in low-resource countries. Inactivated virus vaccines can be given to people with compromised immune systems but might also need cold storage [24].
In inactivated virus vaccines, the genetic material of the virus has been destroyed to stop disease producing capacity. Inactivated virus cannot replicate inside the body, so higher doses are needed. Sometimes, an adjuvant (molecules that stimulate the immune system) is used to help strengthen the immune response. These vaccines generally only induce antibody-mediated immunity (not cell-mediated immunity) [25].
Protein subunit vaccines: Subunit vaccines use pieces of the pathogen - often fragments of protein - to trigger an immune response. Doing so minimises the risk of side effects, but it also means the immune response may be weaker. This is why they often require adjuvants, to help boost the immune response. An example of an existing subunit vaccine is the hepatitis B vaccine [24].
Subunit vaccines use the antigen of the virus without any genetic material, usually with an adjuvant to give a better immune response. Usually made using recombinant expression system (made in a cell without using the virus). With the help of antigenpresenting cells, the antigens are recognized by T helper cells as with a real viral infection. Subunit vaccines generally induce mainly antibody- mediated immunity [25]. Adjuvants can enhance antibody response and also cell-mediated immunity.
Nucleic acid vaccines: Nucleic acid vaccines use genetic material – either RNA or DNA – to provide cells with the instructions to make the antigen. In the case of COVID-19, this is usually the viral spike protein.
Once this genetic material gets into human cells, it uses our cells' protein factories to make the antigen that will trigger an immune response. The advantages of such vaccines are that they are easy to make, and cheap. Since the antigen is produced inside our own cells and in large quantities, the immune reaction should be strong. A downside, however, is that so far, no DNA or RNA vaccines have been licensed for human use, which may cause more hurdles with regulatory approval. In addition, RNA vaccines need to be kept at ultra-cold temperatures, -70?C or lower, which could prove challenging for countries that don’t have specialized cold storage equipment, particularly low- and middle-income countries [24].
RNA vaccines are antigen-coding strands of messenger RNA (mRNA) delivered inside a lipid coat. Once inside cells, the mRNA is translated the protein antigen. The antigen is recognized, inducing an immune reaction [25] and seen by body as if virus inside cell so induces T-helper and cytotoxic T-cells, and antibodies. mRNA also recognized by cells as ‘pathogen’ stimulating strong immune response.
Viral vector vaccines: Viral vector vaccines also work by giving cells genetic instructions to produce antigens. But they differ from nucleic acid vaccines in that they use a harmless virus, different from the one the vaccine is targeting, to deliver these instructions into the cell. One type of virus that has often been used as a vector is adenovirus, which causes the common cold. As with nucleic acid vaccines, our own cellular machinery is hijacked to produce the antigen from those instructions, in order to trigger an immune response. Viral vector vaccines can mimic natural viral infection and should therefore trigger a strong immune response. However, since there is a chance that many people may have already been exposed to the viruses being used as vectors, some may be immune to it, making the vaccine less effective [24].
Viral vector vaccines use a non-coronavirus vector modified to include a gene that encodes a target antigen for examples: adenovirus, measles virus, vesicular stomatitis virus. It can be replicating or non-replicating. Non-replicating; infects a cell and produces SARS-CoV-2 antigen in that cell but not new virus and Replicating; upon infection produces SARS-CoV-2 antigen in that cell and new virus which infects other cells [25]. The SARS-CoV-2 antigen inside cells seen by body as if SARS-CoV-2 infection and induces T helper cells and cytotoxic T cells (Figure 2).
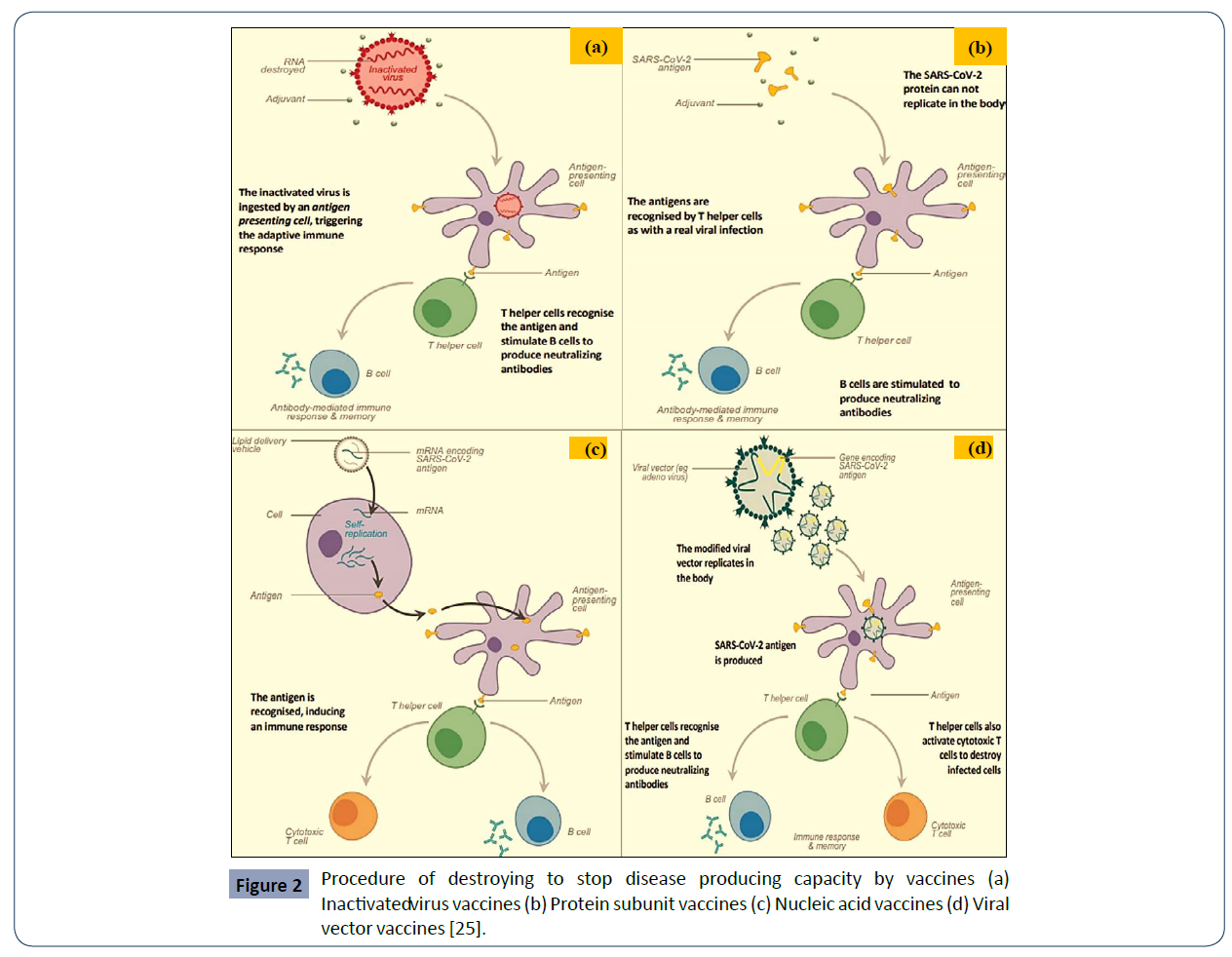
Figure 2 Procedure of destroying to stop disease producing capacity by vaccines (a) Inactivatedv irus vaccines (b) Protein subunit vaccines (c) Nucleic acid vaccines (d) Viral vector vaccines [25].
Advantages and disadvantages
An immunogen is a specific type of antigen that is able to elicit an immune response. The choice of immunogen for vaccines impacts what type of immune response is induced; as well as safety, development time, production time, costs and access to vaccines. In this section mainly discussed the advantages and disadvantages of the immunogens used in COVID-19 vaccines as shown in Table 1 [25].
| Immunogen |
Advantages |
Disadvantages |
| Inactivated virus |
Induces strong antibody response |
Requires large quantities of virus, low or no cellular
response |
| Protein subunit |
May have fewer side effects
than whole virus (redness, swelling at injection site) |
May be poorly immunogenic; complex process |
| Nucleic acid |
Strong cellular immunity;
rapid development |
Relatively low antibody
response |
| Viral vector |
Rapid development, strong cellular response, relatively easy to produce |
Prior exposure to vector virus (eg. adenovirus) may reduce immunogenicity, some vectors require boosting with a
different vector |
Table 1 Immunogen’s advantages and disadvantages used in the vaccines.
Study on age-dependent immune responses against SARS-CoV- 2 after vaccination: In December 2020, the first vaccines for COVID-19 were approved worldwide and the first vaccinations were carried out [26-29]. While the German Standing Committee on 80 Vaccination (STIKO) recommends immunization against SARS-CoV-2, access to the vaccine in Germany and many other countries worldwide at the beginning of 2021 is offered in a prioritization procedure due to limited availability. First, groups of people who are at particularly high risk for severe courses of COVID-19 disease or who are professionally in close contact with such vulnerable people were vaccinated. These two prioritized groups include senior residents of nursing homes aged ≥ 80 years, and 86 their caregivers typically aged ≤ 65 years. A recent, thorough study using mathematical modeling to investigate vaccine prioritization strategies supports the preferential vaccination of the elderly. This study describes a scenario where cumulative incidence rates were minimized when vaccination of the population aged 20-49 years was prioritized, while mortality was decreased when the population aged 60 years or older was prioritized. This model took age- structure, age-related efficacy, and infection-fatality rates into account. They conclude that prioritizing the population aged>60 years, thus directly protecting the vulnerable population, would decrease mortality rates, a strategy that is currently employed by various nations but without the support of recent and thorough data [30].
They conducted a cohort study with two age groups, young vaccines below the age of 60 and elderly vaccines over the age of 80, to compare their antibody responses to the first and second dose of the BNT162b2 COVID-19 vaccination. While the majority of participants in both groups produced specific IgG antibody titers against SARS-CoV-2 spike protein, titers were significantly lower in elderly participants. Although the increment of antibody levels after the second immunization was higher in elderly participants, the absolute mean titer of this group remained lower than the <60 group. After the second vaccination, 31.3% of the elderly had no detectable neutralizing antibodies in contrast to the younger group, in which only 2.2% had no detectable neutralizing antibodies. Their data [31] suggested that lower frequencies of neutralizing antibodies after BNT162b2 vaccination in the elderly population may require earlier revaccination to ensure strong immunity and protection against infection.
Another study was conducted by Melissa K Andrew, Department of Medicine, Dalhousie University joint work with Janet E McElhaney, Health Sciences North Research Institute, Canada [32]. Their results are part of a larger single-blind, randomized, controlled, phase 2/3 trial of the ChAdOx1 nCoV-19 vaccine (which is a replication-defective chimpanzee adenovirus-vector vaccine) with a MenACWY meningococcal vaccine comparison group. The study design was complex, with participants randomly assigned using block randomization to one of ten different groups, and older adults were only enrolled after initial determination of safety in the youngest age group (aged 18–55 years). Participants in the two older age groups (aged 56–69 and ≥ 70 years) were further randomly assigned to receive either one dose (day 0) or two doses (day 0 and a boost dose on day 28) of vaccine. The ChAdOx1 nCoV-19 groups were also sequentially recruited to receive either a low dose or (after demonstration of safety) a standard dose of the vaccine. In this immunogenicity subgroup of the larger study, 560 healthy adults were included, distributed among the three age groups (160 participants aged 18–55 years, of whom 100 received the COVID-19 vaccine; 160 aged 56–69 years, of whom 120 received the COVID-19 vaccine, and 240 aged ≥70 years, of whom 200 received the COVID-19 vaccine). 280 (51%) of 552 analyzed participants were female and the median age in the 18–55 years group was 43·0 years (IQR 33·6–48·0), in the 56–69 years group was 60·0 years (57·5–63·0), and in the 70 years and older group was 73·0 years (71·0–76·0). For 7 days after each dose, participants completed diary cards for solicited local and systemic adverse events. Serious adverse events were recorded and monitored for 1 year. Severity of reactions and adverse events was graded as mild, moderate, or severe, depending on their effect on daily activities. Immune responses were measured using assays of anti-spike protein IgG and neutralising antibody titres for humoral immunity and IFN-γ enzyme-linked immunospot (ELISpot) for cell-mediated immunity.
They found that both local and systemic reactions were more common with ChAdOx1 nCoV-19 than with MenACWY, but decreased with increasing age. For example, in those who received the ChAdOx1 nCoV-19 two standard-dose regimen, 43 (88%) of 49 participants aged 18–55 years, 22 (73%) of 30 aged 56–69 years, and 30 (61%) of 49 aged 70 years and older reported at least one local reaction (most commonly injectionsite pain and tenderness) and 42 (86%) of 49 participants in the 18–55 years group, 23 (77%) of 30 in the 56–69 years group, and 32 (65%) of 49 in the 70 years and older group reported at least one systemic reaction (most commonly fatigue, headache, feverishness, and myalgias; these were graded as severe in seven [5%] of 128 participants after the prime dose and one [1%] of 127 participants after the boost dose). 13 participants had serious adverse events during the study period, none of which were judged to be due to study vaccine. The decrease in local and systemic reactions with increasing age might be explained by the anti-inflammatory response to low-grade chronic inflammation, and suppression of acute inflammatory processes [32].
Immunogenicity was robust and similar across age groups, as long as a boost dose was provided. Anti-spike protein IgG responses at 28 days after the boost dose were similar among the three age groups (in the standard-dose groups: 18–55 years, median 20 713 arbitrary units [AU]/per Ml [IQR 13 898–33 550], n=39; 56–69 years, 16 170 AU/mL [10 233–40 353], n=26; ≥ 70 years, 17 561 AU/mL [9705–3 7796], n=47; p=0·68), and 208 (>99%) of 209 participants in the boost dose groups had neutralizing antibodies by day 14 after the last vaccination. In IFN-γ ELISpot assays enumerating antigen-specific T cells done for those in the prime-boost standard-dose group, T-cell responses peaked at 14 days after a single standard dose and did not increase significantly after a boost dose (18–55 years, median 1187 spot forming cells [SFCs] per million peripheral blood mononuclear cells [IQR 841–2428], n=24; 56–69 years, 797 SFCs [383–1817], n=29; and ≥ 70 years 977 SFCs [458–1914], n=48; p=0·46). The authors stated that these results based on IFN-γ ELISpot will be followed up with a more detailed analysis of other measures of cell- mediated immunity [33-37].
The strengths of the study included a large sample with a wide age range, and a robust trial design. The inclusion of measures of cell-mediated immunity is important given the limitations of relying solely on antibody titres in older adults.
The main study limitations were its single-blind design, the inclusion of few participants older than 80 years, and exclusion of people with substantial underlying chronic illnesses and frailty [38-41]. Overall, Ramasamy and colleagues summaries that the ChAdOx1 nCoV-19 vaccine is better tolerated in older adults than younger adults and has similar immunogenicity across all age groups after a boost dose [32,42].
Conclusion
The concept of immunosenescence (waning of immune responses) is important for understanding vaccine responses in older adults. There is increasing evidence that immunosenescence is not universally or evenly experienced with biological ageing but is part of what contributes to the variability in susceptibility that is seen with frailty and an increasing burden of health conditions. So the story is more complex than simply older age brings immunosenescence. Frailty is increasingly understood to affect older adults' responses to vaccines for infections such as influenza, shingles, and pneumococcus. Even when a measure of frailty has not been included in a study upfront, generation of a robust frailty measure using data already collected is possible. A plan for how to consider frailty in COVID-19 vaccine development is important. Involving geriatricians could bring a key lens to assist with planning these ongoing studies focusing on older adults and interpreting the results. Consideration of the dosing would be important. It is encouraging that more studies in older adult populations are underway and will hopefully bring opportunities to implement nuanced analyses of how underlying health status and frailty affect vaccine safety, reactogenicity, immunogenicity, and efficacy in older adults in real-world settings. Older adults (across the full spectrum of frailty) and those who care about them are eagerly awaiting this progress towards safe and effective COVID-19 vaccines.
Declarations Conflicts of Interest
The author declares no conflicts of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Availability of Data and Materials
Not applicable.
Funding
Not applicable
39213
References
- World Health Organization (2020) WHO coronavirus disease (COVID-19) dashboard, USA. WHO, Geneva.
- Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, et al. (2020) Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol 20: 615-632.
- Chou R, Dana T, Buckley DI, Selph S, Fu R, et al. (2020) Epidemiology of and risk factors for coronavirus infection in health care workers. 2020. Ann Intern Med 173: 120-136..
- Flaxman S, Dana T, Buckley DI, Selph S, Fu R, et al. (2020) Estimating the effects of nonpharmaceutical interventions on COVID-19 in Europe. Nature 584: 257-261.
- Sanche S, Lin YT, Xu C, Romero-Severson E, Hengartner N, et al. (2020) High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis 26: 1470-1477.
- World Health Organization (2020) Draft landscape of COVID-19 candidate vaccines, USA. WHO, Geneva.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, et al. (2020) Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med 383: 2603-2615.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, et al. (2020) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397: 99-111.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, et al. (2020) An mRNA vaccine against SARS-CoV-2-preliminary report. N Engl J Med 383: 1920-1931.
- CDC (2020) Implications of the Emerging SARS-CoV-2 Variant VOC. U.S Department of Health & Human Services, USA.
- Davies NG, Barnard RC, Jarvis CI, Kucharski AJ, Munday J, et al. (2020) Estimated transmissibility and severity of novel SARS-CoV-2 Variant of concern 202012/01 in England. Science 372.
- Widge AT, Rouphael NG, Jackson LA (2020) Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 384: 80-82.
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, et al. (2020) Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 396: 1979-1993.
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, et al. (2020) Safety and immunogenicity of an inactivated SARS-CoV- 2 vaccine, BBIBP-CorV: a randomised, double-blind, placebocontrolled, phase 1/2 trial. Lancet Infect Dis 21: 39-51.
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, et al. (2020) Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 324: 1–10.
- Keech C, Albert G, Cho I, Robertson A, Reed P, et al. (2002) Phase 1-2 trial of a SARS-CoV2 recombinant spike protein nanoparticle vaccine. N Engl J Med 383: 2320–2332.
- Zhang Y, Zeng G, Pan H, Changgui Li C, Hu Y, et al. (2020) Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, doubleblind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 21: 181-192.
- Yang S, Li Y, Dai L, Wang J, He P, et al. (2020) Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD protein vaccine against COVID-19 in adults: pooled analysis of two randomized, double-blind, placebocontrolled, phase 1 and 2 trials. Lancet Infect Dis 21: 1107-1119.
- Zhu FC, Guan XH, Li YH, JY, Jiang T, et al. (2020) Immunogenicity and safety of a recombinant adenovirus type-5- vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 396: 479-488.
- Dagotto G, Yu J, Barouch DH (2020) Approaches and challenges in SARS-CoV-2 vaccine development. Cell Host Microbe 28: 364–370.
- Excler JL, Kim JH (2019) Novel prime-boost vaccine strategies against HIV-1. Expert Rev Vaccines 18:765-779.
- Lu S (2009) Heterologous prime-boost vaccination. Curr Opin Immunol 21: 346-351.
- He Q, Mao Q, An C, Zhang J, Gao F, et al. (2021) Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg Microbes Infect 10: 629-637.
- Gavi (2021) There are four types of COVID-19 vaccines: here’s how they work. Vaccines work, USA.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, et al. (2020) An mRNA Vaccine against SARS-CoV-2 Preliminary Report. N Engl J Med 383: 1920-1931.
- Kaur SP, Gupta V (2020) COVID-19 Vaccine: A comprehensive status report. Virus Res 288: 198114.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, et al. (2020) Safety and Efficacy of the BNT162b2 mRNA Covid-19 416 Vaccine. N Engl J Med 383: 2603-2615.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, et al. (2021) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397: 99-111.
- Bubar KM, Reinholt K, Kissler SM, Lipsitch M, Cobey S, et al. (2021) Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Sci 371: 916-21.
- Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, et al. (2021) Age-dependent immune response to the 2 Biontech/Pfizer BNT162b2 COVID-19 3 vaccination. Clin Infect Dis.
- Andrew MK, McElhaney JE (2020) Age and frailty in COVID-19 vaccine development. Lancet 396: 1942-1944.
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, et al. (2007) Inflammaging and antiinflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128: 92-105.
- McElhaney JE, Andrew MK, Haynes L, Kuchel GA, McNeil SA, et al. (2020) Influenza vaccination: accelerating the process for new vaccine development in older adults. Interdiscip Top Gerontol Geriatr 43: 98–112.
- McElhaney JE, Verschoor CP, Andrew MK, Haynes L, Kuchel GA, et al. (2020) The immune response to influenza in older humans: beyond immune senescence. Immun Ageing 17: 10.
- Joint Committee on Vaccination and Immunisation (2020) Priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI, 25 September 2020. UK Government, UK.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381: 752-762.
- Fulop T, McElhaney J, Pawelec G, Cohen AA, Morais JA, et al. Frailty, inflammation and immunosenescence. Interdiscip Top Gerontol Geriatr 41: 26–40.
- Andrew MK, Shinde V, Ye L, Hatchette T, Haguinet F, et al. (2017) The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 216: 405-414.
- Curran D, Kim JH, Matthews S, Dessart C, Levin MJ, et al. (2020) Recombinant zoster vaccine is efficacious and safe in frail individuals. J Am Geriatr Soc 69: 744-752.
- Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, et al. (2009) Immunological responses to pneumococcal vaccine in frail older people. Vaccine 27: 1628–1636.
- Curran D, Andrew MK, Levin MJ, Turriani E, Matthews S, et al. (2019) Evaluation of two frailty indices, with practical application in a vaccine clinical trial. Hum Vaccin Immunother 15: 2960-2968.







