Keywords
Influenza; H5N1; Pharmaceutical supply chain; Sustainable supply chain management; Oseltamivir phosphate; Tamiflu; Shikimic acid
Introduction
Influenza has been a serious threat to public health and is a key topic in research agenda. In general, there are three main types of influenza virus: A, B and C. Types A and B can cause severe illness while Type C causes mild symptoms. In particular, H5N1 is a virulent subtype of the influenza A virus, which can cause serious illness in humans [1]. In the context of avian influenza (bird flu), it is caused by a type A virus that is spread from bird to bird through respiratory secretions and contact with contaminated droppings [2].
The outbreak of avian influenza is determined by the ability to infect and pass efficiently between humans [3]. Mohan et al. [4] found that transmission of the H5N1 virus can occur in four key methods: (1) bird to bird; (2) bird to animals; (3) bird to humans; and (4) human to human. Some kinds of birds, notably water birds, act as hosts for influenza viruses by carrying the virus in their intestines and shedding it. Infected birds shed virus in nasal secretions, saliva and faeces. Susceptible birds touch with respiratory, contaminated nasal or faecal material from infected birds, and hence, susceptible birds are turned into infected birds.
Death of people has generated from avian influenza virus H5N1 and Tang and Lau [3] have investigated Hong Kong avian influenza circumstances. Hong Kong was the first place attacked by H5N1 infection. In 1997, the transmission of avian H5N1 influenza viruses to 18 humans in Hong Kong with 6 deaths established that avian influenza viruses can transmit to and create lethal infection in humans. The transmission of influenza viruses to mammals and other avian species has been taken into consideration. Since 2003, avian influenza spread among birds worldwide from time to time. The cumulative number of confirmed human cases for avian influenza (H5N1) is summarized in Table 1.
| Country |
2003-2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
Total |
| Azerbaijan |
8 |
0 |
0 |
0 |
0 |
0 |
0 |
8 |
| Bangladesh |
1 |
0 |
2 |
3 |
1 |
0 |
0 |
7 |
| Cambodia |
9 |
1 |
8 |
3 |
26 |
9 |
0 |
56 |
| Canada |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
| China |
38 |
2 |
1 |
2 |
2 |
2 |
2 |
52 |
| Djibouti |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| Egypt |
90 |
29 |
39 |
11 |
4 |
37 |
136 |
346 |
| Indonesia |
162 |
9 |
12 |
9 |
3 |
2 |
2 |
199 |
| Iraq |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
| Lao People’s Democratic Republic |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
| Myanmar |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| Nigeria |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| Pakistan |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
| Thailand |
25 |
0 |
0 |
0 |
0 |
0 |
0 |
25 |
| Turkey |
12 |
0 |
0 |
0 |
0 |
0 |
0 |
12 |
| Vietnam |
112 |
7 |
0 |
4 |
2 |
2 |
0 |
127 |
| Total |
468 |
48 |
62 |
32 |
39 |
52 |
143 |
844 |
Source: World Health Organization (2015); Website: https://www.who.int/en/
Table 1: Cumulative number of confirmed human cases for Avian Influenza (H5N1), 2003-2015.
Influenza, especially H5N1, remains a major global human health issue due to the risk of a major pandemic, and therefore, studies of inhibitors or vaccines of H5N1 have attracted worldwide attention [5]. Oseltamivir phosphate, popularly known as Tamiflu (Figure 1), is one of the most potent orally active neuraminidase inhibitors used for the treatment of human influenza and H5N1 avian flu infections [5-7]. Tamiflu was developed by Gilead Science [6] and Roche [8-10]. It can be orally administered, and, due to the ease of usage, it is a commonly prescribed anti-influenza drug in clinical use. In fact, Tamiflu has been widely used not only as a front-line therapy for H5N1 influenza [7,11,12] and H1N1 influenza [13], but also as a preventive agent against an unpredictable outbreak of pandemic influenza [14] (Figure 1).
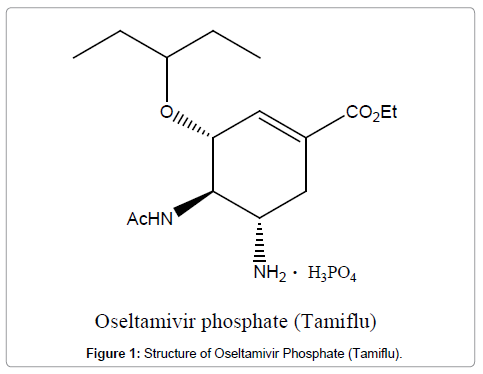
Figure 1: Structure of Oseltamivir Phosphate (Tamiflu).
In view of the importance of Tamiflu in treatment of influenza, related issues and development in pharmaceutical industry should be addressed and updated. In this paper, we will (1) highlight the concepts of Supply Chain Management (SCM) in pharmaceutical industry; (2) describe the recent advance in the synthetic chemistry of Tamiflu, focusing on the new approaches based on inexpensive starting materials. This paper should be of interest to health care study and is expected to provide insights for the drug and supply chain management industry.
Sustainable Supply Chain Management (SSCM)
In the literature, supply chain management (SCM) firstly appeared in the mid-1980s. Starting from the early 1990s, academic scholars have contributed to the structural development of SCM. SCM is evolving functions from ‘operational’ domain to ‘strategic’ domain. In the drug industry, SCM initiatives are growing in popularity due to organizations seek to improve profitability and reduce costs in a competitive environment [15].
In the last two decades, SCM is a broad topic and related studies have been conducted by academicians and business management practitioners. Sustainability is one of the prominent research fields in SCM, namely sustainable supply chain management (SSCM) [16]. According to Seuring and Muller [17], SSCM is defined as “the management of material, information and capital flows as well as cooperation among companies along the supply chain while taking goals from all three dimensions of sustainable development, i.e., economic, environmental and social, into account which are derived from customer and stakeholder requirements”.
Based on the study by Beske et al. [16], we illustrate here the key elements of SSCM practices in the drug industry. Under SSCM, the core elements include: (1) Strategic Orientation means that drug industry requires placing equal importance on all three dimensions (i.e., economic, social and environmental) of sustainability for their decision making. (2) Continuity is concerned about the firms to select qualified supply chain partners and establish long-term relationships with supply chain partners in the drug industry. (3) Collaboration is regarding how to logistically and technically integrate the key information and partners in the process level of SSCM. In the drug industry, joint development could contribute to developing new technologies, processes and products. (4) Risk Management discusses how the firms implement different practices of risk management to mitigate the risks. The various ways include individual monitoring of specific suppliers, pressure group management and standardization of standards and certification to minimize the supply chain risk. (5) Pro-activity describes that the stakeholders are actively engaged and learning from each other in the supply chain. Innovation is the core elements in the dynamic environments of sustainable markets.
As for the case of drug production in the industry, the production cost is the main determinant of the cost of Tamiflu. A series of production cost (i.e., fixed cost relevant with production of clinical trial supplies, infrastructure cost and animal testing during the clinical periods) should be considered [18]. A complex Tamiflu production process is required for manufacturers’ capacities and different suppliers offer different ingredients or components around the world.
Key Findings in the Past Studies
Generally, if the synthesis of a drug can be achieved in a large scale using cheap starting materials and employing a short and efficient route, the production cost would be significantly lowered. The industrial manufacturing process of Tamiflu employs (−)-shikimic acid as the raw material [19]. The commercial synthesis involves a 10- step process of complex chemical reactions [20]. Therefore, design of synthetic routes which have shorter paths and utilize cheaper starting materials would not only lower the cost of production of Tamiflu, but also increase the efficiency of the process. We will here first discuss how the cost of starting materials has affected the cost and the availability of Tamiflu. More details of selected recent breakthrough of research design for the synthesis of Tamiflu, together with their advantages to the cost or efficiency in synthesizing Tamiflu, will be provided in the sections that follow.
The sudden outbreak of swine flu has increased the global demand of shikimic acid which is an industrially interesting compound, as it is used as a key starting material for the synthesis of Tamiflu [21]. However, the high cost and limited availability of shikimic acid isolated from plants such as Chinese star anise seeds (Illicium verum) have detained the use of this valuable building block of the drug [22]. It has been observed that the cost of these starting materials and the cost of Tamiflu are inter-related. The industrial synthesis of Tamiflu uses shikimic acid as a starting material, but the price fluctuates, depending on the supply of star anise [23]. It has been reported that the market value and soaring fears of a Tamiflu shortage have triggered a price surge for star anise in its main production regions of the Guangxi Zhuang Autonomous Region, Guangdong, Yunnan and Fujian provinces in China [24]. For example, according to a wholesaler’s description in Wang’s article, the price of star anise in Guangxi has doubled in a week’s time in 2005: it has risen from 5 yuan (US $60 cents) a kilogram to 8.2 yuan (US $1) a kilogram. While the increased cost of production has increased the cost of Tamiflu, the availability of Tamiflu has also been affected by the low isolation yield of shikimic acid [25]. The production of Tamiflu depended on the isolation of shikimic acid from star anise, and its low isolation yield of 3-7% was held responsible for the world-wide shortage of Tamiflu in 2005 [26].
The pharmaceutical industry is a complex of processes, operations and organizations involved in the discovery, development and production of drugs and medications. Primary manufacturing is one of the key components in a standard pharmaceutical supply chain. The primary manufacturing site is responsible for the synthesis of the active ingredient of a drug. This may involve multi-steps of chemical synthesis and separation stages to build up the complex targets involved [27]. Industrially, Tamiflu is mass produced by the Roche’s process which involves the synthesis of Tamiflu from a naturally occurring compound called shikimic acid [8,10,28,29]. From the point of view of production economy and for the synthesis of such an important and effective drug in treating avian and swine influenza, dependence on a single synthetic route or solely on one specific compound as the starting material poses a high potential for problems of low sustainability and increased cost of production. It is therefore of high importance to develop alternative routes for the synthesis of Tamiflu.
In the past decades, the synthetic chemistry of Tamiflu has attracted tremendous interest and worldwide attention [30-36]. Organic or medicinal chemists have paid various efforts to develop new synthetic routes of Tamiflu. They aimed at developing a synthetic route that allowed for the preparation of large amounts of drug in a short period of time and at low cost. To lower the cost of production, the synthetic routes were designed based on starting materials which are inexpensive and readily available. Increasing overall yields, simplifying operations, such as purifications and isolations of intermediates, and avoiding use of hazardous reagents, were among the other important goals. One remarkable example was reported by Corey et al. in which a short synthetic pathway has been developed for the synthesis of Tamiflu or its enantiomer, with the following advantages: (1) use of inexpensive and abundant starting materials; (2) complete enantio-, regio-, and diastereo-control; (3) avoidance of explosive, azide-type intermediates; (4) good overall yield of 30% (not completely optimized); and (5) scalability [37].
For the commercial or industrial production of Tamiflu, one of the concerns is the limited availability of the starting material. The starting material employed by industrial production, shikimic acid, is a natural product which can be extracted from Chinese star anise, a popular cooking spice among the Eastern countries (e.g., China and India) and also an herb used in traditional Chinese medicine. Shikimic acid, with its structure as shown in Figure 2, has a combination of chirality centers in each molecule and that increases the difficulty and cost for its synthesis by chemical methods [38] (Figure 2).

Figure 2: Structure of Shikimic acid.
Pollack [39] cited an unnamed Roche chemist who said that 13 grams of star anise could produce 1.3 grams of shikimic acid, which in turn could be made into 10 Tamiflu capsules, the amount to treat one person. Thus, one ton of shikimic acid could treat 770,000 people. However, the Times article [39] also cites another expert chemist, Dr Hamied, who disputed that one ton of shikimic acid would yield enough for only 300,000 people at most, and newcomers are not likely to have the same production efficiency as Roche. Therefore, if shikimic acid extracted from Chinese star anise is to be relied as the sole starting material for synthesizing Tamiflu, in order to make 300 million treatments of Tamiflu annually to meet government orders amid fears of a flu pandemic [40], hundreds of tons of Chinese star anise would have to be harvested.
There are recent reports that describe the synthesis or the development of more efficient extraction of shikimic acid from star anise. For example, a concise synthetic route for (−)-shikimic acid from d-ribose-derived enantiomeric aldehydes by employing Barbier reaction and ring-closing metathesis as key steps has been reported [41]. Very recently, a new, practical, rapid, and high-yielding process for the pressurized hot water extraction (PHWE) of multi-gram quantities of shikimic acid from star anise (Illicium verum) using an unmodified household espresso machine has been developed [42]. This operationally simple and inexpensive method enables the efficient and straightforward isolation of shikimic acid and the facile preparation of a range of its synthetic derivatives.
In addition to advancing the extraction of shikimic acid from Chinese star anise or its synthetic routes, organic or medicinal chemists around the world have been actively involved in developing alternative synthetic methods for Tamiflu [30-36]. The cost and availability of the starting material is an important factor to be considered in designing a synthetic route for the mass production of a drug. Synthetic routes for Tamiflu that utilize cheap and readily available compounds as the starting materials have been reported. In the part that follows, we will highlight the recent advance in the synthetic chemistry of Tamiflu using alternative choices of inexpensive or readily available starting materials. Some advantages and disadvantages reviewed in other publications [33,34] will also be described to provide a quick and updated summary and insights for interested parties involved in the drug industry. As the development of the synthetic chemistry of Tamiflu has been periodically reviewed [30-36] and the chemistry behind the synthetic approaches have been discussed in details, the content of our paper will be focused to give a summary account for the drug industry, with the omission of detailed elaboration of the chemistry of reaction principles. This part of our review covers selected reports or examples in recent years from 2008 to 2015, which have utilized inexpensive or readily available starting materials for the chemical synthesis of Tamiflu (Figure 3).
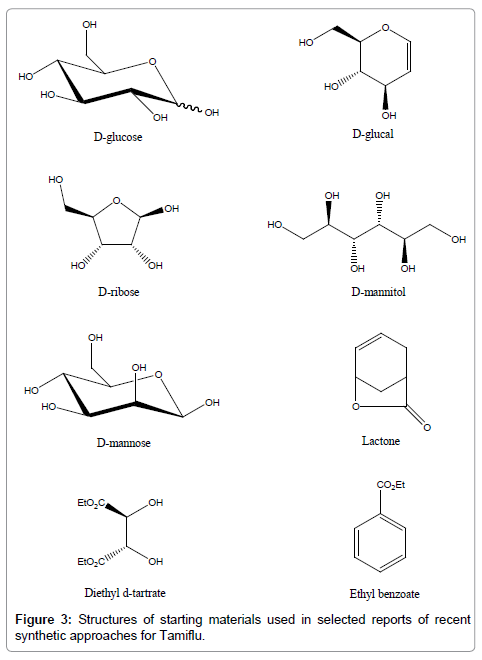
Figure 3: Structures of starting materials used in selected reports of recent synthetic approaches for Tamiflu.
Summary of Literature Review
Very recently, Kongkathip et al. at Kasetsart University in Thailand have reported the synthesis of Tamiflu from commercially available d-glucose via a novel and efficient synthetic route [19]. The synthesis employed a retrosynthetic analysis of Tamiflu from d-glucose (Figure 3) via the key intermediate aziridine cyclohexene. It provides an efficient synthetic route to Tamiflu starting from an inexpensive and abundant material, d-glucose.
Chen et al. at Nanyang Technological University in Singapore have reported a synthesis of Tamiflu by using novel synthetic routes, inexpensive reagents, and the abundant starting material d-glucal (Figure 3), which can also be readily prepared from d-glucose [20]. In addition, this synthetic approach allows late-stage functionalization for the flexible synthesis of Tamiflu analogues. The overall yield for this scheme was 2.6% after 22 linear steps and provided multi-gram quantities of Tamiflu. However, extensive chromatography was required for purifying intermediates [34]. In addition, the adoption of a sealed-tube step at high temperature would also increase the cost and potential hazards for mass production. Together with the need of protecting group chemistry and several oxidation-reduction steps, there are still concerns for this route to be practical at this stage of development for large-scale manufacturing.
Chen et al. at Shionogi and Co., Ltd. in Japan and the Institute of Chemical and Engineering Sciences in Singapore have described an efficient formal synthesis of Tamiflu, which has been achieved in 12 steps with use of the inexpensive and highly abundant d-ribose (Figure 3) as the starting material [43]. This route consists of nine steps to the aziridine intermediate with a 27% overall yield and a 9% overall yield to the final product. The synthetic route was demonstrated on multi-gram scale. In addition to the advantage that the synthesis starts with an inexpensive and readily available compound, the synthetic route does not utilize protecting groups and it makes use of a highly efficient ringclosing metathesis reaction to form the Tamiflu skeleton. However, the need for azide chemistry, the use of highly toxic halogenated solvents, and extensive chromatographic purifications, are still concerns over this synthetic route [34]. Using the same starting material d-ribose, Kongkathip et al. have reported a new synthetic approach towards Tamiflu which comprise a metal (Zn, In)-mediated domino reaction and ring-closing metathesis reaction of the resultant functionalized dienes to produce the Tamiflu skeleton [44]. This synthesis converts inexpensive and readily available d-ribose into Tamiflu in 14 steps and 5% overall yield. The synthesis was demonstrated on multi-gram scale. Some of the disadvantages are the use of azide and hydroxylamine chemistry, the heavy use of halogenated solvents, and the need for extensive chromatographic purifications of intermediates [34]. These disadvantages pose an increased risk of explosive accidents or serious threats to the environment especially when the approach is set for industrial production.
Another readily available compound, d-mannitol (Figure 3), has been employed by Ko et al. at Ewha Womans University in Korea for their synthesis of Tamiflu [45]. A unique feature of this synthetic route is that an acyclic precursor was constructed, which was then cyclized in an intramolecular aldol reaction to form the Tamiflu skeleton. The advantages include the use of inexpensive d-mannitol as starting material and no protection or deprotection sequence was needed. On the other hand, the long synthetic route, extensive use of chromatographic purification, and the need for azide chemistry at high temperature are concerns when adopting the synthetic approach for large-scale production [34]. Starting from the same compound, d-mannitol, Mandai and Oshitari [46] reported that a highly practical asymmetric synthesis of Tamiflu has been accomplished in 18 steps without any chromatographic purification. The synthetic approach features intramolecular aldol condensation of di-aldehyde with a 3-pentyl ether moiety in constructing densely functionalized cyclohexene ring of Tamiflu.
Using inexpensive and abundant d-mannose (Figure 3) as the starting material, Kongkathip et al. at Kasetsart University in Thailand have reported a new asymmetric synthesis of Tamiflu in 13 steps and 5% overall yield [47]. Tamiflu was synthesized from d-mannose through a short and practical synthetic route. A unique feature of the route is that the bulky 3-pentyloxy group and adjacent acetamide of Tamiflu were introduced at an early stage of the synthesis by coppercatalyzed regioselective ring-opening of the 2,3-pentylidene ketal of d-lyxofuranoside. The d-lyxofuranoside ethylphosphonate precursor was then cyclized via an intramolecular Horner-Wadsworth-Emmons reaction to furnish the Tamiflu skeleton.
Trost and Zhang [48] at Stanford University in the United States have communicated a short and efficient synthesis of Tamiflu which requires just eight steps from a commercially available starting material, lactone (Figure 3). The synthesis proceeds with an overall yield of 30%. Key transformations include a novel palladium-catalyzed asymmetric allylic alkylation (Pd-AAA) reaction as well as a chemo-, regio-, and stereo-selective aziridination reaction.
Chan et al. at Sun Yat-sen University and the Hong Kong Polytechnic University in China have reported an azide-free synthesis of Tamiflu that employs inexpensive and abundant diethyl d-tartrate (Figure 3) as starting material [49]. This reported synthesis of Tamiflu from diethyl d-tartrate was accomplished in 11 steps and proceeds in an excellent 21% overall yield. Even though unsafe reagents, such as IBX and MeNO2, have been employed and the synthesis requires chromatographic separation for some of the intermediates, it has the potential to become a scalable route to produce large quantities of drug due to use of inexpensive diethyl d-tartrate, the small number of steps, and lack of protecting group chemistry [34]. The early steps of the synthesis upto intermediate were demonstrated on multi-gram scale. Therefore, this scheme may provide an economical and practical alternative for the synthesis of Tamiflu.
Besides relying on natural compounds as starting materials, bromobenzene, another readily available starting material, has also been employed in the synthesis of Tamiflu [50,51]. However, at later stages, the synthesis required the introduction of the ester functionality via a carbonylation reaction [34]. Hudlicky et al. at Brock University in Canada have reported another synthetic route in which the additional step for introducing the ester functionality was avoided [52]. A short chemo-enzymatic formal synthesis of Tamiflu has been achieved starting from an inexpensive compound, ethyl benzoate (Figure 3). The reported example is a symmetry-based chemo-enzymatic synthetic approach. The key steps involve a toluene dioxygenase-mediated dihydroxylation, Hetero-Diels-Alder cycloaddition, and generation of C4 acetamido functionality [34]. The reported synthesis of Tamiflu, which is achieved in ten steps, has the main advantage that it starts from a readily available source, ethyl benzoate.
Conclusion
The recent advance in synthetic approaches to Tamiflu reported by different research groups of organic chemistry community is a valuable asset to the drug industry. It has been discussed that various efforts have been put by researchers to address one of the concerns, the limited availability of starting materials, for the industrial production of the effective drug Tamiflu. In this article, the elegant synthetic designs for Tamiflu reported by various chemists in recent years from 2008 to 2015 have been addressed. Among the examples highlighted, the method communicated by Trost and Zhang [48], which requires just eight steps from the commercially available lactone, is considered the most promising one for developing into an industrial trial in terms of time and cost effectiveness. Notably, other designs suggested by various experts, such as those utilizing cheap starting materials or involving short and practical processes suggested by Kongkathip et al. [19] or Weng et al. [49], are also worth undergoing further development. Even though many of the synthetic schemes may not have good scalability at this stage of development, they suggest potential or alternative paths for the synthesis of Tamiflu that, eventually, may lead to the development and implementation of new practical commercial processes. Since different synthetic routes may share the same intermediates and may be complementary to some extents [53], it is possible that a novel and practical large-scale methodology will be derived from a combination of chemistry and creativity behind the reported approaches. Together with the highlighted concepts of sustainable supply chain management (SSCM), this article should be of interest to health care study and is expected to provide insights for the drug and supply chain management industry.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing and Conflicting Interests
The authors declare no conflicts of interest.
16117
References
- Webster RG, Monto AST, Braciale J, Lamb RA (1998) Textbook of Influenza. Blackwell Science: Oxford, UK, pp: 219-264.
- Gstraunthaler T (2008) Planning for judgment day: restrictions of government planning for avian influenza. Disaster Prev Manag 17: 199-211.
- Tang O, Lau YY (2013) Logistics aspects of avian influenza pandemic in Hong Kong. International Journal of Logistics Systems and Management 14: 110-130.
- Mohan U, Viswanadham N, Trikha T (2009) Impact of avian influenza in the Indian poultry industry: a supply chain risk perspective. International Journal of Logistics Systems and Management 5: 89-105.
- Moscona A (2005) Neuraminidase inhibitors for influenza. N Engl J Med 353: 1363-1373.
- Kim CU, Lew W, Williams MA, Liu HT, Zhang LJ, et al. (1997) Influenza Neuraminidase Inhibitors Possessing a Novel Hydrophobic Interaction in the Enzyme Active Site: Design, Synthesis, and Structural Analysis of Carbocyclic Sialic Acid Analogues with Potent Anti-Influenza Activity. J Am Chem Soc 119: 681-690.
- Hurt AC, Selleck P, Komadina N, Shaw R, Brown L, et al. (2007) Susceptibility of highly pathogenic A(H5N1) avian influenza viruses to the neuraminidase inhibitors and adamantanes. Antiviral Research 73: 228-231.
- Rohloff JC, Kent KM, Postich MJ, Becker MW, Chapman HH, et al. (1998) Practical total synthesis of the anti-influenza drug GS-4104. J Org Chem 63: 4545-4550.
- Karpf M, Trussardi RJ (2001) New, Azide-Free Transformation of Epoxides into 1,2-Diamino Compounds: Synthesis of the Anti-Influenza Neuraminidase Inhibitor Oseltamivir Phosphate (Tamiflu). J Org Chem 66: 2044-2051.
- Abrecht S, Harrington P, Iding H, Karpf M, Trussardi R, et al. (2004) The synthetic development of the anti-influenza neuraminidase inhibitor oseltamivir phosphate (Tamiflu®): a challenge for synthesis & process research. CHIMIA International Journal for Chemistry 58: 621-629.
- Laver G, Garman E (2001) The origin and control of pandemic influenza. Science 293: 1776-1777.
- Abbott A (2005) Avian flu special: what’s in the medicine cabinet? Nature 435: 407-409.
- Bloom JD, Gong LL, Baltimore D (2010) Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 328: 1272-1275.
- Nie LD, Wang FF, Ding W, Shi XX, Lu X (2013) A novel azide-free asymmetric synthesis of oseltamivir phosphate (Tamiflu) starting from Roche’s epoxide. Tetrahedron: Asymmetry 24: 638-642.
- Dharni K, Sharma RK (2015) Supply chain management in food processing sector: experience from India. International Journal of Logistics Systems and Management 21: 115-132.
- Beske P, Land A, Seuring S (2014) Sustainable supply chain management practices and dynamic capabilities in the food industry: a critical analysis of the literature. International Journal of Production Economics 152: 131-143.
- Seuring S, Muller M (2008) From a literature review to a conceptual framework for sustainable supply chain management. J Clean Prod 16: 1699-1710.
- DiMasi JA, Hansen RW, Grabowski HG (2003) The price of innovation: new estimates of drug development costs. Journal of Health Economics 22: 151-185.
- Kongkathip B, Akkarasamiyo S, Kongkathip N (2015) A new and efficient asymmetric synthesis of oseltamivir phosphate (Tamiflu) from d-glucose. Tetrahedron 71: 2393-2399.
- Ma J, Zhao Y, Ng S, Zhang J, Zeng J, et al. (2010) Sugar-based synthesis of Tamiflu and its inhibitory effects on cell secretion. Chemistry - A European Journal 16: 4533-4540.
- Tripathi P, Rawat G, Yadav S, Saxena RK (2015) Shikimic acid, a base compound for the formulation of swine/avian flu drug: statistical optimization, fed-batch and scale up studies along with its application as an antibacterial agent. Antonie van Leeuwenhoek 107: 419-431.
- Rawat G, Tripathi P, Saxena RK (2013) Expanding horizons of shikimic acid. Appl Microbiol Biotechnol 97: 4277-4287.
- Satoh N, Akiba T, Yokoshima S, Fukuyama T (2009) A practical synthesis of (-)-oseltamivir. Tetrahedron 65: 3293-3245.
- Wang GW, Hu WT, Huang BK, Qin LP (2011) Illicium verum: A review on its botany, traditional use, chemistry and pharmacology. J Ethnopharmacol 136: 10-20.
- Ressmann AK, Gaertner P, Bica K (2011) From plant to drug: ionic liquids for the reactive dissolution of biomass. Green Chem 13: 1442-1447.
- Shah N (2004) Pharmaceutical supply chains: key issues and strategies for optimization. Computers and Chemical Engineering 28: 929-941.
- Federspiel M, Fischer R, Hennig M, Mair HJ, Oberhauser T, et al. (1999) Industrial Synthesis of the Key Precursor in the Synthesis of the Anti-Influenza Drug Oseltamivir Phosphate (Ro 64-0796/002, GS-4104-02): Ethyl (3R,4S,5S)-4,5-epoxy-3-(1-ethyl-propoxy)-cyclohex-1-ene-1-carboxylate. Org Proc Res Dev 3: 266-274.
- Abrecht S, Federspiel MC, Estermann H, Fischer R, Karpf M, et al. (2007) The synthetic-technical development of oseltamivir phosphate TamifluTM: a race against time. CHIMIA International Journal for Chemistry 61: 93-99.
- Farina V, Brown JD (2006) Tamiflu: the supply problem. Angewandte Chemie International Edition 45: 7330-7334.
- Shibasaki M, Kanai M (2008) Synthetic strategies for oseltamivir phosphate. Eur J Org Chem 1839-1850.
- Gong J, Xu W (2008) Different synthetic strategies of oseltamivir phosphate: a potent influenza neuraminidase inhibitor. Curr Med Chem 15: 3145-3159.
- Magano J (2009) Synthetic approaches to the neuraminidase inhibitors zanamivir (Relenza) and oseltamivir phosphate (Tamiflu) for the treatment of influenza. Chem Rev 109: 4398-4438.
- Magano J (2011) Recent synthetic approaches to oseltamivir phosphate (Tamiflu™) for the treatment of influenza. Tetrahedron 67: 7875-7899.
- Zhang T, Lu H, Zhang FM, Cheng J, Liu T (2013) Recent progress in the synthesis of Tamiflu. Chinese J Org Chem 33: 1235-1243.
- Li NG, Shi ZH, Tang YP, Shi QP, Zhang W, et al. (2014) Recent progress on the total synthesis of (–)-oseltamivir phosphate (Tamiflu) for the treatment of influenza disease. Curr Org Chem 18: 2125-2138.
- Yeung YY, Hong S, Corey EJ (2006) A short enantioselective pathway for the synthesis of the anti-influenza neuramidase inhibitor oseltamivir from 1,3-butadiene and acrylic acid. J Am Chem Soc 128: 6310-6311.
- Yamatsugu K, Kanai M, Shibasaki M (2009) An alternative synthesis of Tamiflu®: a synthetic challenge and the identification of a ruthenium-catalyzed dihydroxylation route. Tetrahedron 65: 6017-6024.
- Pollack A (2005) Is Bird Flu Drug Really So Vexing? Debating the Difficulty of Tamiflu. The New York Times.
- Kancharla PK, Doddi VR, Kokatla H, Vankar YD (2009) A concise route to (-)-shikimic acid and (-)-5-epi-shikimic acid, and their enantiomers via Barbier reaction and ring-closing metathesis. Tetrahedron Lett 50: 6951-6954.
- Just J, Deans BJ, Olivier WJ, Paull B, Bissember AC, et al. (2015) New method for the rapid extraction of natural products: efficient isolation of shikimic acid from star anise. Org Lett 17: 2428-2430.
- Osato H, Jones IL, Chen A, Chai CLL (2010) Efficient formal synthesis of oseltamivir phosphate (Tamiflu) with inexpensive d-ribose as the starting material. Org Lett 12: 60-63.
- Wichienukul P, Akkarasamiyo S, Kongkathip N, Kongkathip B (2010) An efficient synthesis of oseltamivir phosphate (Tamiflu) via a metal-mediated domino reaction and ring-closing metathesis. Tetrahedron Lett 51: 3208-3210.
- Ko JS, Keum JE, Ko SY (2010) A synthesis of oseltamivir (Tamiflu) starting from d-mannitol. J Org Chem 75: 7006-7009.
- Mandai T, Oshitari T (2009) Efficient asymmetric synthesis of oseltamivir from d-mannitol. Synlett 783-786.
- Chuanopparat N, Kongkathip N, Kongkathip B (2012) A new and efficient asymmetric synthesis of oseltamivir phosphate (Tamiflu) from d-mannose. Tetrahedron Letters 53: 6209-6211.
- Trost BM, Zhang T (2008) A concise synthesis of (-)-oseltamivir. Angew Chem Int Ed Engl 47: 3759-3761.
- Weng J, Li YB, Wang RB, Li FQ, Liu C, et al. (2010) A practical and azide-free synthetic approach to oseltamivir from diethyl d-tartrate. J Org Chem 75: 3125-3128.
- Shie JJ, Fang JM, Wong CH (2008) A concise and flexible synthesis of the potent anti-influenza agents Tamiflu and Tamiphosphor. Angew Chem Int Ed Engl 47: 5788-5791.
- Matveenko M, Willis AC, Banwell MG (2008) A chemoenzymatic synthesis of the anti-influenza agent Tamiflu®. Tetrahedron Lett 49: 7018-7020.
- Sullivan B, Carrera I, Drouin M, Hudlicky T (2009) Symmetry-based design for the chemoenzymatic synthesis of oseltamivir (Tamiflu) from ethyl benzoate. Angew Chem Int Ed Engl 48: 4229-4231.
- World Health Organization (2015) Available from: https://www.who.int/en/ Accessed Dec 2015.









