Keywords
Curcumin; Sulfanilamide; Renal; Nephrotoxicity
Abbreviations
CUR: Curcumin; Sd: Sulfa Drugs; MDA: Malondialdehyde; GSH: Glutathione; FA: Folic acid; CAT: Catalase; SOD: Superoxide dismutase.
Introduction
The management of several drugs usually accompany renal damage. Sulfonamides are broadly administered for inflammatory-mediated human diseases [1]. Some examinations indicated reduction in renal function in sulfa-treated patients. Sulfa-induced renal damage has typically been introduced as interstitial nephritis, glomerulonephritis, nephritic syndrome and acute renal failure [2]. A Dihydropteroate synthetase enzyme that is responsible for folic acid (FA) production has been confirmed by structural activity relationship (SAR) of sulfanilamide. FA is very close of the ancestors of bacteria DNA and RNA [3]. In accordance to easily excretion of Sd in urine there are many harmful infections have been initiated in urinary tract. Sulfa drugs possess more fixation than re-absorption of water from the filtrate by the kidneys [4]. Furthermore, the accumulation of sulfa drugs leads to decrease in renal excretion and this remarkably increases the nephrotoxic risk of their action up to severe renal nephritis and necrosis [5]. Sulfa drug treatment for many days cause acute toxicity since the kidneys flop to evacuate the sulfa accurately which it can lead to renal failure [6]. Therapy of sulfa with high dose has been seen among of treating many diseases such as toxoplasmic encephalitis in HIV patients [7]. Meanwhile, sulfa nephropathy has become increasingly common [8]. CUR is a yellow-orange macromolecule which is exhibited in turmeric, with widespread uses as an additive in some foods like potato chips, curry, and mustard as well as important in several drugs and cosmetics [9-12]. It is considered as one of vital class of anticancer [13] and antioxidant agents [14]. CUR treatment was reported for preventing renal lesions of diabetic rats [15] and protected from renal cell line oxidative [16,17]. Furthermore, curcumin has been attenuated nephrotoxicity that caused via cisplatin [18]. From retrospective cohort study that has been performed. The main goal of this work is to inspect the protective effects for CUR on induced renal toxicity which is produced by sulfanilamide experimentally. Figure 1 displays the chemical structures of curcumin and sulfa drug.
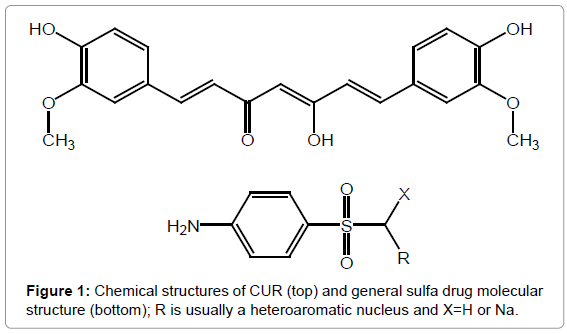
Figure 1: Chemical structures of CUR (top) and general sulfa drug molecular structure (bottom); R is usually a heteroaromatic nucleus and X=H or Na.
Experimental
Methodology
Sulfa drug and CUR were obtained from Sigma Aldrich. Curcumin was taken orally after solubilize in corn oil. 380 ± 40 g weight Wistar Albino male rats were cast off in an experiment. Albino rats were kept under Appropriate conditions "temperature, humidity-controlled, light-cycled quarters" and haphazardly divided into five groups. The study pro-tocol was approved by Animal house of the National Research Center.
Each of five groups of examined rats were consists of six animals. Saline solution of sulfanilamide was prepared and injected i.p. for each dose of "1000 mg/kg/day". Also, CUR was liquefied in corn oil and was injected i.p. dose of "200 mg/kg". Selection dose of sulfanilamide was attributed to previous literature [19,20]. Meanwhile, CUR dose was also referred to studies based on antioxidant effect [21]. The first group was served as control and injected by 1 mL isotonic saline containing i.p., second group rats were injected with a single dose of sulfanilamide. Third group rats received Sd+CUR. Curcumin was administered 1/2 hour after the received sulfanilamide. Where, fourth group rats were injected by CUR alone. Meanwhile, fifth group received sulfanilamide+corn oil with curcumin.
Male rats have been injected by ketamine to anesthetized, after 24 h receiving Sd. Some biochemical parameters were measured by taking blood samples from vena cava. A high dose of ketamine was sacrificed by all rats.
Finally, abdominal middle-line was incised then quickly kidneys were removed and washed with cold saline solution. One of the two kidneys was stored in formalin solution for the histopathological test and the second was stored at -80°C to evaluate its renal glutathione (GSH), activities of glutathione peroxidase (GSH-Px), malondialdehyde, superoxide dismutase (SOD) and catalase (CAT).
Biochemical determining
Urea, Potassium, sodium and creatinine were estimated from serum testers [22] Biochemical assays were assessed using an Olympus Auto analyzer (Tokyo, Japan).
In "150 mM KCL in ice-cold tamponade 300 mg kidney tissue" was added containing to determine malondialdehyde which is referred to thiobarbituric acid that measured by spectrophotometer [23].
Spectrophotometric method was used to determine GSH, which is dependent on Allman's reagent [24]. Glutathione peroxidase activity was assessed via detecting oxidation of reduced NADPH at 340 nm [25]. Activity of CAT was measured through the initial rate of H2O2 disappearance at 245 nm [26]. In the meantime, SOD activity was assigned by superoxide anion that produced with xanthine and xanthine oxidase [27].
Histological determination
The method of Houghton has been employed to analyze every section of kidney sections as semi-quantitative, the lesion grades were ranged from zero to four [28].
Results
Increasing of creatinine levels and plasma urea has been shown in rats after treatment with sulfanilamide alone comparing with control. Meanwhile, treatment of wistar rate with curcumin and sulfanilamide inhibit the increasing of creatinine levels and plasma urea less than 0.01 has been shown in Table 1.
| Parameter |
Group |
| Control |
Sulfonamide |
Sulfa+Curcumin |
Curcumin |
Sulfa+Vehicle |
| Creatinine (mg/dL) |
0.23 ± 0.05 |
1.35 ± 0.43a,*** |
0.33 ± 0.06b,** |
0.20 ± 0.01 |
1.15 ± 0.4 |
| BUN (mg/dL) |
18.9 ± 2.4 |
86.1 ± 1.7a,*** |
27.1 ± 8.0b,** |
20.4 ± 1.2 |
77.3 ± 6.1 |
| Na+ (mmol/L) |
139.2 ± 2.8 |
146 ± 2.5a,* |
141.1 ± 3.5b,* |
138.4 ± 1.2 |
142 ± 2.5 |
| K+ (mmol/L) |
5.0 ± 0.1 |
4.8 ± 0.8a,* |
4.9 ± 0.3b,* |
2.8 ± 0.2 |
5.1 ± 0.1 |
Values are expressed as mean ± SD for six rats in each group.
Groups: control, Sd; sulfanilamide 1000 mg/kg, Sd + CUR; sulfanilamide 1000 mg/kg + curcumin, CUR; curcumin, Sd + vehicle; sulfanilamide + corn oil.
a Compared with control group
b Compared with Sd group.
* P>0.05, ** P<0.05 and *** P<0.01.
Table 1: Biochemical experimental for rat groups.
Albino male rats, which treated with curcumin, have been showing increasing for GSH level, antioxidant activity and decreasing in malondialdehyde level. Meanwhile, rats, which are treated with sulfanilamide has been shown a decreasing of GSH level, CAT, SOD and GSH-Px activates in comparing with control see in Table 2.
| Parameter |
Group |
| Control |
sulfonamide |
Sulfa+Curcumin |
Curcumin |
Sulfa+Vehicle |
| MDA (nmol/g wet tissue) |
35.3 ± 5.9 |
60.4 ± 4.9a,** |
34.8 ± 6.9b,* |
36.1 ± 4.6 |
63.5 ± 5.3 |
| GSH (µ/g wet tissue) |
1.9 ± 0.2 |
0.96 ± 0.1a,** |
1.7 ± 0.5b,* |
1.7 ± 0.2 |
0.84 ± 0.3 |
| NO (nmol/g wet tissue) |
44.1 ± 11.2 |
92.6 ± 12.6a,** |
52.2 ± 9.5b,** |
46.5 ± 8.6 |
96.4 ± 8.4 |
| GSH-Px (IU/mg protein) |
8.57 ± 1.6 |
7.1 ± 0.8a,** |
8.39 ± 0.56b,* |
8.6 ± 0.7 |
6.9 ± 0.4 |
| CAT (k/s/mg protein) |
28 ± 0.6 |
20 ± 1.5a,** |
28 ± 2.1b,* |
29 ± 0.5 |
19 ± 2.3 |
| SOD (U/mg protein) |
159 ± 1.6 |
118.4 ± 2.1a,** |
127 ± 4.3b,* |
160 ± 1.8 |
114.5 ± 3.4 |
Values are expressed as mean SD for six rats in each group. CUR; curcumin, Sd; sulfanilamide 1000 mg/kg, MDA; malondialdehyde, GSH; reduced glutathione, GSH-Px; glutathione peroxidase, CAT; catalase and, SOD; superoxide dismutase.
Statistics: Mann–Whitney U-test.
a Compared with control group.
b Compared with Sd group.
* P<0.05 and ** P<0.01.
Table 2: Data for calibration curve of Cefprozil.
Antioxidant enzymes like "SOD, CAT, peroxidation of lipid and GSH" which recognized as "MDA levels" were exaggerated by CUR. Moreover, it was found that the double impact via treating with sulphanilamide antioxidant enzyme activities were decreased meanwhile, MDA levels were increased compared with control. Furthermore, on treatment via CUR it was showing a remarkable accumulative for antioxidant enzymes activities and the level of GSH, but reducing the MDA level "p<0.05" as listed in Table 2. In the control group, Light microscope assessment was displayed standard morphology of renal parenchyma with well-defined glomeruli and of kidney tubules (Figure 2A). It was observed that after an animal injected by sulfanilamide there was the morphology of "tubular epithelial degeneration, cell desquamation and necrosis" was changed as showed in Figure 2B. Kidney of rate that treated with sulfanilamide showed debris in proximal tube cellular and cortical interstitial overcrowding. Meanwhile, it was noticed that cellular desquamation was inhibited and glomeruli kept a healthier morphology "grade of tubular necrosis: 0–2" when rats was injected by sulfanilamide and CUR as recorded in Figure 2C.
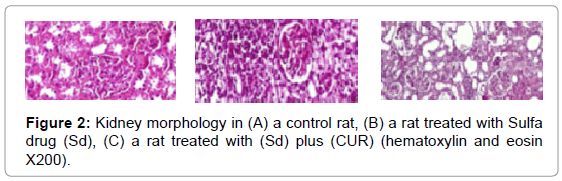
Figure 2: Kidney morphology in (A) a control rat, (B) a rat treated with Sulfa drug (Sd), (C) a rat treated with (Sd) plus (CUR) (hematoxylin and eosin X200).
Discussion
To inhibit renal injury that caused by sulfanilamide there are several drugs have been utilized like vitamin E, melatonin and so on [21]. Increasing of creatinine and plasma urea levels in these rats that administrated by sulfanilamide have been inhibited by treating this rats with curcumin, which decreases ischemia-reperfusion prompted an escalation in serum creatinine levels [28]. Moreover, sulfanilamide toxicity came from its metabolism in the liver and extrahepatic tissues and excreted by kidney as a soluble water form [29]. Whenever, administration of high dose of sulfanilamide leads to production of large amounts of metabolites, which increases the toxicity, leaving large amounts of unrestricted reactive metabolite that bind with cellular protein macromolecules covalently [30]. Furthermore, sulfanilamide has been attenuated nephrotoxicity which is referred to its reactive metabolite [31]. A fact of antioxidants stimulates the synthesis of GSH, which is a remark of sulfanilamide-induced renal injury prominent to tissue necrosis and ultimately to tissue dysfunction was disordered [32]. From literature survey, it was found GSH level plays an important rule to estimate GSH peroxidase and reductase, leads to inhibit Adriamycin treatment and decrease of defense mechanism for kidney antioxidant, increase free radical induced by kidney damage in nephrotoxicity [33]. Moreover, it was endorsed that lipid peroxidation may be a causative factor in the enhancement of renal toxicity. It is probable that the activity of curcumin in inhibiting the membrane damage is moderately associated with its capability to forage lipid peroxidation initiating agents [34]. Herein, we also observed that treatment of Albino rate with sulfanilamide alone has a remarkable increasing in Malondialdehyde levels in their renal tissue than control. In this work, consequences of clinical investigation displayed a vibrant prove of nephrotoxicity following the treatment by sulfanilamide in an overdose. A sever renal tubular necrosis was a strong relation to clinical change. These point of view concurrence with those of the past examination, explain renal histological change following the treatment by of overdose sulfanilamide [19]. It is clear that inhibition of nephrotoxicity that caused by sulfanilamide by administration curcumin to get rid of nitrogen mono and dioxide, singlet oxygen and superoxide radical [35- 38]. Finally, an impact of this investigation, curcumin administration has the reverse effect for treating sulfanilamide which causes decrease of endogenous antioxidant, which is confirmed by the abatement in SOD, CAT, and GSH-Px activity.
Therapeutic doses of SD administered to human are amongst 3-6 g/day. As a high dose of SD is managed for the chronic mode, it might lead to cell defensive mechanism to nonstop oxidative stress. Curcumin might be able to be managed as a supplementary agent in sulfa-treated patients. The proposed mechanism of sulfa-induced renal damage and the possible therapeutic effects of CUR are shown in Figure 3.
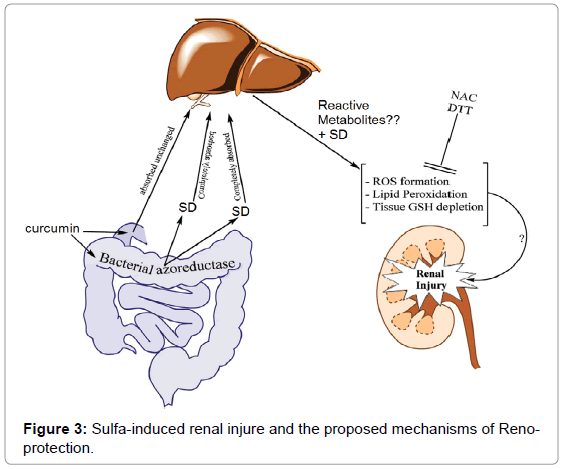
Figure 3: Sulfa-induced renal injure and the proposed mechanisms of Reno-protection.
Conclusion
From this perspective study, it has been included that curcumin might be haveing a potential hopeful specialist against tentatively instigated nephrotoxicity of sulfanilamide by means of its free radical- searching and antioxidant properties. Assist examinations are expected to show the effect of curcumin on sulfanilamide-initiated nephrotoxicity. Malondialdehyde (MDA), glutathione (GSH) level and antioxidant enzyme activity were measured in the renal tissue. Where, urea, creatinine levels were determined in the blood. Sulfa drug administration caused elevated antioxidant enzyme activity, and levels of renal Malondialdehyde. Sd markedly was reduced the elevation of MDA levels, elevation antioxidant enzyme activity and maintained altered renal morphology normally in rats administrated Sulfa drug.
Availability of Data and Materials
All materials and data are available in the manuscript.
Competing Interests
Authors announce that there are no conflicts of interest.
Declarations
Ethics approval and consent to participate. Authors approve all Ethics and consent to participate.
Funding
Author approve that there is no funding for this work
Contribution of Authors
The authors declare that this work was done by their names in this article and all liabilities pertaining to claims relating to the content of this article will be borne by them.
Acknowledgements
I would like to thank Division of Biochemistry Staff, Faculty of Applied Science, SA for their excellent technical assistance during the work.
24427
References
- Kabanov A, Batrakova E, Mahajan V, Haney MJ (2017) Compositions and methods for gene therapy. US Patent No. 9,789,205.
- Kabha M, Dekalo S, Barnes S, Mintz I, Matzkin H, et al. (2016) Sulfadiazine-induced obstructive nephropathy presenting with upper urinary tract extravasation. J Endourolcase Reports 2: 159-161.
- Projan SJ (2002) New (and not so new) antibacterial targets-from where and when will the novel drugs come? Curr Opin Pharmacol 2: 513-522.
- Krüger-Thiemer E (2017) Pharmacokinetics and dose-concentration relationships. Proc 3rd Int Pharmacol Meeting 7: 63-113.
- Gilmartin C, Pai AB, Hughes D, Hilal N (2016) Risky business: Lessons from medication misadventures in chronic kidney disease. Physician Assistant Clin 1: 55-76.
- Perazella MA (2018) Pharmacology behind Common drug nephrotoxicities. Clinical J Amer Soc Nephrol 7: 1897-1908.
- Zhang Z, Hever A, Bhasin N, Kujubu DA (2018) Secondary syphilis associated with membranous nephropathy and acute hepatitis in a patient with HIV: A case report. Perm J 22: 17-62.
- Nazzal L, Blaser MJ (2018) Does the receipt of antibiotics for common infectious diseases predispose to kidney stones? A cautionary note for all health care practitioners. J Amer Soc Nephrol 29: 1590-1592.
- Okada K, Wangpoengtrakul C, Tanaka T, Toyokuni S, Uchida K, et al. (2001) Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J Nutr 131: 2090-2095.
- Joe B, Vijaykumar M, Lokesh B (2004) Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr 44: 97-111.
- Mahmood K, Zia KM, Zuber M, Salman M, Anjum MN (2015) Recent developments in curcumin and curcumin based polymeric materials for biomedical applications: A review. Int J Biolog Macromol 81: 877-890.
- Gaffer H, Mashaly H, Abdel-Rhman SH, Hammouda M (2017) Synthesis of novel dyes based on curcumin for the creation of antibacterial silk fabrics. Pigment Resin Technol 46: 478-484.
- Gafner S, Bergeron C, McCollom MM, Cooper LM, McPhail KL, et al. (2004) Evaluation of the efficiency of three different solvent systems to extract triterpene saponins from roots of Panax quinquefolius using high-performance liquid chromatography. J Agri Food Chem 52: 1546-1550.
- Kempaiah R, Srinivasan K (2004) Influence of dietary curcumin, capsaicin and garlic on the antioxidant status of red blood cells and the liver in high-fat-fed rats. Ann Nutr Metabol 48: 314-320.
- Venkatesan N, Punithavathi D, Arumugam V (2000) Curcumin prevents adriamycin nephrotoxicity in rats. British J Pharmacol 129: 231-234.
- Tirkey N, Kaur G, Vij G, Chopra K (2005) Curcumin, a diferuloylmethane, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol 5: 15.
- Balogun E, Foresti R, Green CJ, Motterlini R (2003) Changes in temperature modulate heme oxygenase-1 induction by curcumin in renal epithelial cells. Biochem Biophys Res Commun 308: 950-955.
- Antunes LMG, Darin JDAC, Maria de Lourdes PB (2001) Effects of the antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol Res 43: 145-150.
- Abraham P (2005) Vitamin C may be beneficial in the prevention of paracetamol-induced renal damage. Clin Exp Nephrol 9: 24-30.
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, et al. (2000) Characterization of SB‐269970‐A, a selective 5‐HT7 receptor antagonist. British J Pharmacol 130: 539-548.
- Chuang SE, Cheng AL, Lin JK, Kuo ML (2000) Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem Toxicol 38: 991-995.
- Lassnigg A, Donner E, Grubhofer G, Presterl E, Druml W, et al. (2000) Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Amer Soc Nephrol 11: 97-104.
- Cekmen M, Ilbey Y, Ozbek E, Simsek A, Somay A, et al. (2009) Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Cheml Toxicol 47: 1480-1484.
- Panraksa Y, Siangproh W, Khampieng T, Chailapakul O, Apilux A, et al. (2018) Paper-based amperometric sensor for determination of acetylcholinesterase using screen-printed graphene electrode. Talanta 178: 1017-1023.
- Türkoğlu S, Celik S, Keser S, Türkoğlu İ, Yilmaz Ö (2017) The effect of Pistacia terebinthus extract on lipid peroxidation, glutathione, protein, and some enzyme activities in tissues of rats undergoing oxidative stress. Turkish J Zool 41: 82-88.
- Yagmurca M, Erdogan H, Iraz M, Songur A, Ucar M, et al. (2004) Caffeic acid phenethyl ester as a protective agent against doxorubicin nephrotoxicity in rats. Clin Chim Acta 348: 27-34.
- Ozbek E, Cekmen M, Ilbey YO, Simsek A, Polat EC, et al. (2009) Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF-kB pathways. Renal Failure 31: 382-392.
- Bayrak O, Uz E, Bayrak R, Turgut F, Atmaca AF, et al. (2008) Curcumin protects against ischemia/reperfusion injury in rat kidneys. World J Urol 26: 285-291.
- Gu J, Cui H, Behr M, Zhang L, Zhang QY, et al. (2005) In vivo mechanisms of tissue-selective drug toxicity: Effects of liver-specific knockout of the NADPH-cytochrome P450 reductase gene on acetaminophen toxicity in kidney, lung, and nasal mucosa. Mole Pharmacol 67: 623-630.
- Bessems JG, Vermeulen NP (2001) Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 31: 55-138.
- Lu AY (2001) Covalent binding of chemical residues: Health impact. Biol React Intermed VI 500: 657-661.
- Werner C, Tiegs G, Wendel A (2017) Manipulation of liver glutathione status-A double-edged sword. CRC Press, pp: 21-28.
- Beladi-Mousavi SS, Hajibabaei K, Tamadon MR, Rafieian-Kopaei M (2016) Relationship between free radicals and risk of kidney diseases; the role of antioxidants and their reaction mechanisms. Ann Res Antioxidants.
- Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K (2002) Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomed 9: 232-238.
- Manjunatha H, Srinivasan K (2006) Protective effect of dietary curcumin and capsaicin on induced oxidation of low‐density lipoprotein, iron‐induced hepatotoxicity and carrageenan‐induced inflammation in experimental rats. FEBS J 273: 4528-4537.
- El-Kashef DH, El-Kenawi AE, Suddek GM, Salem HA (2015) Protective effect of allicin against gentamicin-induced nephrotoxicity in rats. Int Immunopharmacol 29: 679-686.
- Amalraj A, Pius A, Gopi S, Gopi S (2017) Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives-A review. J Traditional Complemen Med 7: 205-233.
- Jain SK, Rains J, Jones K (2006) Effect of curcumin on protein glycosylation, lipid peroxidation, and oxygen radical generation in human red blood cells exposed to high glucose levels. Free Radical Biol Med 41: 92-96.









