COVID-19; Antiviral drugs; Antiparasitic drugs; Immunoboosters
Introduction
As of March 11th, 2020, COVID-19 was declared as a major pandemic, a public health emergency by the World Health Organization (WHO). The latest report as of May 7th, 2020, shows that more than 3.75 million cases of COVID-19 have been reported in over 187 countries and territories, resulting in more than 263,000 deaths all around the world [1]. More than 1.24 million people have recovered. COVID-19 is an infectious disease due to a newly found novel coronavirus also known as SARSCOV- 2, Severe Acute Respiratory Syndrome Coronavirus, with its first case originating from Wuhan, China.
Coronaviruses are a randomized group of positive-sense single-stranded RNA viruses that lead to both upper and lower respiratory tract infections in humans and mammals. It also shows effects on other animals, i.e. diarrhea in cows and pigs [2]. Coronaviruses were first discovered in the 1930s when an acute respiratory infection of domesticated chickens was shown to be caused by the Infectious Bronchitis Virus (IBV) a type of coronavirus affecting animals. It has spike-like projections on its outer surface which form the appearance of a crown over a phospho-bilayer of proteins. Belonging to the family Coronaviridae having a size that approximately ranges from 26 to 32 kilobases, one of the largest among RNA viruses [3-5].
The most common symptoms of COVID-19 are high fever (hyperthermia), dry cough, and tiredness. Other symptoms that are less common and may affect some patients include aches and pains, nasal congestion, headache, shortness of breath, conjunctivitis, sore throat, diarrhea, loss of taste or smell, and rash on skin or discoloration of fingers or toes. The decreased level of oxygen in the body is also an indicator. These symptoms are usually mild in the early phase and progress gradually. Some people become infected but only carry very mild symptoms or are completely asymptomatic. Complications include Pneumonia, Bronchitis, Pulmonary Hypertension, Emphysema, alveolar dysfunction, fibrosis in the lungs, etc. It also leads to cardiac and other serious abnormalities. There are seven different types of coronaviruses leading to mild and severe infections as shown below.
Four human coronaviruses produce symptoms caused by them which are generally mild
• Human coronavirus 229E (HCov-229E), α-Cov
• Human coronavirus NL63 (HCov-NL63), α-Cov
• Human coronavirus HKU1 (HCov-HKU1), β-Cov
• Human coronavirus OC43 (HCov-OC43), β-Cov
Three human coronaviruses produce potentially severe symptoms
• Severe acute respiratory syndrome coronavirus 2 (SARSCov- 2), β-Cov
• Middle East respiratory syndrome-related coronavirus (MERSCov), β-Cov
• Severe acute respiratory syndrome coronavirus (SARS-Cov), β- Cov
The spread of COVID-19 primarily spreads from person to person through small droplets from the nose or mouth, which are expelled or inhaled when a person with COVID-19 coughs, sneezes, or speaks. These droplets do not travel far and quickly sink to the ground. People can contract COVID-19 if they inhale the droplets of an infected person. These droplets can also land on objects and surfaces (fomites) such as handles, tables, doorknobs, and clothes. The uninfected person can become infected by touching these fomites, then touching their eyes, nose, or mouth, and other mucosa orifices. This is why it has been recommended to wash your hands regularly with soap and water or clean with alcohol-based hand rub for about 30 seconds as directed by the WHO.
Rapid diagnosing kits are avoided by the Indian Council of Medical Research (ICMR) due to inaccurate results.
Diagnosing criteria is based on the results observed after testing through various methods and parameters which are written below
• Physical evaluation of sign & symptoms
• RT-PCR testing
• Serological testing
• Nasopharyngeal swab testing for both upper and lower tract infection
• Imaginary testing
• Blood testing
• Isothermal nucleic amplification testing
There is no specific vaccine or approved drug available to treat COVID-19 but there are a lot of scientists working to find a progressive solution to treat the major epidemic COVID-19. Various drugs are claimed to be effective to treat patients but also have contraindications or ADR. The major drugs being considered are explained below based on clinical trials and their efficacy according to my literature studies. All the drugs aim to inhibit the replication of the genome in the early phase.
Remdesivir
Remdesivir is a broad-spectrum antiviral drug (with developing code GS-441524). Officially synthesized and developed by Gilead science was previously used for treating Ebola and claimed to be affected in treating COVID-19 as it enters in the third phase and In-vitro studies show that remdesivir can inhibit coronaviruses such as SARS-Cov and MERS-Cov replication. In an in-vitro test conducted utilizing epithelial cell cultures of a primary human airway, at which remdesivir was effective against Bat-Covs, prepandemic Bat- Covs, and circulating contemporary human-Cov in primary in human lung cells. One study shows that the drug remdesivir and interferon-beta were superior to the drug combinations of lopinavir, ritonavir, and interferon-beta both in vitro and in a MERS-Cov mouse model significantly [6-11]. On 17 March 2020, the drug was provisionally approved for use in COVID-19 patients having the critical condition in the Czech Republic, and on 18th, March 2020 [12], the WHO has launched a four-arm clinical trial, which formally known as the recovery trial, the largest globally trials of different drugs on groups of patients including Remdesivir. Remderevir is an experimental drug and had not been licensed or approved at the time of writing this paper.
Mechanism of action
Remesedivir is an adenosine analog and it is a monophosphoramidate prodrug. It metabolized into its actual active form, GS-441524, which obscures viral RNA polymerase and evades the proofreading by viral exonuclease, cause a decrease in viral RNA production. The antiviral mechanism of the drug remdesivir is a delayed chain cessation of nascent viral RNA. Current research trials of the drug on COVID-19 have revealed that the drug is effective against certain key enzymes of SARS-Cov-2, causes COVID-19 [12]. According to a recent research paper published on the 13th April 2020 in the journal Science Daily, by the University of Alberta and Faculty of Medicine & Dentistry, scientists have proved that Remdesivir is highly effective in inhibiting the replication mechanism of the coronavirus that leads to the novel COVID-19 [7]. The observations are followed closely by research demonstrating how the drug acts against the Middle East Respiratory Syndrome (MERS) virus, a related type of coronavirus. Clinical studies are still ongoing to understand whether the prodrug terminates RNA chains or it causes mutations in the RNA. By Using Ebola virus studies, it was noted that the prodrug, Remdesivir can inhibit the action of RNA-dependent RNA polymerase, causing the elongation of the synthesized chain. 200 MG of Remesedivir through I.v. route TDS was given to the patients who are enrolled in clinical trials. Early results from Phase III trials conducted in Chicago indicate that Remdesivir may be beneficial for the patients infected with COVID-19. Slightly clinical improvement was observed in 36 of 53 patients treated with compassionate-use Remdesivir Early results from Phase III trials in Chicago indicate that Remdesivir may be beneficial (Figure 1).
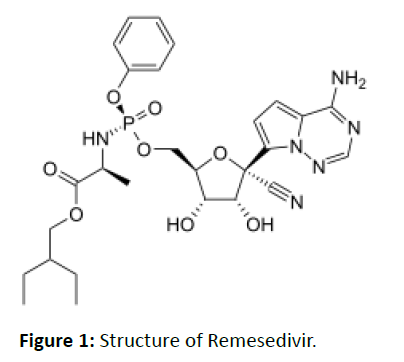
Figure 1: Structure of Remesedivir.
ADR’s and contraindications linked to use of Remesedivir:
• Respiratory failure
• Elevated levels of liver enzymes
• Hypotension
• Thrombipenia
• Lowers the level of RBC's in our blood
Hydroxychloroquine/Chloroquine
Hydroxychloroquine is a derivate of chloroquine an antimalarial drug used to treat malaria but also used to treat other ailments i.e. in the treatment of rheumatoid arthritis, lupus, and porphyria cutanea tarda [13-17]. It is taken orally. Available in different dosage forms used according to the therapeutically need of patients. It is also being experimented with as a treatment for coronavirus disease 2019 (COVID-19). It is on the WHO's List of Essential Medicines, the safest and most effective medicines needed in a health system to treat various diseases. It has both an anti-spirochete activity and an anti-inflammatory activity. Chloroquine has been found to have immunomodulatory activity and could effectively inhibit SARSCov- 2 in vitro. Clinical controlled trials have shown that chloroquine was effective in the treatment of patients with COVID-19 but also have contraindications that Hydroxychloroquine and chloroquine can lead to abnormal heart rhythms such as QT interval [18] prolongation and a dangerously rapid heart rate known as ventricular tachycardia. The risk factors may increase when these medicines are combined with other medicines used to prolong the QT interval, which includes the antibiotic azithromycin, which is also being used in some COVID-19 patients without FDA approval for this condition [19]. However, the initial margin between the therapeutic and toxic dose is narrow and chloroquine poisoning has been associated with cardiovascular disorders that can be life-threatening Hence it also passes in-vitro trials but failed at clinical trials. Hydroxychloroquine is not yet approved for the treatment of COVID-19. In vitro antiviral activity of chloroquine has been identified that the growth of many different viruses has been inhibited in cell culture by both chloroquine and hydroxychloroquine, including the SARS coronavirus, some evidence for activity in mice has been found for a large variety of viruses, including the human coronavirus [20]. The major advantage of this drug is that it is cheap in cost and easily available.
Mechanism of action of hydroxychloroquine
TLR Hydroxychloroquine acts on antigen-presenting cells and increases their pH, it blocks toll-like receptors on Plasmacytoid Dendritic Cells (PDCs) in inflammatory conditions. Toll-Like Receptor 9 (TLR 9) are cellular receptors for microbial products that induce inflammatory responses through activation of the innate immune system, which helps in recognizing DNAcontaining immune complexes, directly leads to the production of interferon, and causes the dendritic cells to mature and present antigen to T cells [19]. By decreasing signaling, Hydroxychlorquinone reduces the activation of dendritic cells and the inflammatory process. Used in the treatment of patients with novel COVID-19. It acts by increasing the endosomal pH and then interacts with the glycosylation of cellular receptors of SARS-Cov and thereby it can block viral infection. Besides, it also inhibits the enzyme quinone reductase-2, which is involved in sialic acid biosynthesis that makes this agent a broad antiviral agent. It is important to note that both Human Corona Virus HCov-O43 and orthomyxoviruses use sialic acid moieties as a binding receptor. Changes in the pH of lysosomes and likely inhibits catharsis, which leads to the formation of the auto phagosome holding cleave SARS-Cov-2 spike protein. SARS-Cov-2 utilizes the similar surface receptor ACE2 which is also utilized by hydroxychloroquine, it is believed that chloroquine can also interfere with ACE2 receptor glycosylation thus prevents SARSCov- 2 attachment to the target cells (Figure 2) [21].

Figure 2: Structure of hydroxy chloroquinone.
ADR’S and contraindications related to COVID-19:
• Cardiac diseases
• Allergic reactions
• Retinopathy and macular toxicity
• Psoriasis
• Hypokalemia
Lopinavir-Ritonavir
Lopinavir was formally developed by Abbott in an attempt to improve upon the company's previous protease inhibitor, the drug ritonavir, specifically concerning its serum protein-binding properties (decreasing the involvement by serum on protease enzyme inhibition) and its HIV resistance profile (reducing the propensity of the virus to develop resistance to the drug). It is given in combination with a small fixed dose of the drug ritonavir as it has insufficient bioavailability when administered alone. Like several HIV protease inhibitors, by lower doses of ritonavir, its randomized blood levels are greatly increased, it is a potent inhibitor of intestinal and hepatic cytochrome P450 3A4 present in the liver, which would otherwise reduce drug levels through the catabolism process. Co-administering strategy of lopinavir with doses of ritonavir sub-therapeutic concerning HIV inhibition; hence, the drug lopinavir was only formulated and marketed as a fixed-dose combination with ritonavir. An accurate combination of lopinavir/ritonavir with auxiliary IFNB treatment shows improved clinical parameters in marmosets and mice infected with MERS-Cov. But in humans, it doesn't show any significant effect and yet it is not officially approved till now to treat COVID-19. Standard dose is 400 mg/100 mg PO BID (Figure 3).

Figure 3: Ritonavir.
Mechanism of action
Lopinavir and ritonavir both drugs are protease inhibitors, block viral replication. Lopinavir seems to be the agent that acts on the virus. Ritonavir is a CYP3A inhibitor that functions primarily to reduce the metabolism of lopinavir, thereby boosting lopinavir levels. During the production of the viruses in our body, new proteins for the viruses are made. Some of them are structural proteins, that, is, proteins that form the body of the virus. Other proteins are enzymes that manufacture DNA and other components for the new viruses in our bodies. Protease is the enzyme that is responsible for the new structural proteins and enzymes. The drug lopinavir blocks the action of protease and results in the formation of defective or immature viruses that are unable to infect the body's cells. As a result of which, the number of viruses in the body (the viral load) decreases (Figure 4) [22].

Figure 4: Lopinavir.
ADR’S and contraindications:
• Hyperglycemia, hypertriglyceridemia
• Renal failure
• Anemia, leukopenia, neutropenia
• Pancreatitis
• Hepatotoxicity
• Cardiac disease (cardiomyopathy, structural heart disease, ischemic heart disease, QT prolongation).
Ivermectin
It was first discovered in 1975 Ivermectin is a drug used to treat many types of parasite infestations in both humans as well as animals. This includes head lice, scabies, river blindness (onchocerciasis), ascariasis, strongyloidiasis, trichuriasis, and lymphatic filariasis It can be taken by oral route in form of capsules or applied topically skin as an ointment for external contagion. Ivermectin belongs to the ivermectin family of drugs. Taking a single dose of ivermectin reduces microfilaraemia by 98%-99% after 1-2 months. Ivermectin cannot kill adult worms. Single Oral dose of ivermectin capsule taken once or twice a year for the adult of the lifespan of 10-15 year the worms are advisable, which is required to protect the individual from river blindness. In recent years, ivermectin has shown to have antiviral activity against a broad range of viruses. According to in vitro studies it is originally associated as an inhibitor of the interlinkage between the Integrase Protein (IN) Human Immunodeficiency Virus-1 (HIV-1) and the Importin (IMP) α/β1 heterodimer responsible for IN nuclear import inside the cell. Recent studies on SARS-Cov proteins have disclosed a potential role for IMPα/β1 during infection in signal-dependent nucleocytoplasmic shutting of the SARS-Cov Nucleocapsid. Ivermectin's nuclear transport repressive activity may be powerful against SARS-Cov-2. Ivermectin-associated neurotoxicity, particularly in patients having hyper inflammatory state possible with COVID-19. In inclusion to the drug interactions with potent CYP3A4 inhibitors (eg, ritonavir) needs careful consideration of administered drugs [23]. Finally, evidence suggests that ivermectin plasma levels with meaningful activity against COVID-19 would not be achieved without potentially toxic level increases in ivermectin doses in humans.
Mechanism of action
It acts by killing, interfering, and muscle function, in particular by amplifying inhibitory neurotransmission. Ivermectin acts by binding to Glutamate-Gated Chloride Channels (GluCls) in the membranes of muscle cells and invertebrate nerve, which leads to expand permeability to chloride ions, resulting in cellular hyper-polarization, followed by paralysis and death. GluCls are invertebrate-specific members of the Cys-loop family of ligandgated ion channels present in neurons and myocytes. With the nervous system. Inhibits the replication of SARS-Cov-2 in a specific species of monkey kidney cell culture with an IC50 [22]. But can’t have any positive effect on humans to treat Covid-19 (Figure 5).

Figure 5: Structure of Ivermectin.
Adr’s and contraindications:
• Neurotoxicity
• CNS Depression
• Kidney Disease
• Tiredness
• Loss of appetite
Convalescent Plasma Therapy
Scientists and researchers are finding out various avenues to return up with medical treatment that helps fight the novel coronavirus. Treatment that's focused immediately is convalescent plasma therapy [24-26]. The convalescent plasma features a previous history of effectiveness within the treatment of infectious diseases. The FDA has easy access to convalescent plasma, during which antibody-rich products are collected from eligible donors who have recovered from COVID-19 recently. Clinical results convalescent plasma has not yet been shown to be effective in COVID-19 [27]. The FDA states that it's compulsory to identify its safety and efficacy via clinical trials before routinely administering convalescent plasma to treating patients with COVID-19. This therapy has successfully experimented within the past and now becomes a ray of hope within the fight against the novel coronavirus pandemic. This therapy focuses on using antibodies from the blood of a recovered COVID-19 patient to treat those critically affected by the virus. The therapy can also be used to immunize those who are at a high risk of contracting the virus-like families of patients, doctors, and other high-risk contacts i.e. individuals working intuned with infected patients. Antibodies are developed during a patient by the body's natural immune response to a faraway pathogen or in this case, the novel coronavirus. These antibodies are highly specific to the invading pathogen affecting the body. After collecting the donated blood is then checked for the presence of the opposite disease-causing agents like hepatitis C, hepatitis B, HIV, etc. For effective plasma therapy "a sufficient amount of antibody must be administered. When given to a susceptible person, then this antibody will circulate within the blood, reach tissues, and provide protection against infection. Relying on the antibody amount and composition, the protection conferred by the transferred antibodies can last from weeks to months." Various shreds of evidence show that convalescent plasma from patients who have recovered from viral infections is often used as a treatment without the occurrence of severe adverse events. But according to haemovigilance if the plasma isn't compactable in the receiver's body then it's getting to cause various reactions. Therefore, it would be worthwhile to see the safety and efficacy of convalescent plasma transfusion in SARS-Cov-2 infected patients [28].
In the case of SARS-Cov-2, the anticipated mechanism of action by which the passive antibody therapy would mediate protection is viral neutralization. However, other mechanisms could even be possible, like antibody-dependent cellular cytotoxicity and/or phagocytosis. Typical Possible sources of antibody for SARS-Cov-2 are human convalescent sera from individuals who have recovered from COVID-19, mabs, or preparations were generated in certain animal hosts, like genetically engineered cows that produce human antibody. Although many kinds of preparations are or will soon be under development, the only antibody type that's currently available for immediate use is that found in human convalescent sera [29-32].
Safety precautions to be taken to protect you and others from COVID-19, as indicated by WHO
• Wash your hands regularly with soap and water, or clean them with alcohol-based hand rub sanitizer.
• Maintain at least a 1-meter distance between you and people coughing or sneezing.
• Avoid touching your eyes, mouth, and nose
• Cover your mouth and nose when coughing or sneezing.
• Avoid eating junk or unhealthy food.
• Eat fruits and vegetables after washing 2-3 times.
• Avoid contact with COVID-19 affected patients. If visited anyone then go for a test.
• If you have a fever, cough, and difficulty breathing, seek medical attention, but call by telephone in advance if possible and follow the directions of your local health authority.
• Keep up to date on the latest information from trusted sources, such as WHO or your local and national health authorities, and don’t believe in myths.
• Use antiseptics and disinfectants on things used continuously.
• Wear gloves when you are holding something or having contact with the surface.
• Don't use unnecessary drugs without consulting with doctors.
• Avoid cold drinks and other soda beverages.
Immunoboosters that Help in Increasing Immune Power Against Covid-19
Citrus fruits
They are rich in vitamin-c and help to fight against a variety of flu andi nfections, helpful in collagen formation, and have autoxidizing properties. The example of citrus fruits is orange, lemon, grapes, etc. [33,34].
Wheatgrass
Wheatgrass act as a natural Immune booster is a potent source of potassium, dietary fiber, vitamin A, vitamin C, vitamin E (alpha-tocopherol), vitamin K, pantothenic acid, iron, zinc, copper, manganese, thiamin, riboflavin, niacin, vitamin B6 and selenium. It helps to fight a variety of infections and is also used to treat cancer.
Turmeric
It is a natural immunity booster for cold and flu prevention regimen! A natural way to help boost the immune system by increasing the immune modulation capacity of the body. Use turmeric in periods of stress or during flu season to help give your immune system a little boost. Turmeric includes diarylheptanoids. Curcumin is the main active ingredient present in turmeric. It has powerful anti-inflammatory effects and is a very strong antioxidant used commonly in daily routine [35,36].
Ginger
Ginger is a well-known remedy for cramping and travel sickness. It’s also a great ingredient for boosting our immune response due to its anti-inflammatory, antioxidant and antimicrobial properties.
Tulsi
Tulsi (Holy basil) acts as a natural immune system booster and keep infections away. Protects against all infections from viruses, bacteria, fungi, and protozoa. Tulsi leaves extract increases the T helper cells and natural killer cells activity, boosting the immune system.
Oregano leaves
It is a natural Immune booster consists of highly phenolic compounds thymol. Which has very good results against respiratory tract infections as in COVID-19 for prevention purposes? It is also a good antioxidant.
Garlic
It contains active constituent allicin which boosts the diseasefighting response of some types of white blood cells in the body when they encounter viruses, such as the viruses that cause the common cold or flu.
Rose petals
It has a variety of properties. Rose tea is a non-calorie hot beverage that consists of Vitamin C a potent immune booster. It helps to fight respiratory tract infection, the body infectionfighting power decrease, by drinking mild-warm Indian rose tea can aid in easy recovery.
Bee honey
It consists of flower nectar and has ant oxidative and immunity-boosting properties.it helps in increasing antibodies and helps to maintain various ailments.
Giloy
It is known as the powerhouse of antioxidants which helps to fight from free-radicals, keep your cells healthy, and get rid of diseases. It helps to remove toxins, purifies blood, and fights bacteria that leads to diseases and it also help to treat chronic fever.
Liquorice
It is also known as LIQUORICE helps to boost immunity and keeps the respiratory tract healthy. Works as a bronchodilator and expectorant.
Green tea
It helps to prevent lung infection and has varieties of properties used in weight management and as an antioxidant.
A Natural Drink to Boost Immunity to Fight Against Various Infections
Shows effective results when used in individuals for continuous one week (medications conditions may vary the result). It is beneficial to boost immunity and making the body more aggressive to fight against a variety of infections. After observations individuals who used this drink twice a day were found to be more aggressive and are less prone to infection during studies in comparison with common individuals [36-40].
• Take 150 ml water in a pan
• Add the following ingredients:-
• 5-6 Tulsi leaves
• 0.5 GM Oregano leaves
• 4-5 Rose petals
• Small Portion of Dried Ginger
• 1 Dried Small mulethi or liquorice root
• Small portion of raw turmeric
• 3 gm Green tea
• A 5 inch of cleaned giloy stem.
After adding this all boil it at a medium flame until you observe a strong pleasant smell or for 5 minutes with continuous stirring. Then refine it and pour it into a glass and add 1 tbsp HONEY in it when it becomes lukewarm and drink it twice a day (You may avoid the ingredient if allergic to any of them).
Conclusion
It can be concluded that there is no approved treatment for the complete recovery of COVID-19. Therefore, preventative measures play a major role in controlling the disease burden. Two major preventative measures include proper hand hygiene and sanitization and secondly, by boosting immunity. The drugs being studied may potentially show positive effects in upcoming trials but also have contraindications. Thus, the disease is difficult to treat with no therapeutically satisfactory response of drugs, as Resemdivir approaches to the clinical trial phase 3 and Hydroxychloroquine leads to ADR’S when given with azithromycin. The continued focus is to find therapeutically effective and safe drugs and maintain preventative measures.
40126
References
- COVID-19 pandemic (2019) ongoing global pandemic of coronavirus disease.
- Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, et al. (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. Mbio 9: 00221.
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, et al. (2017)Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 9: 3653.
- Tawfiq JAA, Homoud AHAL, Memish ZA (2020) Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis 34: 101615
- Warren TK, Jordan R, Ray AS, Mackman RL, Soloveva V, et al. (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531: 381-385.
- Zablon FM (2020) Remdesivir- Definition, Mechanism of Action, Uses, Synthesis.
- Eleanor M (2020) Study finds remdesivir effective against a key enzyme of coronavirus that causes COVID-19.
- Centers for Disease Control and Prevention (2021) Clinical Questions about COVID-19: Questions and Answers.
- University of Alberta (2011) Serving those injured in service to Canada.
- Jones L (2020) SPOT THE SIGNS Six new possible coronavirus symptoms added to official US list.
- Chauhan A, Tikoo A (2015) The enigma of the clandestine association between chloroquine and HIV-1 infection. HIV Med 16: 585–590.
- Chinese Clinical Trial Register (2021) The world health organization international clinical trials registered organization registered platform.
- Singh AK, Singh A, Shaikh A,Singh R, MisraA (2020) Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr 14: 241-246.
- Gautreta P, Lagier JC, Parola P, Hoang VT, Meddeb L, et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 56: 105949.
- Ogbru O (2020) lopinavir and ritonavir (Kaletra): Potential COVID-19 Drug.
- Cennimo DJ (2021) Coronavirus Disease 2019 (COVID-19) Treatment & Management
- Ivermectin (2021) Ivermectin is a medication used to treat parasite infestations
- Caly L, Druce D, Catton MG, Jans DA, Wagstaff KM (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research 178: 104787.
- Anand A (2020) What is Plasma Therapy: A possible treatment for coronavirus?
- Chen L, Xiong J, Bao L, Shi Y (2020) Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis 20: 398-400.
- Beigel JH, Voell J, Kumar P, Raviprakash K, Wu H, et al. (2018) Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from trans chromosomic cattle: a phase 1 randomized, double-blind, single-dose-escalation study. Lancet Infect Dis 18: 410-418.
- Casadevall A, Pirofski LA (2020) The convalescent sera option for containing COVID-19. J Clin Invest 130: 1545-1548.
- Bergman SJ (2021) COVID-19 Treatment: Investigational Drugs and Other Therapies.
- Warren Tk, Jordan R, Lo MK, Ray AS (2016) Therapeutic Efficacy of the Small Molecule GS-5734 against Ebola Virus in Rhesus Monkeys. Nature 531: 7594
- Maurya D, Sharma D (2020) Evaluation of Traditional Ayurvedic Preparation for Prevention and Management of the Novel Coronavirus (SARS-CoV-2) Using Molecular Docking Approach.
- Recipe Of The Immunity-Boosting Kadha Shared By A COVID-19 Survivor Becomes Viral
- Saha S (2020) This Rose-Mulethi Tea May Help Induce Better Digestion, Immunity And Weight Loss.