Keywords
Pontine Myelinolysis; Osmotic demyelination syndrome; Central pontine myelinolysis; Extrapontine myelinolysis; Hyponatremia; Demyelination; Prostate
Abbreviations
PM: Pontine Myelinolysis; ODS: Osmotic Demyelination Syndrome; CPM: Central Pontine Myelinolysis; EPM: Extrapontine Myelinolysis; BBB: Blood Brain Barrier; MRI: Magnetic resonance imaging; GABA: Gamma-aminobutyric acid
Introduction
Pontine myelinolysis (PM) was first described in 1959 by Adams et al. in alcoholic patients [1]. Also known as osmotic demyelination syndrome (ODS), PM is subdivided into central pontine myelinolysis (CPM) and extrapontine myelinolysis (EPM) [2]. Each is identified at the level of demyelination either centered within the pons [1] or outside the pons [3], respectively. While the etiology of ODS is poorly understood, it is believed that rapid correction of sodium in chronic hyponatremia is implicated as primary factor [1].
Epidemiology of pontine myelinolysis
ODS, though uncommon, has been reported at a rate of 0.4- 0.56% for patients admitted to neurology services and 0.05% of all admitted in a general hospital [4-6]. Underdiagnosis of ODS has been reported in recent literature; Newell et al. identified 0.3-1.1% of patients with unsuspected CPM during autopsies, with an even greater percentage of CPM in patients with liver transplant and chronic liver disease [7].
Clinical diagnosis of pontine myelinolysis
When trying to diagnosis a patient with PM, examining physical symptoms and neurological complications may often be the simplest route. The most common symptoms seen in PM patients are tremor and quadriplegia [8-12] as well as depressed level of consciousness [13]; additional clinical diagnosis and neurological complications are outlined in Tables 1 and 2, respectively. In patients suspected of early PM, the new concept of fundoscopy may reveal optic disc changes suggestive of raised intracranial hypertension.
| Clinical Diagnosis of Pontine Myelinolysis |
| Clinical Diagnosis |
Reference |
| Muscle weakness |
[14-17] |
| Tremors and quadriplegia |
[8-12] |
| Slowed speech and poor enunciation |
[18-20] |
| Swallowing difficulty |
[19,20] |
Table 1: Clinical diagnosis of pontine myelinolysis.
| Neurological Complications of Pontine Myelinolysis |
| Author |
Neurological Complications |
| Choe et al. |
Difficulty in chewing, swallowing, walking, and communicating, progressing to a decreased level of consciousness [13] . |
| Choe et al. |
Pseudobulbar palsy, dysarthria, dysphagia, and facial diplegia[13] . |
| Cuvellier et al. |
Cerebellar syndrome, somnolence, hemiparesis, and facial paralysis [21]. |
| di Rocco et al. |
Symptoms of labile speech [22]. |
| Kawahara et al. |
Seizure, mental change, and quadriparesis[23] . |
| Kepes et al. |
Generalized seizures and decreased level of consciousness [24]. |
| Matsuoka et al. |
Progressively decreased level of consciousness and eventually coma [25]. |
| Ranger et al. |
Decreased level of consciousness and severe hyponatremia [26]. |
| Tsutsumi et al. |
Level of consciousness progressively deteriorated with loss of verbal response via spastic paresis [27] . |
Table 2: Neurological complications of pontine myelinolysis.
Pathogenesis of pontine myelinolysis
Despite PM’s relatively ambiguous pathogenesis, it is believed that rapid correction of hyponatremia plays a pivotal role [28]. Hyponatremia causes glial cells to swell via selective aquaporin channels [29]. Treatment of chronic hyponatremia, using a hypertonic saline solution, causes brain cell dehydration and the loss of vital electrolytes, in addition to organic osmolytes such as myo-inositol, taurine, glutamine, glutamate, creatine, phosphocreatine, and glycerophosphorylcholine [30]. Failure to compensate for increasing plasma tonicity within cells results in osmotic stress [31], all the while preventing the reabsorption of organic osmolytes into the cell [30]. According to studies by Rojiani et al., [32,33], the opening of the blood brain barrier (BBB) and the generation of edema may play a role in the early stages of the disease. Stress on the BBB as a result of osmotic shrinkage results in the opening of tight junctions [34]. This is of particular concern in oligodendrocytes, which appear to be the most susceptible cells to this form of damage [11]. Todd et al., suggested that plasminogen activator, complement proteinase, cytokines, immunoglobulins, and neural proteases released from endothelial cells may lead to oligodendrocyte degeneration [35], ultimately causing the destruction of myelin [11] (Figure 1).
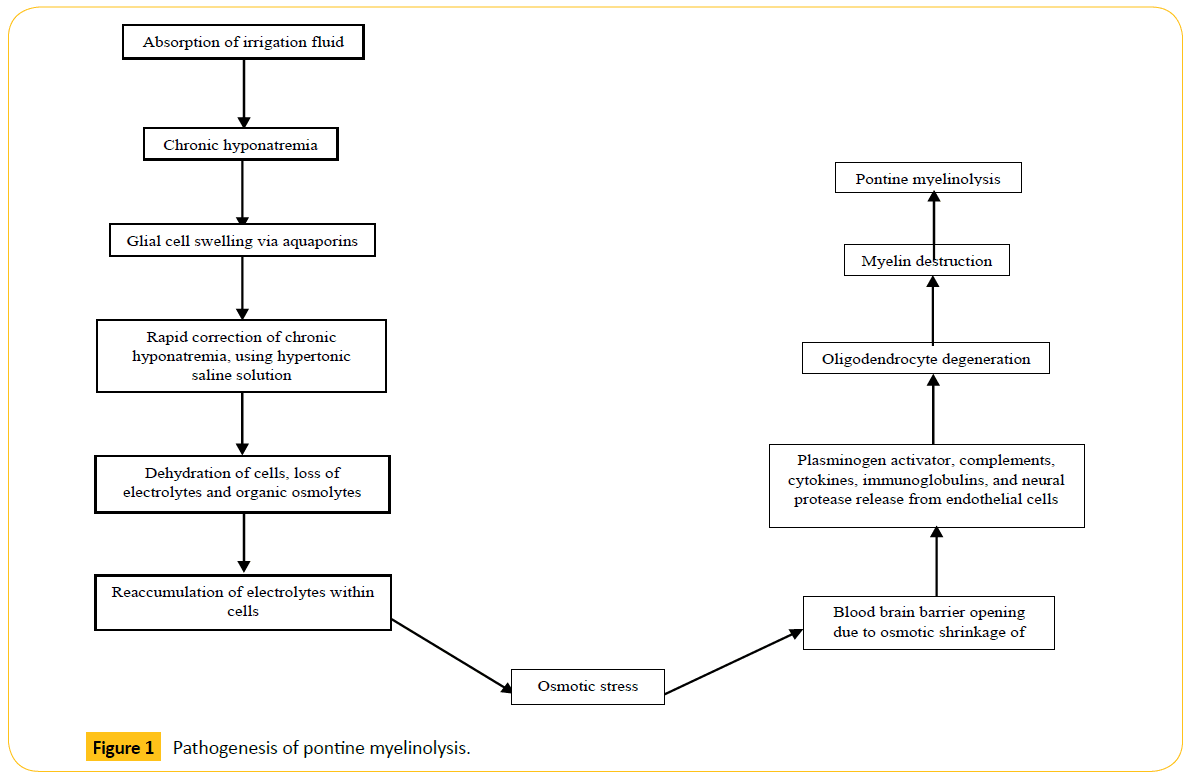
Figure 1: Pathogenesis of pontine myelinolysis.
Onset of Hyponatremia
Hyponatremia is defined by the excess of water in relation to the serum sodium concentration; Table 3 depicts its severity levels [36]. Absorption of irrigation fluid, a surgical necessity for improving operating field vision, is known to induce iatrogenic hyponatremia [37]. The irrigation fluid must be generally electrolyte free to allow cutting with a resectoscope, despite a danger of absorption [37]. Glycine 1.5%, mannitol, or sorbitol are common solutes added to decrease hypo-osmolality and prevent hemolysis in the event of reabsorption [37]. For absorption to occur, the driving force of fluid must exceed the venous pressure of approximately 1.5 kPa [38]. Fluid absorption can occur in all operations utilizing irrigation fluid. Irrigation fluid is used most commonly in transurethral resection of the prostate (TURP) and transcervical resection of the endometrium (TCRE) [37]. Absorption can range between 300-1000 mL in 8% of TURP operations [39] and approximately 450 mL in TCRE procedures [40]. Moreover, photoselective vaporization of the prostate via green light laser surgery is a minimally invasive technique [41] that delivers more advantages than TURP [42]. However, absorption of irrigation fluid is still possible in patients who undergo high power 532 nm laser vaporization of the prostate [43]. Fluid absorption can elicit cardiopulmonary and neurological symptoms, while ethanol breath tests yield early detection [43].
| Severity of Hyponatremia |
| Levels |
Serum concentration: mmol•L-1 |
| Severe |
< 120 |
| Moderate |
120-124 |
| Mild |
125-134 |
| Normal |
135-144 |
Table 3 Severity of hyponatremia.
Etiology of pontine myelinolysis
Rapid correction of hyponatremia is one of the many potential causes of PM. Uchida et al., explains where a patient develops CPM secondary to liver transplant [44]. Wadhwa et al., identified a case where an alcoholic patient showed signs of EPM [45]. CPM can also be a rare manifestation of Wilson’s disease [46] and celiac disease [8]. According to Wu et al., EPM can arise from primary adrenal insufficiency. This author described a 49 year old female who presented with primary adrenal insufficiency. The patient was given an isotonic saline solution to treat for adrenal insufficiency and hyponatremia. Rapid correction showed demyelination of the bilateral basal ganglia and the thalamus, using MRI [47]; additional causes are outlined in Table 4.
| Etiology of Pontine Myelinolysis |
| Causes |
Reference |
| Liver transplant |
[44,48-50] |
| Alcohol |
[45,51] |
| Hypophosphatemia |
[52] |
| Type 1 diabetes |
[53] |
| Gestational diabetes |
[54] |
| Hypernatremia |
[55,56] |
| Wilson’s disease |
[46] |
| Hyperglycemia |
[55,57] |
| Celiac disease |
[8] |
| Primary Adrenal Insufficiency |
[47] |
Table 4 Etiology of pontine myelinolysis.
Differential diagnosis of pontine myelinolysis
According to Falini et al., acute multiple sclerosis lesions can be seen as hyperintense lesions on T2-weighted sequences and accompanied by BBB breakdown showing contrast on T1-weighted images. This is sometimes perceived as PM, as demyelination is implicated at the pons, basal ganglia, midbrain, thalamus, and subcortical white matter [58]. Using magnetic resonance imaging (MRI), PM is marked by prolongation of T1 and T2 relaxation times in myelinolytic regions [58].
Histopathology of pontine myelinolysis
Intramyelinitic splitting, vacuolization, and rupture of myelin sheaths are key in identifying histopathological finding of an individual with CPM [59]. Progression of CPM results in macrophages findings at the site myelin debris [59]. Pietrini et al., identified an acute stage of demyelination and large macrophagic infiltration, but no significant inflammatory reaction [60].
Autonomic neuropathy and pontine myelinolysis
Autonomic neuropathy and PM can be found occurring simultaneously in patients. A 2008 case study by Tilikete et al., identified a 51 year old patient with primary position upbeat nystagmus and internuclear ophthalmoplegia. The diagnosis, using MRI, showed a demyelination of the pons may have resulted in this action [61]. A 1999 article by Susa et al., discussed a 29 year old woman with acute intermittent porphyria-an autosomal dominant disease caused by a deficiency of porphobilinogen deaminase-suffered severe hyponatremia and was treated with heme arginate. MRI showed CPM and EPM, and cortical laminar necrosis, all of which are not common in acute intermittent porphyria [62].
Magnetic resonance imaging of pontine myelinolysis
MRI has streamlined the diagnosis of PM by tracing the evolution of the lesion and pairing its progression or regression with clinical features [63]. Prominent signal characteristics include T1- hypotensive, T2-hypertensive, and FLAIR-hypertensive [5]. Table 5 highlights several brain regions affected by PM.
| Magnetic Resonance Imaging of Brain Regions for Pontine Myelinolysis Diagnosis |
| Brain Region |
Reference |
| Pons |
[23,49,51,56,64] |
| Basal ganglia |
[13,22,26] |
| Thalamus |
[26] |
| Cerebellum |
[26] |
| Middle cerebellar peduncle |
[44] |
| Corpus callosum |
[57] |
Table 5: MRI of different brain regions for pontine myelinolysis.
Prevention and treatment of pontine myelinolysis
Several options surround the prevention and treatment of PM. A hallmark of prevention is proper management of hyponatremia post-absorption of irrigation fluid. Hyponatremia can be treated using hypertonic saline solution [30]. Treatment can, however, pose a challenge as rapid correction can lead to PM [3]. Gradually increasing the sodium concentration, from 4-6 mmol.L-1 in any 24 hour period, is the most favorable therapy to treat a patient with severe hyponatremia while preventing any unwanted side effects [65].
A unique situation in which rapid correction of hyponatremia without consequence of PM may be embraced by azotemic, patients with large amounts of nitrogenous waste products in blood, patients [66]. Dhrolia et al., conducted a study on 52 azotemic patients, who had undergone hemodialysis to rapidly treat hyponatremia [66]. Despite the known assumption that rapid correction will elicit PM, MRI verified that none of the 52 patients with azotemia developed neurological conditions. Azotemia may protect the brain from osmotic demyelination by rapidly changing urea concentration. Urea can act as an effective osmole in dialysis disequlibrium syndrome [66].
A separate method for treating PM involves the administration of intravenous immunoglobin (IVIg). Murthy et al., highlighted three PM cases in which the patient received IVIg over the course of a few days and began to recover [67]. Despite the unclear mechanism, a possible explanation involves the reduction of myelinotoxic substances and promotion of remyelination [68]. Plasmaphersis (PS) is another option in treating PM. Saner et al., identifies a patient, who underwent liver transplant and developed CPM. The patient was immediately placed PS and IVIg for six days. The patient showed signs of recovery after the treatment [69]. It is believed that PS may also reduce myelinotoxic substances, thereby leading to clinical improvement [69]. Table 6 provides additional pharmacological treatment options.
| Treatment for Pontine Myelinolysis |
| Drug Name or Class |
Identity/Function |
Reference |
| Levodopa |
Dopamine precursor that can cross the BBB to increase the concentration of dopamine. |
[6,70-72] |
| Amantadine |
Weak antagonist of N-methyl-D-aspartate -type glutamate receptor that blocks reuptake of dopamine. |
[72] |
| Anticholinergic |
Blocks acetylcholine from binding to its receptor in the central and peripheral nervous system. |
[6,73,74] |
| Pramipexole |
Dopamine agonist that activates dopamine D2, D3, and D4 receptors. |
[75] |
| Clonazepam |
Benzodiazepine drug mainly prescribed for epilepsy and panic disorder. |
[6,76] |
| Baclofen |
Derivative of gamma-aminobutyric acid (GABA). It is an agonist of GABAB receptors used for treating spastic movement and addiction. |
[6,11,77] |
| Botulinum toxin |
Neurotoxin produced from bacterium Clostridium botulinum that prevents the release of acetylcholine. |
[6] |
| Bromocriptine |
Dopamine agonist. Potent agonist at dopamine D2 receptors and various serotonin receptors. |
[78] |
| Tiapride |
Selectively blocks dopamine D2 and D3 receptors. Treats various neurological and psychiatric disorders. |
[79] |
| Perphenazine |
Antipsychotic drug and dopamine antagonist. |
[79] |
| Pergolide |
Dopamine receptor agonist that binds to dopamine D2 and D1 receptors and various serotonin receptors. |
[72] |
| Haloperidol |
Antipsychotic drug. |
[76] |
Table 6 Treatments for pontine myelinolysis.
Conclusion
Pontine myelinolysis (PM) develops primarily from rapid treatment of hyponatremia, but also occasionally from liver transplantation and alcohol abuse. The effect of PM can be seen through histological examination and MRI-deduced regional brain effects. Upon treating chronic hyponatremia with hypertonic saline, the disequilibrium of organic osmolytes may play a key role in the pathogenesis of PM. Hyponatremia is caused from absorption of irrigation fluid during operations such as transurethral resection of the prostate (TURP). Prevention of PM must be conducted by gradually increasing sodium concentration 4-6 mmol.L-1 in any 24-hour period. Additional PM treatment mandates the use of immunoglobin, plasmaphersis, or select neurological drugs.
Acknowledgment
We gratefully acknowledge literature research assistance from Mrs. Wendy Isser and Ms. Grace Garey. We would also like to acknowledge the works for Dr. Kelly Warren and Mr. Jason Gandhi, for their assistance with critiquing and applying logical reasoning to literature, and assisting with performing medline searches.
Compliance with Ethical Standards
The authors declare they have no conflict of interest.
8094
References
- Adams RD, Victor M, Mancall EL (1959) Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 81: 154-72.
- Alleman AM (2014) Osmotic demyelination syndrome: central pontine myelinolysis and extrapontine myelinolysis. Semin Ultrasound CT MR 35: 153-159.
- Wright DG, Laureno R, Victor M (1979) Pontine and extrapontine myelinolysis. Brain 102: 361-385.
- de Souza A, Desai PK (2012) More often striatal myelinolysis than pontine? A consecutive series of patients with osmotic demyelination syndrome. Neurol Res 34: 262-271.
- Kallakatta RN (2011) Clinical and functional outcome and factors predicting prognosis in osmotic demyelination syndrome (central pontine and/or extrapontine myelinolysis) in 25 patients. J NeurolNeurosurg Psychiatry 82: 326-331.
- Bhoi KK, Guha G PA, Barma P, Misra AK, Garai PK (2007) Reversible parkinsonism in central pontine and extrapontine myelinolysis: a report of five cases from India and review of the literature. Neurol Asia 12: 101-109.
- Newell KL, Kleinschmidt-DeMasters BK (1996) Central pontine myelinolysis at autopsy; a twelve year retrospective analysis. J NeurolSci 142: 134-139.
- Sharma P, Sharma S, Panwar N, Mahto D, Kumar P, et al. (2014) Central pontine myelinolysis presenting with tremor in a child with celiac disease. J Child Neurol 29: 381-384.
- Sadeh M, Goldhammer Y (1993) Extrapyramidal syndrome responsive to dopaminergic treatment following recovery from central pontine myelinolysis. EurNeurol 33: 48-50.
- Huq S, Wong M, Chan H, Crimmins D (2007) Osmotic demyelination syndromes: central and extrapontine myelinolysis. J ClinNeurosci 14: 684-688.
- Seah AB, Chan LL, Wong MC, Tan EK (2002) Evolving spectrum of movement disorders in extrapontine and central pontine myelinolysis. Parkinsonism RelatDisord 9: 117-119.
- Vermetten E, Rutten SJ, Boon PJ, Hofman PA, Leentjens AF (1999) Neuropsychiatric and neuropsychological manifestations of central pontine myelinolysis. Gen Hosp Psychiatry 21: 296-302.
- Choe WJ, Cho BK, Kim IO, Shin HY, Wang KC (1998) Extrapontine myelinolysis caused by electrolyte imbalance during the management of suprasellar germ cell tumors. Report of two cases. Childs NervSyst 14: 155-158.
- Post B, van Gool WA, Tijssen MA (2009) Transient Parkinsonism in isolated extrapontine myelinolysis. NeurolSci 30: 325-328.
- Shintani M, Yamashita M, Nakano A, Aotani D, Maeda K, et al. (2005) Central pontine and extrapontine myelinolysis associated with type 2 diabetic patient with hypokalemia. Diabetes Res ClinPract 68: 75-80.
- Burneo J, Vizcarra D, Miranda H (2000) Central pontine myelinolysis and pregnancy: a case report and review of literature. Rev Neurol 30: 1036-1040.
- Alberca R, Iriarte LM, Rasero P, Villalobos F (1985) Brachial diplegia in central pontine myelinolysis. J Neurol 231: 345-346.
- Rodríguez J, Benito-León J, Molina JA, Ramos A, Bermejo F (1998) Central pontine myelinolysis associated with cyclosporin in liver transplantation. Neurologia 13: 437-440.
- Dieterle L, Büchler G, Pfitzer F (1992) Central pontine myelinolysis. Dtsch Med Wochenschr 117: 332-336.
- Copeland PM (1989) Diuretic abuse and central pontine myelinolysis. PsychotherPsychosom 52: 101-105.
- Cuvellier JC, Soto Ares G, de Sèze C, Santos C, Cuisset JM, et al. (2000) Radiology case of the month. Extrapontine myelinolysis. Arch Pediatr 7: 75-77.
- Di Rocco F, Caldarelli M, Di Rocco C (2001) Extrapontine reversible myelinolysis in a child operated on for craniopharyngioma. PediatrNeurosurg 34: 166-167.
- Kawahara I (2009) Reversible clinical and magnetic resonance imaging of central pontine myelinolysis following surgery for craniopharyngioma: serial magnetic resonance imaging studies. Neurol Med Chir (Tokyo) 49: 120-123.
- KEPES JJ, REECE CA, OXLEY DK (1965) CENTRAL PONTINE MYELINOLYSIS IN A 7-YEAR-OLD BOY. J NeurolNeurosurg Psychiatry 28: 39-47.
- Matsuoka T, Miyoshi K, Saka K, Hayashi S, Kageyama N (1965) Central pontine myelinolysis. (A report of three cases). ActaNeuropathol 5: 117-132.
- Ranger A (2010) Osmotic myelinolysis with malignant cerebellar edema occurring after DDAVP-induced hyponatremia in a child. PediatrNeurosurg 46: 318-323.
- Tsutsumi S, Yasumoto Y, Ito M (2008) Central pontine and extrapontine myelinolysis in an infant associated with the treatment of craniopharyngioma: case report. Neurol Med Chir (Tokyo) 48: 351-354.
- Lampl C, Yazdi K (2002) Central pontine myelinolysis. EurNeurol 47: 3-10.
- King JD1, Rosner MH (2010) Osmotic demyelination syndrome. Am J Med Sci 339: 561-567.
- Norenberg MD (2010) Central pontine myelinolysis: historical and mechanistic considerations. Metab Brain Dis 25: 97-106.
- Martin RJ (2004) Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J NeurolNeurosurg Psychiatry 75 Suppl 3: iii22-28.
- Rojiani AM, Prineas JW, Cho ES (1994) Electrolyte-induced demyelination in rats. 1. Role of the blood-brain barrier and edema. ActaNeuropathol 88: 287-292.
- Rojiani AM, Cho ES, Sharer L, Prineas JW (1994) Electrolyte-induced demyelination in rats. 2. Ultrastructural evolution. ActaNeuropathol 88: 293-299.
- Sterns RH, Thomas DJ, Herndon RM (1989) Brain dehydration and neurologic deterioration after rapid correction of hyponatremia. Kidney Int 35: 69-75.
- Todd AS (1972) Endothelium and fibrinolysis. Atherosclerosis 15: 137-140.
- Snell DM, Bartley C (2008) Osmotic demyelination syndrome following rapid correction of hyponatraemia.Anaesthesia 63: 92-95.
- Hahn RG (1996) Ethanol monitoring of irrigating fluid absorption. Eur J Anaesthesiol 13: 102-115.
- Hulten JBM, Engberg A, Hjertberg H, Svedberg J (1984) The pressure in the prostatic fossa and fluid absorption. Scand J UrolNephrol 82: 33-43.
- Hahn R, Berlin T, Lewenhaupt A (1988) Irrigating fluid absorption and blood loss during transurethral resection of the prostate studied with a regular interval monitoring (RIM) method. Scand J UrolNephrol 22: 23-30.
- Magos AL, Baumann R, Lockwood GM, Turnbull AC (1991) Experience with the first 250 endometrial resections for menorrhagia. Lancet 337: 1074-1078.
- Herrmann TR, Liatsikos EN, Nagele U, Traxer O, Merseburger AS; EAU Guidelines Panel on Lasers, et al. (2012) EAU guidelines on laser technologies. EurUrol 61: 783-795.
- Reich O, Bachmann A, Siebels M, Hofstetter A, Stief CG, et al. (2005) High power (80 W) potassium-titanyl-phosphate laser vaporization of the prostate in 66 high risk patients. J Urol 173: 158-160.
- Hermanns T, Grossmann NC, Wettstein MS, Fankhauser CD, Capol JC, et al. (2015) Absorption of irrigation fluid occurs frequently during high power 532 nm laser vaporization of the prostate. J Urol 193: 211-216.
- Uchida H (2014) Central pontine myelinolysis following pediatric living donor liver transplantation: a case report and review of literature. Pediatr Transplant 18: E120-E123.
- Wadhwa J, Ananthakrishnan R, Sadashiv S, Hamide A (2013) Extrapontine myelinolysis: rare manifestation of a well-known disorder. BMJ Case Rep 2013.
- Verma R, Rai D (2013) Central pontine myelinolysis associated with Wilson disease in a 7-year-old child. BMJ Case Rep 2013.
- Wu JW, Wang PN, Lirng JF, Hsu RW, Chen WT (2014) Extrapontine Myelinolysis in a patient with Primary Adrenal Insufficiency. ActaNeurol Taiwan 23: 146-152.
- Fukazawa K, Nishida S, Aguina L, Pretto E Jr (2011) Central pontine myelinolysis (CPM) associated with tacrolimus (FK506) after liver transplantation. Ann Transplant 16: 139-142.
- Cui R, Fayek S, Rand EB, Feygin T, Khrichenko D, et al. (2012) Central pontine myelinolysis: a case report and clinical-pathological review. Pediatr Transplant 16: E251-256.
- Cartier RL (2010) Central pontine myelinolysis after liver transplantation. Report of five cases. Rev Med Chil 138: 1264-1271.
- Sakai T, Tomimoto H (2014) Central pontine myelinolysis developed during alcohol withdrawal in a chronic alcoholic with hyperosmolar hyperglycemic state. RinshoShinkeigaku 54: 116-123.
- Turnbull J, Lumsden D, Siddiqui A, Lin JP, Lim M (2013) Osmotic demyelination syndrome associated with hypophosphataemia: 2 cases and a review of literature. ActaPaediatr 102: e164-168.
- Petzold S, Kapellen T, Siekmeyer M, Hirsch W, Bartelt H, et al. (2011) Acute cerebral infarction and extra pontine myelinolysis in children with new onset type 1 diabetes mellitus. Pediatr Diabetes 12: 513-517.
- Lee IW, Su MT, Kuo PL, Chang CM (2010) Gestational diabetes and central pontine myelinolysis with quadriplegia: a case report. J Matern Fetal Neonatal Med 23: 728-731.
- Hegazi MO, Mashankar A (2013) Central pontine myelinolysis in the hyperosmolar hyperglycaemic state. Med PrincPract 22: 96-99.
- Chang KY (2014) Plasma exchange successfully treats central pontine myelinolysis after acute hypernatremia from intravenous sodium bicarbonate therapy. BMC Nephrol 15: 56.
- Guerrero WR, Dababneh H, Nadeau SE (2013) Hemiparesis, encephalopathy, and extrapontine osmotic myelinolysis in the setting of hyperosmolar hyperglycemia. J ClinNeurosci 20: 894-896.
- Falini AKC, Pontesilli S, Rovaris M, Scotti G (2001) Differential diagnosis of posterior fossa multiple sclerosis lesions--neuroradiological aspects. NeurolSci 22: S79-S83.
- Ruzek KA, Campeau NG, Miller GM (2004) Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol 25: 210-213.
- Pietrini V, Mozzani F, Crafa P, Sivelli R, Cademartiri F, et al. (2010) Central pontine and extrapontine myelinolysis despite careful correction of hyponatremia: clinical and neuropathological findings of a case. NeurolSci 31: 227-230.
- Tilikete C, Milea D, Pierrot-Deseilligny C (2008) Upbeat nystagmus from a demyelinating lesion in the caudal pons. J Neuroophthalmol 28: 202-206.
- Susa S, Daimon M, Morita Y, Kitagawa M, Hirata A, et al. (1999) Acute intermittent porphyria with central pontine myelinolysis and cortical laminar necrosis. Neuroradiology 41: 835-839.
- Laubenberger J, Schneider B, Ansorge O, Götz F, Häussinger D, et al. (1996) Central pontine myelinolysis: clinical presentation and radiologic findings. EurRadiol 6: 177-183.
- Mascarenhas JV, Jude EB (2014) Central pontine myelinolysis: electrolytes and beyond. BMJ Case Rep 2014.
- Sterns RH, Hix JK, Silver S (2010) Treatment of hyponatremia. CurrOpinNephrolHypertens 19: 493-498.
- Dhrolia MF, Akhtar SF, Ahmed E, Naqvi A, Rizvi A (2014) Azotemia protects the brain from osmotic demyelination on rapid correction of hyponatremia. Saudi J Kidney Dis Transpl 25: 558-566.
- Murthy SB (2013) Osmotic demyelination syndrome: variable clinical and radiologic response to intravenous immunoglobulin therapy. NeurolSci 34: 581-584.
- Rodriguez M, Miller DJ, Lennon VA (1996) Immunoglobulins reactive with myelin basic protein promote CNS remyelination. Neurology 46: 538-545.
- Saner FH, Koeppen S, Meyer M, Kohnle M, Herget-Rosenthal S, et al. (2008) Treatment of central pontine myelinolysis with plasmapheresis and immunoglobulins in liver transplant patient. TransplInt 21: 390-391.
- Wu YC PG, Cheng CA, Lin CC, Huang WS, Hsueh CJ (2009) 99m-Tc-TRODAT-1 and123I-IBZM SPECT studies in a patient with extrapontine myelinolysis with parkinsonian features. Ann Nucl Med 23:409-412.
- Imam YZ, Saqqur M, Alhail H, Deleu D (2012) Extrapontine myelinolysis-induced parkinsonism in a patient with adrenal crisis. Case Rep Neurol Med 2012: 327058.
- Tomita I, Satoh H, Satoh A, Seto M, Tsujihata M, et al. (1997) Extrapontine myelinolysis presenting with parkinsonism as a sequel of rapid correction of hyponatraemia. J NeurolNeurosurg Psychiatry 62: 422-423.
- Srimanee D, Bhidayasiri R, Phanthumchinda K (2009) Extrapontine myelinolysis in preoperative sellar region tumor: report of two cases. J Med Assoc Thai 92: 1548-1553.
- Gupta R GD, Sangal A, Kukreti R, Singhal A (2007) Extrapontine myelinolysis without striatal involvement presenting as pathological crying, reversible parkinsonism and dystonia. Delhi Psy J 10:150-153.
- Gujjar A, Al-Mamari A, Jacob PC, Jain R, Balkhair A, et al. (2010) Extrapontine myelinolysis as presenting manifestation of adrenal failure: a case report. J NeurolSci 290: 169-171.
- Salerno SM, Kurlan R, Joy SE, Shoulson I (1993) Dystonia in central pontine myelinolysis without evidence of extrapontine myelinolysis. J NeurolNeurosurg Psychiatry 56: 1221-1223.
- Sajith J, Ditchfield A, Katifi HA (2006) Extrapontine myelinolysis presenting as acute parkinsonism. BMC Neurol 6: 33.
- Koussa S, Nasnas R (2003) Catatonia and Parkinsonism due to extrapontine myelinolysis following rapid correction of hyponatremia: a case report. J Neurol 250: 103-105.
- Seiser A, Schwarz S, Aichinger-Steiner MM, Funk G, Schnider P, et al. (1998) Parkinsonism and dystonia in central pontine and extrapontine myelinolysis. J NeurolNeurosurg Psychiatry 65: 119-121.






