Keywords
Dengue; Co-infected; COVID-19
Introduction
Dengue fever is a geographically widespread viral disease transmitted by mosquito species Aedes aegypti or Aedes albopictus. It is an endemic disease in many tropical and sub- tropical countries [1,2]. The prevalence of dengue infection is rising globally with 100 to 400 million cases and 22000 deaths per year. Recent reports estimate that it is an alarming situation in Asian countries, contributing 70%-75% of the burden of disease, especially in developing countries such as Pakistan, India, Bangladesh etc., [3,4]. In Pakistan, the recent outbreaks of dengue infection were recorded in 2018-2019 that revealed about a 15-fold rise in cases from 2018 (3,204) to 2019 (47,120). Out of 47,120, confirmed cases 12,053 and 33 deaths were reported from Sindh province [5,6].
In late 2019, a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged from Wuhan, China and spread to 216 countries with 117,476,803 confirmed cases and 2,606,136 deaths worldwide. The World Health Organization (WHO) has declared the coronavirus disease 2019 (COVID-19) as a "Pandemic". Until now, Pakistan has reported 672,931 confirmed COVID-19 cases and 14,530 deaths [7]. This infection presents clinically with symptoms similar to dengue infection including headache, fever, nausea, skin rashes, vomiting, muscular pain, respiratory distress etc., [3,8]. Therefore, it is a challenge for healthcare professionals to discriminate dengue and COVID-19 on early clinical presentation.
Recently, few countries have reported the co-infection of dengue and COVID-19, especially in dengue-endemic countries. These include Pakistan, Brazil, India, France etc., [9-12]. Co-infection is defined as the occurrence of dengue fever and acquiring COVID-19 infection during the incubation period of dengue. Generally, the incubation period of dengue is 7-10 days; simultaneous infection of SARS-CoV-2 during this incubation period produces a co-infection. However, few studies from Singapore, Thailand, and Indonesia [13-17] etc., reported that it is the misdiagnosis of COVID-19. SARS-CoV-2 positive cases initially misdiagnosed as dengue due to the false-positive results of dengue antibodies (IgM, IgG), but worsening of patient symptoms addresses the physician to test for COVID-19. The initial misdiagnosis as dengue infection leads to the spread of COVID-19 infection because of delay in case isolation and patient management.
Serological testing is an easy, useful and convenient way for rapid diagnosis of dengue. Numerous kits for dengue antigen or antibody detection were introduced several years ago. Based on serology, dengue infection is classified into primary and secondary infection. Primary infection is defined as a rise of IgM but not the IgG in the blood sample which is drawn within the early onset of infection (3-5 days). Whereas secondary infection is defined as the presence of IgG in the serum sample which is drawn within the early onset of infection (3-5 days). In secondary infection, dengue IgM may be positive or negative depending on the time interval of sample collection. IgM may be positive in the early phase of secondary infection [18,19]. However, for the confirmation of viral infection Nucleic Acid Amplification Test (NAAT) is performed using the Polymerase Chain Reaction (PCR) method [20]. But these advanced techniques are expensive and not commonly used in diagnosis as the cases are treated symptomatically, especially in low-income countries where diagnostic capabilities are limited.
Pakistan is currently facing a COVID-19 pandemic that has spread across the country. Therefore, it can be estimated that Pakistan is also at high risk of possible co-infection or cross-reactivity. Therefore, this study aims to determine the current situation of co-infection or cross-reactivity in a tertiary care hospital in Karachi, Pakistan. Co-infection may lead to the severity and boost up the mortality rate by activating the Antibody-Dependent Enhancement (ADE) and cross-reactivity leads to inappropriate diagnosis and treatment. Furthermore, liver profile changes will be determined to identify whether the co-infected patients have severe liver damage. As liver is the main target organ of dengue as well as SARS-CoV-2 infection. Lastly, the serological profile of co-infected cases will be explored to classify the chances of cross- reactivity of DENV with COVID-19 antibodies.
Methodology
This was a retrospective study conducted at The Indus Hospital Karachi, Sindh Pakistan. The study approval was exempted by the Institutional Review Board (IRB) of the Interactive Research and Development (IRD_IRB_2021_01_015). The data was extracted from the Indus hospital electronic Management Information System (HMIS) in Microsoft Excel format, from the period of 1st- Jan -2020 to 31st- Dec -2020.
Selection criteria of co-infected cases
Dengue positive cases detected on dengue NS1 antigen/dengue IgM/IgG antibodies along with COVID 19 RT-PCR positive during Dengue fever incubation period were selected as co-infected cases and were included in the study.
Diagnosis of dengue infection
Dengue NS1 antigen: It is a qualitative screening test of dengue infection by nonstructural protein NS1 antigen which is secreted in dengue infected patient sample. The test was performed on SD BIOLINE, standard diagnostics, Kenya test kit. It is a rapid detection method based on immune-chromatographic technique and performed on plasma or serum sample.
Dengue IgG and IgM: To differentiate the type of infection, IgM and IgG antibody assays were performed by immune- chromatography strip method (SD BIOLINE, Standard Diagnostics, and Kenya). SD BIOLINE test kit has greater sensitivity and specificity 94.2% and 96.4% for the classification of dengue cases into primary and secondary dengue infection respectively.
Diagnosis of SARS-CoV-2 infection by RT-PCR
For qualitative screening of the SARS-CoV-2 virus, the nasopharyngeal swab samples were collected, the test was performed on a fully automated Cobas 6800 instrument (Roche Molecular Diagnostics, USA). Interpretation of results was done on amplification of target genes as COVID-19 positive or negative.
Liver function tests
The liver function tests include Total and Direct Bilirubin and liver enzymes i.e. Alanine aminotransferase (ALT), Gamma-Glutamyl Transferase (GGT) and Alkaline Phosphatase (ALP). Total and direct bilirubin was tested to assess the intrahepatic and extrahepatic cholestatic condition, whereas Alanine Aminotransferase (ALT) was performed to evaluate the synthetic functions of the liver. However, Alkaline Phosphatase (ALKP) and Gamma-Glutamyl Transferase (GGT) were measured to assess the hepatobiliary diseases. All the tests were performed on an Abbott Alinity C automated clinical chemistry analyzer that works on the principle of spectrophotometry. The cutoff values of each test results were considered based on reference ranges used by the laboratory.
Statistical analysis
The data was analyzed on Statistical Package for the Social Sciences (SPSS), Version 21.0. The chi-square test/Fisher exact test was applied for association studies.
Results
In the one-year study period, a total of 16950 cases were suspected of dengue infection. Out of them, only 1753 cases were confirmed to have dengue infection having antigen/ antibody positive (NS1, IgM, IgG). Among the confirmed dengue cases, suspicion of COVID-19 was raised in (146%, 8.44%). The diagnosis of COVID-19 virus infection was confirmed by RT-PCR. The co-infection was provisionally confirmed in individuals that show positive results of COVID-19 RT-PCR within 10 days of dengue positive results of antigen/antibody test. However, (38%, 2.11%) showed the confirmed co-infection of dengue-COVID-19, whereas few of them (4%, 0.22%) showed dengue and COVID-19 infection at different intervals of the selected period of the year, ranging from 17,30,63,90 days respectively (Figure 1).
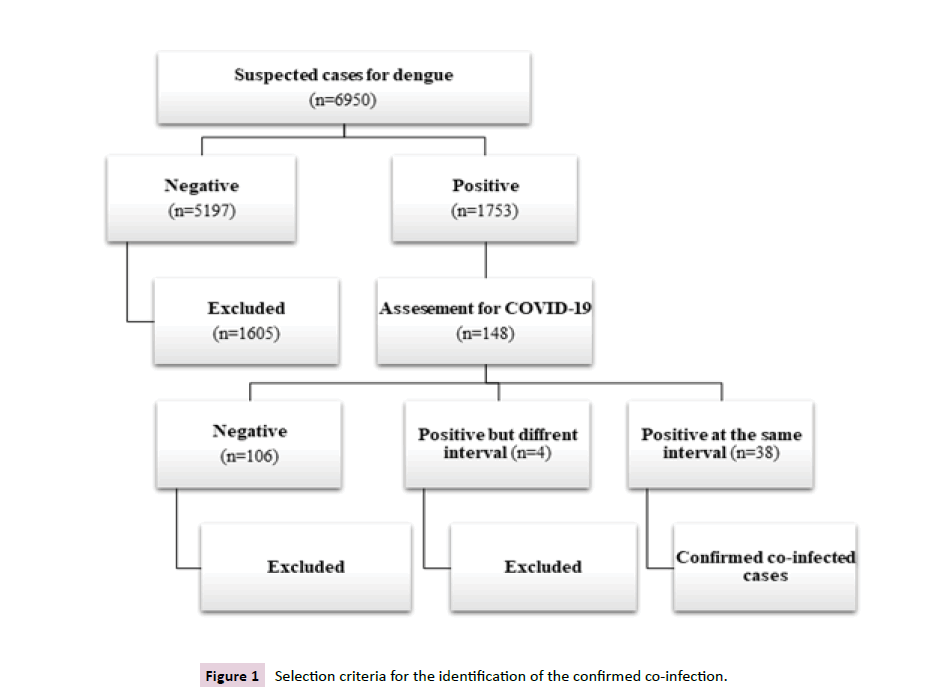
Figure 1 Selection criteria for the identification of the confirmed co-infection.
Frequency and trend of dengue and co-infection
The highest number of confirmed dengue cases were reported in Oct-Dec (36.28%) followed by July-Sep (24.30%), and Jan-March (23.44%), whereas the smallest number of cases was reported in the interval of April-Jun (15.97%). A significant decline in the number of reported dengue cases was observed in April-Jun as compared to other month quartiles. In comparison, the frequency and trend of co-infected cases (38%, 2.11%) were calculated, which showed that the highest number of co-infected cases in Oct-Dec followed by July-Sept, then April-Jun. While no case was reported in the Jan-march quartile (Figure 2).

Figure 2 Frequency and interquartile trends of dengue and dengue COVID-19 co-infection.
Distribution of co-infected cases according to age and gender
The demographic data i.e. age and gender distribution of co- infected cases (38%, 2.11%) was assessed. It was found that, among the co-infected cases, most of them were found in the age group of 61-80 years followed by the 40-60 years of age i.e. older age group (Figure 3A). However, according to the gender distribution, (23%, 61%) were male whereas (15%, 39%) were females. So, male predominance was observed in the co-infected cases (Figure 3B).

Figure 3 Demographics (age, gender) distribution of coinfection.
Moreover, the association of the co-infection with demographical variables i.e. age groups and gender were calculated by comparing the two groups (I) Dengue with COVID-19 positive and (II) Dengue positive but COVID-19 negative. It was found that there is a significant positive association between the co-infection and age groups (p-value<0.029). While no association was observed between the gender and the co-infection cases (p-value<0.889) as shown in Table 1.
| Variables |
Number of co-infected cases (%) |
Number of dengue + COVID-19 - cases (%) |
P-value |
| Age (years) |
| <20 |
2(5.3) |
17(16.0) |
0.029 |
| 20-40 |
4(10.5) |
31(29.2) |
| 41-60 |
14(36.8) |
25(23.6) |
| 61-80 |
17(44.7) |
30(28.8) |
| >81 |
1(2.6) |
3(2.8) |
| Gender |
| Male |
23(61.5) |
46(43.4) |
0.889 |
| Female |
15(39.5) |
60(56.6) |
Table 1: Association of demographic variables with co-infection.
Liver function test of co-infected cases
Liver function test is recognized as a marker to predict the severity of dengue and COVID-19 infections. Therefore, any alteration of liver function test will be used to verify whether the co-infected cases have a severe or non-severe liver infection. It was observed that the frequency to order liver function tests was very high in co-infection. In this retrospective study, we successfully obtain the data of Liver Function Tests (LFTs) of thirty-six co-infected patients out of thirty-eight. Among these co-infected cases (36), the majority showed normal levels of liver function tests in comparison to reference values, that includes total bilirubin (31%,86.1%), direct bilirubin (27%, 75%), ALT (22%, 61.1%) GGT (34%, 94.4%), ALP (31%, 86.1%). Whereas a small number of cases, the total bilirubin (5%, 13.9%), direct bilirubin (9%, 25%), ALT (14%, 38.9%), (2%, 5.6%), GGT (2%, 5.6%) ALP (5%, 13.9%) revealed mild variations. The association studies in comparing the group (I) Dengue-COVID-19 positive and group (II) Dengue positive but COVID-19 negative showed no significant association with the liver function test as shown in Table 2.
| Liver function test |
Levels |
Ranges |
Number of co-infected cases (%) |
Number of dengue+ COVID-19 - cases (%) |
P-value |
| Total bilirubin (mg/dl) |
Normal |
0.3-1.2 |
31(86.1) |
73(78.5) |
0.599 |
| Altered |
>1.2 |
5(13.9) |
20(21.5) |
| Direct bilirubin (mg/dl) |
Normal |
0-0.5 |
27(75) |
66(71.7) |
0.94 |
| Altered |
>0.5 |
9(25) |
26(28.3) |
| ALT (U/L) |
Normal |
≤45 |
22(61.1) |
62(67.4) |
0.23 |
| Altered |
45-999 |
14(38.9) |
27(29.3) |
| Severe |
1000 |
0(0) |
3(3.3) |
| GGT (U/L) |
Normal |
<55 |
34(94.4) |
77(83.7) |
0.2899 |
| Altered |
>55 |
2(5.6) |
15(16.3) |
| ALP (U/L) |
Normal |
<150 |
31(86.1) |
68(73.1) |
0.174 |
| Altered |
>150 |
5(13.9) |
25(26.9) |
Table 2: Liver function tests of coinfected cases.
Serological testing of co-infected cases
Serological testing is necessary to determine the type of dengue infection. The co-infected cases were grouped based on the serotypes of dengue. Among the 38 co-infected cases (31%, 81.57%) were seropositive for only anti-DENV-IgG antibody, whereas few co-infected cases (5%, 13.15%) were positive for serology anti-DENV IgM and IgG antibodies. However, only two co-infected cases were associated with primary dengue infection having positive serotype of only DENV-NS1 antigen (2.63%) and IgM positive (2.63%) respectively (Table 3).
| Type of dengue |
Serological testing |
Number of cases (%) |
| Primary dengue |
NS-1 |
1(2.63) |
| IgM |
1(2.63) |
| NS1 & IgM |
0(0) |
| Secondary dengue |
IgG |
31(81.6) |
| IgM & IgG |
5(13.2) |
| NS1, IgM & IgG |
0(0) |
Table 3: Dengue serological profile of the coinfected cases.
Discussion
Dengue fever is a high burden endemic disease in the developing countries of Asia, where mostly the diagnosis is based on clinical symptoms and lab testing of Dengue NS-1 antigen or Dengue serology. Confirmatory test like reverse-transcriptase polymerase chain reaction (RT-PCR) is not usually required to establish the diagnosis. Unfortunately, the recently introduced COVID-19 infection created an issue of misdiagnosis of the disease due to similar signs and symptoms.
COVID-19 infection can be associated with multiple bacterial and viral co-infections [20,21]; nevertheless, co-infection of SARS- CoV-2 with dengue virus is still scarce. It is still unclear about the frequency and severity of dengue-SARS-CoV-2- co-infection as there is no retrospective or meta-analysis data published so far. Therefore, the current study designed to identify the co-infected cases of dengue-COVID-19 in the tertiary care hospital of Pakistan located in Karachi. The data for the year 2020 of dengue was collected; filtered and co-infected DENV-COVID-19 cases were identified. Results suggested that, like other nations, Pakistan is also suffering from the situation of co-infection. This study reports a total of 38 cases of dengue-SARS-CoV2 co-infection in a tertiary care hospital of Pakistan and this was not the first study of co- infection from Pakistan. Siddiqui et al., report the cases of DENV- COVID-19 co-infection [9]. Few studies reported that this state of co-infection is basically due to cross-reactivity of SARS-CoV-2- DENV antibodies and vice versa which leads to misdiagnosis as reported from other countries [12,13].
Furthermore, the frequency and trend of co-infected cases were studied. The highest number of cases was found in Oct-Dec followed by July-Sep and lastly in April-June quartiles. Similarly, the “High Alert” for dengue is the monsoon season (July-Dec), whereas, another report stated Sept-Oct is the utmost vulnerable period for the spread of dengue in Pakistan [22,23]. Conversely, for COVID-19, the situation was diverse, the major outbreaks of SARS-CoV-2 observed in May-July and Nov-Dec [7]. These simultaneous outbreaks of DENV and COVID-19 in the same quartile (Oct-Dec) increased the number of co-infected cases, as in this quartile highest number of co-infected cases was reported. However, no case was reported in the Jan-March quartile that may due to late onset of COVID-19 infection in Pakistan, as the first case diagnosed on 26th February 2020. Secondly, in the first quartile, there were no appropriate diagnostic facilitates available for COVID-19 testing.
Additionally, demographical variables (age, gender) and their association with co-infected cases were explored in comparison of co-infected and non-co-infected cases. Results revealed that out of thirty-eight cases (14%,36.8%) and (17%,44.7%) overall about four-fifth of, co-infected cases fell in age groups of 41-60 and 61-80 years of age respectively. As stated earlier, Dengue and COVID-19 virus affect more in the older age group as compared to the adults' group. But extensive former investigations suggest that dengue affects more in the age group of 20-60 years as compared to age >60 years [24,25], whereas chances of getting COVID-19 infection rise with the increase of age (>60 years) [26]. On the other hand, no significant association was found with gender whereas in dengue male preponderance is common [27]. The overall results are suggesting that the older age group as compared to adults are more prone to DENV-COVID-19 co- infection however males and females can equally get co-infected.
The liver is the major target organ of damage by dengue and COVID-19 infection. A Liver Function Tests (LFTs) serves as a biomarker of severity in both infections [28,29]. So, in this study, LFTs profile was assessed to predict the severity of co- infected cases. The basic idea is that in co-infection the liver derangement is expected to be augmented when two viruses are affecting the liver at the same time as discussed in Antibody- Dependent Enhancement (ADE) response. ADE is represented by the binding of non-neutralizing antibodies with dengue virus followed by entrance of the antigen-antibody complex in the cell by fcy receptor that increases the viral load and thus the severity of disease which is enhanced in secondary infection [30]. In contrast, it was found that most of the co-infected cases have normal LFTs. However, a small number of cases showed variations in LFTs, but the association with co-infected cases was non-significant. However, it has been confirmed from previous studies that the liver function tests significantly altered in dengue patients especially in secondary dengue as compared to primary dengue infection and it leads to liver failure [29,31,32]. The majority of cases found in this study were secondary dengue co- infected with COVID-19 but about two-third (22%, 61.1%) of co- infected cases displayed the normal ALT levels, where some cases (14%, 38.9%) showed slightly elevated levels. Not a single case showed the ALT levels >1000 U/L which indicates that none of the cases had severe dengue infection according to WHO criteria (In severe dengue infection ALT>1000 U/L) [33]. In addition, recently Saddique, et al., Teotônio, et al., compared the diagnostic parameters of the DENV-COVID-19 co-infected cases and only COVID-19 infected cases, which also revealed, co-infection might be significantly affecting haemoglobin, neutrophils, and monocytes levels but no significant changes were observed in liver enzymes [9,34]. These results in agreement with the previous meta-analysis concluded that there is no difference in clinical disease severity between viral co-infections and single infection [35]. To understand this complexity, one phenomenon is of viral interference, i.e. one virus competitively blocking the entry and replication of another virus via ACE2 receptors in a single individual, SARS-CoV-2 over DENV, as SARS-CoV-2 have additional proteins [36] and causing competitive inhibition because both viruses ultimately are targeting endothelial cells [37]. Contrarily, these results (Age group, Gender, LFTs) are also highlighting alternative postulation, chances of false-positive results of dengue serology. The confirmed co-infected RT-PCR positive cases might have false-positive dengue serology due to cross-reactivity, as in these cases dengue infection was confirmed based on dengue serology results.
For further understanding the cross-reactivity of antigen or antibodies, we explored the serology of co-infected cases, which indicated that mainly the secondary dengue infection having Anti- DENV-IgG may be associated with cross-reactivity. As only five cases report Anti-DENV-IgG and IgM positivity, one was only IgM positive, and one was NS1-Ag positive, whereas thirty-one cases were showed the positive serology of IgG only. Results of this study, supposed to be in concurrence with former investigations, where the cross-reaction of IgM and IgG was found more as compared to NS-1 Ag. For instance, Yan et al. report two cases of false- positive dengue serology (IgG, IgM) [13], Likewise Nasomsong, et al., Ratnarathon, et al., positive dengue serology (IgM and IgG) and negative NS1-Ag in the SARS-CoV-2 infected cases. Cross- reactivity was confirmed by negative results of RT-PCR of dengue [14,15]. Conversely, Kembuan, G.J stated that co-infection and cross-reactivity both persist in the population as five cases were reported to have a mixed infection. But after repeat lab testing, four cases showed negative results of dengue serology, whereas one case was again positive for dengue serology and SARS-CoV2 (RT-PCR) [16]. Similarly, Masyeni, et al., reported co-infection of DENV-COVID-19 in one patient having positive NS1-Ag and SARS−COV-2 positive by RT-PCR. Although, repeat testing showed two cases had false-positive results [17]. Additionally, the false- positive dengue serology was also reported in a European study when only one serum sample was used multiple times by enzyme- linked immunosorbent assay (ELISA; PanBio) [38]. Furthermore, comparison studies between rapid diagnostic test (RDT) kits of dengue and COVID-19 also revealed the possibility of cross- reactivity of antibodies [39,40]. The above-mentioned studies suggested that SARS-CoV-2 and DENV might have antigenic structural similarities as all the antibody kits are manufactured to detect antibodies against the specific protein antigen i.e. Abbott diagnostic kits of dengue detect antibodies against the envelope protein. To further understand this concept, In-silico study was performed that also confirmed the similarity of outer proteins of DENV and SARS-CoV-2 [41]. Additionally, shreds of evidence come from computational docking study that declared DENV antibodies that are manufactured based on envelope proteins of DENV bind to Receptor-Binding Domain (RBD) of amino acid residues of SARS-CoV-2 that are crucial for interaction with ACE2 receptors in SARS-CoV-2 infection [42]. Overall reports are pieces of evidence that there is a possibility of cross-reactivity of IgG and IgM in these cases too. On the other hand, the chances of false-positive results in molecular testing are rare, these cases may only be having SARS-CoV-2 infection, but misdiagnosed as co-infection. On the other hand, several studies confirm that co-infection and cross-reactivity both events are running in the community [16,17,40]. Therefore, this study suggest that clinicians should consider both options i.e, cross-reactivity or co-infection when diagnosing dengue and SARS-CoV-2 infection because misdiagnosis of SARS-CoV-2 may play a significant role in the spread of SARS-CoV-2 infection due to delay in case isolation, misdiagnosis and contact tracing.
At last, considering both assumptions this study depicts that if it is co-infection then mainly secondary dengue is associated with COVID-19 infection and if it is cross-reactivity, the risk of cross-reactivity of antibodies IgM and IgG with COVID-19 is high as compared to dengue NS-1 Ag. On the contrary, the NS-1 antigen is a non-structural protein that cannot be expressed in SARS-CoV-2 infection as SARS-CoV-2 have structural proteins only [36]. Therefore, it can be hypothesized that where NS-1 antigen positivity found in SARS-CoV-2 cases, these cases may confirm to be real co-infected but no such strong evidences are available to prove this hypothesis. Extensive studies are needed to clarify this multifarious condition. But according to our understanding probability of cross-reactivity may be high as compared to co- infection as in this study only a single case was NS-1 antigen positive.
Conclusion
This study strongly supports the concurrence of cross-reactivity between DENV and SARS-CoV-2 antibodies which may be found in these co-infected cases. If it is co-infection, secondary dengue infection is more involved in co-infection with COVID-19 as compared to primary dengue infection and may not be causing severe variations in the liver function tests. Furthermore, antigen/antibody kit-based tests are not a reliable method for the diagnosis of dengue infection; it must be confirmed by the RT- PCR especially in secondary dengue infection. Co-infection and cross-reactivity both are serious health issues that need in-depth attention from the researchers to introduce a new standard method or technique for differentiation of dengue and SARS- CoV-2 infections in the future.
Limitation
The limitations of this retrospective study are the inability to confirm the diagnosis of dengue infection by RT-PCR or virus isolation method. Secondly, due to the large size of dengue cases, comparison of LFTs of co-infected cases with the dengue cases was not possible.
Acknowledgement
We would like to acknowledge the Electronic medical record department of The Indus Hospital for providing us patients’ data.
Author’s Contribution
Conceptualization and designing of the study, Maliha Zubairy, Fatima Kanani writing draft preparation of the manuscript, Maliha Zubairy and Soma Vankwani, Revising and editing, Soma Vankwani, Maliha Zubairy and Fatima Kanani. All authors agreed to the final version of the manuscript.
39032
References
- Rana MS, Alam MM, Salmn M, Ikram A (2019) Prevention and control of escalating dengue epidemics in Pakistan. J Med Viral 92: 927-928.
- Lwande OW, Obanda V, Lindsdet A, Ahlm C, Evander M, et al. (2020) Globe-trotting Aedes aegypti and Aedes albopictus: Risk factors for arbovirus pandemics. Vector Borne Zoonotic Dis 20: 71-81.
- Butt MH, Ahmad A, Misbah S, Mallhi TH, Khan YH (2020) Dengue fever and COVID‐19 co-infection; a threat to public health for coepidemic in Pakistan. J Med Virol 93: 671-672.
- Rana MS, Ikram A, Alam MM, Salman M (2021) Novel coronavirus outbreak in Pakistan: Beware of dengue. J Formos Med Assoc 120: 765.
- Centers for Disease Control and Prevention (2020) National center for emerging and zoonotic infectious DIseases (NCEZID), Division of vector-borne diseases (DVBD).
- Saddique A, Rana MS, Alam MM, Ikram A, Usman M, et al. (2020) Emergence of co-infection of COVID-19 and dengue: A serious public health threat. J Infect 81: e16.
- Bicudo N, Bicudo E, Costa JALP, Barra GB (2020) Co-infection of SARS-CoV-2 and dengue virus: a clinical challenge. Braz J Infect Dis 24: 452-454.
- Mahajan NN, Kesarwani SN, Shinse SS, Nayak A, Deepak N (2020) Co‐infection of malaria and dengue in pregnant women with SARS‐CoV‐2. Int J Gynecol Obset 151: 459-462.
- Verduyn M, Allou N, Gazaille V, Andre M, Desroche T, et al. (2020) Co-infection of dengue and COVID-19: A case report. Negl Trop Dis 14: e000847.
- Yan G, Lee KC, Lam MTL, Yan B, Chua XY, et al. (2020) Covert COVID-19 and false-positive dengue serology in Singapore. Lancet Infect Dis 20: 536
- Ratnarathon, A.C., Pongpirul K, Pongpirul AW, Charoenpong L, Prasithsirikul W (2020) Potential dual dengue and SARS-CoV-2 infection in Thailand: A case study. Case Rep 6: E04175.
- Nasomsong W, Luvira V, Phiboonbanakit D (2020) Case Report: Dengue and COVID-19 Co-infection in Thailand. Amer J Trop Med Hygiene 110: 487-489.
- Kembuan GJJI (2020) Dengue serology in Indonesian COVID-19 patients: Co-infection or serological overlap? ID Cases 22: e00927.
- Masyeni S, Santosoc SM, Widyaningsih DP, Asmara WGD, Nainu F, et al. (2021) Serological cross-reaction and co-infection of dengue and COVID-19 in Asia: Experience from Indonesia. Int J Infec Dise 102: 152-154.
- Changal KH, Ab Raina H, Raina A, Raina M, Bashir R, et al. (2016) Differentiating secondary from primary dengue using IgG to IgM ratio in early dengue: An observational hospital based clinico-serological study from North India. BMC Infect Dis 16: 1-7.
- Lima JRC, Rouquayrol ZM, Callado MRM, Guedes FIM, Pessoa C (2012) Interpretation of the presence of IgM and IgG antibodies in a rapid test for dengue: Analysis of dengue antibody prevalence in Fortaleza City in the 20th year of the epidemic. Rev Soc Bras Med Trop 45: 163-167.
- Lanciotti RS, Calisher HC, Gubler JD, Chang JG, Vorndam VA (1992) Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microb 30: 545-551.
- Contou D, Claudinon A, Pajot O, Micaëlo M, Flandre LP, et al. (2020) Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intens Care 10: 1-9.
- Haqqi A, Awan AU, Ali M, Saqib NAM, Ahmed H, et al. (2021) COVID‐19 and dengue virus coepidemics in Pakistan: A dangerous combination for an overburdened healthcare system. J med virol 93: 80-82.
- National Institute of Health (2020) Seasonal awareness and alert letter.
- Raza FA, Rehman US, Khalid R, Ahmad J, Ashraf S, et al. (2014) Demographic and clinico-epidemiological features of dengue fever in Faisalabad, Pakistan. Plos one 9: e89868.
- Khan J, Khan I, Ghaffar A, Khalid B (2018) Epidemiological trends and risk factors associated with dengue disease in Pakistan (1980–2014): A systematic literature search and analysis. BMC Pub Health 18: 1-13.
- Centers for disease control and prevention report: At increased risk for severe illness, COVID-19 update. (2021).
- Anker M, Arima Y (2011) Male–female differences in the number of reported incident dengue fever cases in six Asian countries. Western Pac Surveill Response J 2: 17-23.
- Piano S, Dalbeni A, Vettore E, Benfaremo D, Mattioli M, et al. (2020) Abnormal liver function tests predict transfer to intensive care unit and death in COVID‐19. Liver Int 40: 2394-2406.
- Chia PY, Thein LT, Ong XWS, Lye CD, Leo SY (2020) Severe dengue and liver involvement: an overview and review of the literature. Exp Rev Anti-infective Ther 18: 181-189.
- Harapan H, Ryan M, Yohan B, Abidin SR, Nainu F, et al. (2021) Covid‐19 and dengue: Double punches for dengue‐endemic countries in Asia. Rev Med Virol 31: e2161.
- Shastri PS, Gupta P, Kumar R (2020) A prospective 3 year study of clinical spectrum and outcome of dengue fever in ICU from a tertiary care hospital in North India. Indian J Anaesth 64: 181-186.
- Khurram M, Qayyum W, ul Hassan SJ, Mumtaz S, Bushra HT (2014) Dengue hemorrhagic fever: Comparison of patients with primary and secondary infections. J of infec pub health 7: 489-495.
- Dengue guideliness for diagnosis, treatment, prevention, control. World Health Organization (WHO). (2009).
- Teotônio IMSN, De Carvalho JL, Castro LC, Nitz N, Hagström L, et al. (2021) Clinical and biochemical parameters of COVID-19 patients with prior or active dengue fever. Acta Tropica 214: 105782.
- Asner SA, Science ME, Tran D, Smieja M, Merglen A, et al. (2014) Clinical disease severity of respiratory viral co-infection versus single viral infection: A systematic review and meta-analysis. PloS one 9: e99392.
- Henrina J, Putra ICS, Lawrensia S, Handoyono QF, Cahyadi A (2020) Coronavirus disease of 2019: A mimicker of dengue infection?. SN Comp Clinical Med 2: 1-11.
- Kumar N, Sharma S, Barua S, Tripathi BN, Rouse BT (2018) Virological and immunological outcomes of coinfections. Cli micro rev 31: e00111-17.
- Wichmann O, Stark K, Shu PY, Niedrig M, Frank C, et al. (2006) Clinical features and pitfalls in the laboratory diagnosis of dengue in travellers. BMC infec dise 6: 1-8.
- Santoso MS, Masyeni, S Haryanto S, Yohan B, Hibberd ML (2021) Assessment of dengue and COVID-19 antibody rapid diagnostic tests cross-reactivity in Indonesia. Vir j 18: 1-5.
- Nath H, Mallick A, Roy S, Sukla S, Basu K, et al. (2020) Dengue antibodies can cross-react with SARS-CoV-2 and vice versa-Antibody detection kits can give false-positive results for both viruses in regions where both COVID-19 and Dengue co-exist. MedRxiv.
- Lustig Y, Keler S, Kolodny R, Ben-Tal N, Atias-Varon D, et al. (2020) Potential antigenic cross-reactivity between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and dengue viruses. Clin infect dis 2: 1-6.
- Nath H, Mallick A, Roy S, Sukla S, Biswas S (2021) Computational modelling supports that dengue virus envelope antibodies can bind to SARS-CoV-2 receptor binding sites: Is pre-exposure to dengue virus protective against COVID-19 severity?. Compu Struc Biotech J 19: 459-466.








